Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Participants
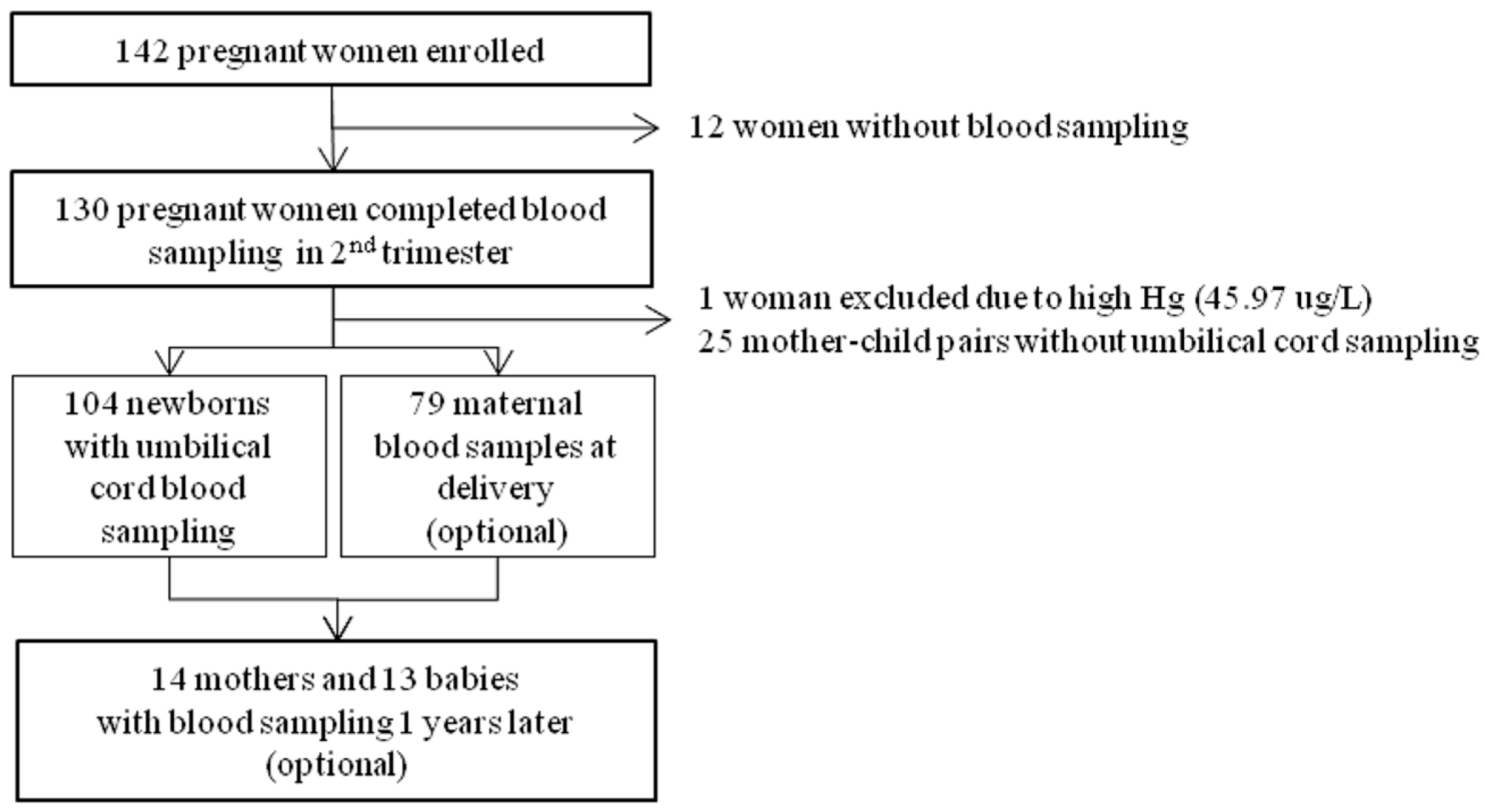
2.2. Questionnaire
2.3. Heavy Metals Analysis
| Parameters | Conditions |
|---|---|
| RF Power (kW) | 1.55 |
| Carrier gas flow rate (L/min) | 1.15 |
| Sample depth (mm) | 8.0 |
| Sampler and skimemer cones | Nickel |
| Spray chamber temperature (°C) | 2 |
| Nebulizer type | Concentric nebulizer |
| Sample uptake rate (RPS) | 0.5 |
| He flow rate (mL/min) | 4.5 |
| Analytical masses | 111Cd, 208Pb |
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Participants
| Category | Characteristics | Mean | ±Std | Min | Max |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Age (year) | 31.8 | ±4.0 | 22.0 | 46.0 | |
| Gestational weeks at recruitment (week) | 20.7 | ±4.3 | 13.0 | 28.0 | |
| Gestational weeks at delivery (week) | 38.8 | ±1.2 | 34.6 | 41.1 | |
| Infant’s characteristics | n | (%) | |||
| Sex | |||||
| boy | 66 | (63.5) | |||
| girl | 38 | (36.5) | |||
| Delivery methods | |||||
| vaginal | 65 | (62.5) | |||
| C/S | 39 | (37.5) | |||
| Birth order | |||||
| first | 53 | (51.0) | |||
| over second | 51 | (49.0) | |||
| Hospitalization after birth | |||||
| no | 78 | (75.0) | |||
| yes | 26 | (25.0) |
3.2. Maternal Blood Levels of Heavy Metals at Different Sampling Times
| Heavy Metals | Periods | N | GM | G. Std | Min | P10 | P25 | P50 | P75 | P90 | Max | p Value * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead | at 2nd trimester | 104 | 1.02 | ±1.39 | 0.50 | 0.65 | 0.81 | 1.03 | 1.26 | 1.58 | 2.20 | 0.7703 |
| (µg/dL) | at delivery | 79 | 1.03 | ±1.34 | 0.51 | 0.67 | 0.85 | 1.02 | 1.27 | 1.51 | 1.82 | |
| after 1 year | 13 | 1.08 | ±1.34 | 0.66 | 0.81 | 0.89 | 1.02 | 1.48 | 1.58 | 1.62 | ||
| Cadmium | at 2nd trimester | 104 | 0.61 | ±1.51 | 0.26 | 0.38 | 0.44 | 0.60 | 0.81 | 1.01 | 2.01 | 0.6437 |
| (µg/L) | at delivery | 79 | 0.61 | ±1.58 | 0.24 | 0.33 | 0.45 | 0.59 | 0.84 | 1.09 | 2.80 | |
| after 1 year | 13 | 0.54 | ±1.52 | 0.23 | 0.35 | 0.39 | 0.61 | 0.79 | 0.84 | 0.86 | ||
| Total mercury | at 2nd trimester | 104 | 2.97 | ±1.45 | 1.34 | 1.92 | 2.34 | 2.89 | 3.71 | 5.05 | 8.41 | 0.0079 |
| (µg/L) | at delivery | 79 | 2.66 | ±1.40 | 1.36 | 1.75 | 2.14 | 2.66 | 3.13 | 4.26 | 8.50 | |
| after 1 year | 13 | 3.63 | ±1.48 | 2.23 | 2.36 | 2.56 | 3.42 | 4.90 | 5.87 | 7.77 | ||
| Methylmercury | at 2nd trimester | 104 | 2.39 | ±1.45 | 1.02 | 1.54 | 1.86 | 2.25 | 2.89 | 4.18 | 6.96 | 0.0010 |
| (µg/L) | at delivery | 79 | 2.16 | ±1.42 | 1.02 | 1.40 | 1.72 | 2.18 | 2.59 | 3.32 | 6.82 | |
| after 1 year | 13 | 3.25 | ±1.49 | 1.88 | 1.95 | 2.41 | 2.86 | 4.26 | 5.26 | 7.03 |
3.3. Blood Levels of Heavy Metals in the Children at Different Sampling Times
| Heavy Metals | Periods | N | GM | G. Std | Min | P10 | P25 | P50 | P75 | P90 | Max | p Value * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lead | at birth | 104 | 0.71 | ±1.42 | 0.47 | 0.57 | 0.73 | 0.88 | 1.05 | 0.20 | 1.58 | 0.1161 |
| (µg/dL) | at 1 year old | 14 | 0.85 | ±1.73 | 0.46 | 0.55 | 0.84 | 1.49 | 1.90 | 0.39 | 2.14 | |
| Cadmium | at birth | 104 | 0.01 | ±5.31 | 0.00 | 0.00 | 0.02 | 0.04 | 0.06 | 0.00 | 0.22 | 0.0222 |
| (µg/L) | at 1 year old | 14 | 0.03 | ±3.35 | 0.00 | 0.02 | 0.04 | 0.08 | 0.13 | 0.00 | 0.18 | |
| Total mercury | at birth | 104 | 4.44 | ±1.49 | 2.61 | 3.32 | 4.35 | 5.58 | 7.24 | 2.08 | 12.06 | <0.0001 |
| (µg/L) | at 1 year old | 14 | 1.51 | ±1.52 | 0.90 | 1.22 | 1.45 | 1.99 | 2.53 | 0.64 | 3.49 | |
| Methylmercury | at birth | 104 | 3.67 | ±1.51 | 2.25 | 2.59 | 3.56 | 4.57 | 5.87 | 1.79 | 11.25 | <0.0001 |
| (µg/L) | at 1 year old | 14 | 1.48 | ±1.48 | 0.85 | 1.28 | 1.45 | 2.03 | 2.16 | 0.71 | 3.25 |
3.4. Ratios of Heavy Metal Levels in the Newborns to those in the Mothers
| Heavy Metals | N | Mean | Std | Min | P25 | Median | P75 | Max |
|---|---|---|---|---|---|---|---|---|
| Lead | 104 pairs * | 0.76 | ±0.31 | 0.19 | 0.56 | 0.72 | 0.89 | 2.52 |
| 79 pairs ** | 0.72 | ±0.25 | 0.33 | 0.55 | 0.66 | 0.87 | 1.80 | |
| Cadmium | 104 pairs | 0.04 | ±0.05 | 0.00 | 0.01 | 0.03 | 0.05 | 0.42 |
| 79 pairs | 0.04 | ±0.05 | 0.00 | 0.01 | 0.02 | 0.06 | 0.37 | |
| Total mercury | 104 pairs | 1.56 | ±0.49 | 0.64 | 1.26 | 1.49 | 1.77 | 4.01 |
| 79 pairs | 1.76 | ±0.43 | 1.09 | 1.49 | 1.68 | 1.90 | 4.26 | |
| Methylmercury | 104 pairs | 1.62 | ±0.56 | 0.69 | 1.22 | 1.57 | 1.90 | 4.43 |
| 79 pairs | 1.81 | ±0.52 | 1.25 | 1.45 | 1.73 | 1.95 | 4.89 |
3.5. Correlation between Maternal Blood Levels of Heavy Metals and Newborn Blood Levels of Heavy Metals at Birth
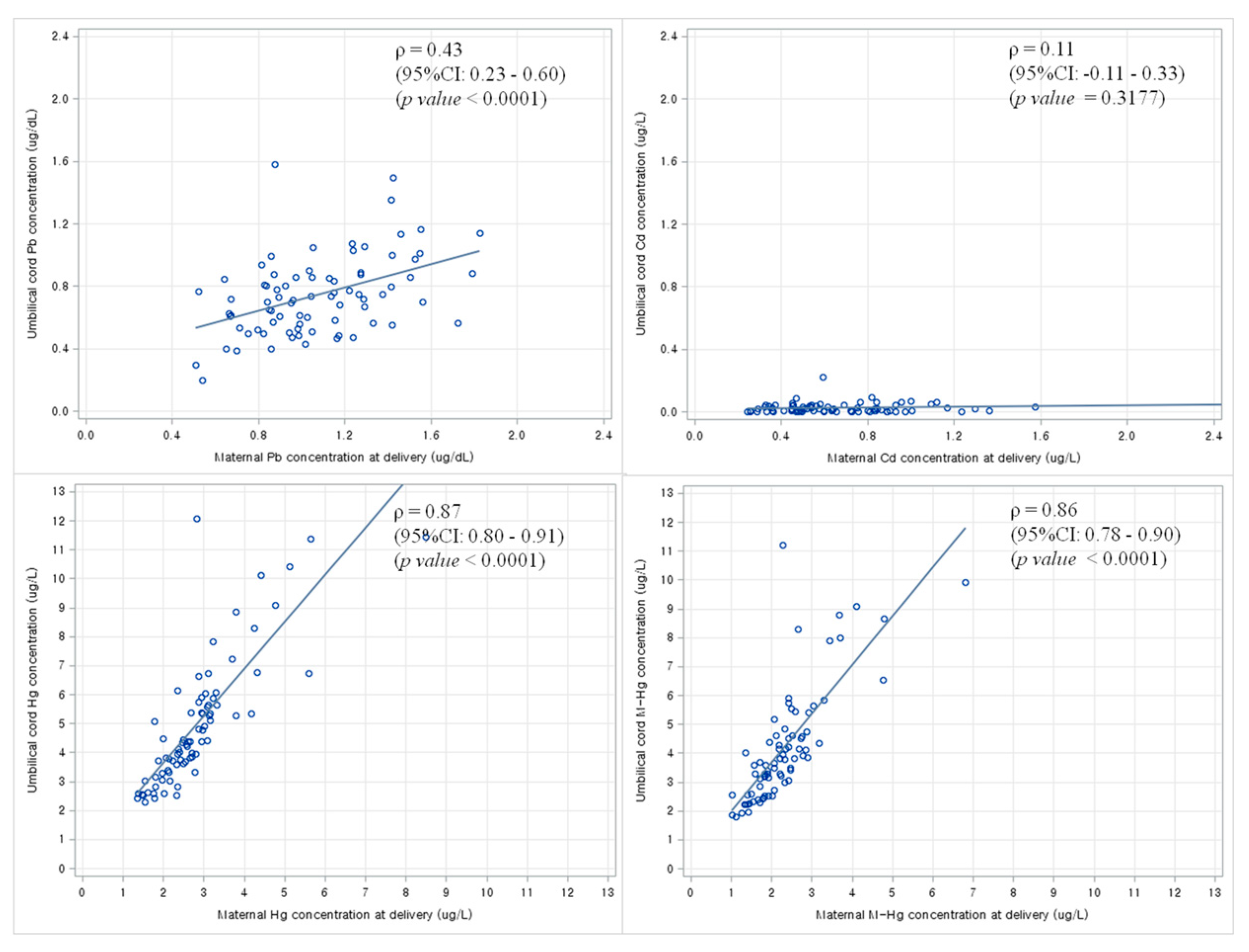
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicology Profile for Lead; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2007.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2012.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Mercury; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1999.
- National Academy of Science (NAS). Pesticides in the Diets of Infants and Children; National Academic Press: Washington, DC, USA, 1993. [Google Scholar]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Graziano, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2198–2206. [Google Scholar] [PubMed]
- Borja-Aburto, V.H.; Hertz-Picciotto, I.; Rojas Lopez, M.; Farias, P.; Rios, C.; Blanco, J. Blood lead levels measured prospectively and risk of spontaneous abortion. Am. J. Epidemiol. 1999, 150, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Avila, M.; Peterson, K.E.; Gonzalez-Cossio, T.; Sanin, L.H.; Aro, A.; Schnaas, L.; Hu, H. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch. Environ. Health 2002, 57, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H.L.; Rabinowitz, M.; Leviton, A.; Linn, S.; Schoenbaum, S. The relationship between prenatal exposure to lead and congenital anomalies. Jama 1984, 251, 2956–2959. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, L.; Li, Z.; Liu, J.M.; Ye, R.; Ren, A. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a chinese population. Reprod. Toxicol. 2013, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.T.; Bellinger, D.C.; Shaywitz, B.A. A quantitative analysis of prenatal methyl mercury exposure and cognitive development. Am. J. Prev. Med. 2005, 29, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nakagawa, H.; Honda, R.; Tanebe, K.; Saito, S.; Teranishi, H.; Tawara, K. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup. Environ. Med. 2002, 59, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Esquinas, E.; Perez-Gomez, B.; Fernandez-Navarro, P.; Fernandez, M.A.; de Paz, C.; Perez-Meixeira, A.M.; Gil, E.; Iriso, A.; Sanz, J.C.; Astray, J.; et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Golding, J.; Emond, A.M. Lead, cadmium and mercury levels in pregnancy: The need for international consensus on levels of concern. J. Dev. Orig. Health Dis. 2014, 5, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Kim, H.; Kim, K.N.; Ha, E.; Park, H.; Ha, M.; Kim, Y.; Chang, N. The association of maternal food intake and blood lead levels in pregnant and their newborns. Mol. Cell. Toxicol. 2008, 4, 61–65. [Google Scholar]
- Kim, B.M.; Lee, B.E.; Hong, Y.C.; Park, H.; Ha, M.; Kim, Y.J.; Kim, Y.; Chang, N.; Kim, B.N.; Oh, S.Y.; et al. Mercury levels in maternal and cord blood and attained weight through the 24 months of life. Sci. Total Environ. 2011, 410–411, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Hong, Y.S.; Kim, D.S.; Han, M.S.; Yu, B.C.; Kim, Y.W.; Roh, M.S.; Lee, H.J.; Lee, J.W.; Kwak, J.Y.; et al. Mercury concentrations of maternal and umbilical cord blood in Korean pregnant women: Preliminary study. Korean J. Occup. Environ. Med. 2007, 19, 268–275. [Google Scholar]
- Kim, B.G.; Jo, E.M.; Kim, G.Y.; Kim, D.S.; Kim, Y.M.; Kim, R.B.; Suh, B.S.; Hong, Y.S. Analysis of methylmercury concentration in the blood of Koreans by using cold vapor atomic fluorescence spectrophotometry. Ann. Lab. Med. 2012, 32, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Parker, J.D.; Mendola, P. Blood lead and mercury levels in pregnant women in the United States, 2003–2008. NCHS Data Brief 2010, 52, 1–8. [Google Scholar] [PubMed]
- Butler Walker, J.; Houseman, J.; Seddon, L.; McMullen, E.; Tofflemire, K.; Mills, C.; Corriveau, A.; Weber, J.P.; LeBlanc, A.; Walker, M.; et al. Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in arctic Canada. Environ. Res. 2006, 100, 295–318. [Google Scholar] [CrossRef] [PubMed]
- You, C.H.; Kim, B.G.; Kim, Y.M.; Lee, S.A.; Kim, R.B.; Seo, J.W.; Hong, Y.S. Relationship between dietary mercury intake and blood mercury level in Korea. J. Korean Med. Sci. 2014, 29, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Murata, K.; Kubota, M.; Nakai, K.; Satoh, H. Mercury and heavy metal profiles of maternal and umbilical cord RBCs in Japanese population. Ecotoxicol. Environ. Saf. 2010, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Engstrom, K.; Ballester, F.; Franforte, E.; Alhamdow, A.; Pisa, F.; Tratnik, J.S.; Mazej, D.; Murcia, M.; Rebagliato, M.; et al. Polymorphisms in abc transporter genes and concentrations of mercury in newborns—Evidence from two mediterranean birth cohorts. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Curren, M.S.; Liang, C.L.; Davis, K.; Kandola, K.; Brewster, J.; Potyrala, M.; Chan, H.M. Assessing determinants of maternal blood concentrations for persistent organic pollutants and metals in the eastern and western Canadian arctic. Sci. Total Environ. 2015, 527–528, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.H.; Smith, A.E. An assessment of the cord blood: Maternal blood methylmercury ratio: Implications for risk assessment. Environ. Health Perspect. 2003, 111, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch. Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Breen, J.G.; Eisenmann, C.; Horowitz, S.; Miller, R.K. Cell-specific increases in metallothionein expression in the human placenta perfused with cadmium. Reprod. Toxicol. 1994, 8, 297–306. [Google Scholar] [CrossRef]
- Harville, E.W.; Hertz-Picciotto, I.; Schramm, M.; Watt-Morse, M.; Chantala, K.; Osterloh, J.; Parsons, P.J.; Rogan, W. Factors influencing the difference between maternal and cord blood lead. Occup. Environ. Med. 2005, 62, 263–269. [Google Scholar] [CrossRef] [PubMed]
- 2014 Birth Registration from Korean Statistics. Available online: http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parentId=A#SubCont (accessed on 24 October 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Chung, J.-Y.; An, H.S.; Park, S.Y.; Kim, B.-G.; Bae, J.W.; Han, M.; Cho, Y.J.; Hong, Y.-S. Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea. Int. J. Environ. Res. Public Health 2015, 12, 13482-13493. https://doi.org/10.3390/ijerph121013482
Kim Y-M, Chung J-Y, An HS, Park SY, Kim B-G, Bae JW, Han M, Cho YJ, Hong Y-S. Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea. International Journal of Environmental Research and Public Health. 2015; 12(10):13482-13493. https://doi.org/10.3390/ijerph121013482
Chicago/Turabian StyleKim, Yu-Mi, Jin-Young Chung, Hyun Sook An, Sung Yong Park, Byoung-Gwon Kim, Jong Woon Bae, Myoungseok Han, Yeon Jean Cho, and Young-Seoub Hong. 2015. "Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea" International Journal of Environmental Research and Public Health 12, no. 10: 13482-13493. https://doi.org/10.3390/ijerph121013482





