Association of Roadway Proximity with Indoor Air Pollution in a Peri-Urban Community in Lima, Peru
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Setting
2.2. Study Homes
2.3. Home Inspection and Questionnaires
2.4. Pollutant Monitoring
2.5. Allergen Dust Collection
2.6. Biostatistical Methods
| Housing Characteristics | Mean | Min | Max |
|---|---|---|---|
| Household distance to nearest main road (m) | 148.6 | 34.8 | 358.0 |
| % Households less than 100 m from main road | 36% | ||
| Total rooms per home | 7.2 | 3 | 14 |
| Total bedrooms per home | 3.7 | 1 | 8 |
| Total windows per home | 5.2 | 1 | 15 |
| No. windows facing road | 2.4 | 0 | 5 |
| No. doorways to outside | 1.9 | 0 | 5 |
| % Bedrooms without window | 32% | ||
| % Homes with cleanliness ratings: * | |||
| Below average | 10% | ||
| Average | 57% | ||
| Above average | 33% | ||
| Principal material of roof | |||
| Iron or Tin | 60% | ||
| Cement | 36% | ||
| Brick | 4% | ||
| Principal material of residential walls | |||
| Cement | 52% | ||
| Iron | 24% | ||
| Wood | 16% | ||
| Brick | 8% | ||
| % Homes that cook indoors by fuel type | |||
| Gas | 70.8% | ||
| Charcoal | 8.3% | ||
| Electricity | 8.3% | ||
| Firewood | 4.2% | ||
| Unreported | 8.4% | ||
| % Indoor kitchens with ventilation | 76% | ||
| % Homes with pets/animals indoors | |||
| Dog | 42% | ||
| Cat | 16% | ||
| Bird | 16% | ||
| Chicken | 8% |
3. Results
3.1. Household Characteristics
3.2. Outdoor Environmental Exposures
| Pollutant | Indoor | Mean (Range) | Outdoor | Mean (Range) | I vs. O * |
|---|---|---|---|---|---|
| n | n | (p-Value) | |||
| PM2.5 (μg/m3) | 39 | 20.0 (5.7–55.4) | 42 | 16.9 (8.5–40.5) | 0.14 |
| Summer | 20 | 20.8 (5.7–32.8) | 19 | 20.2 (11.2–40.5) | 0.47 |
| Winter | 19 | 19.2 (9.1–55.4) | 23 | 14.3 (8.5–21.5) | 0.23 |
| S vs. W * | (p-value) | 0.21 | <0.001 | ||
| BC (μg/m3) | 39 | 7.6 (3.5–21.1) | 35 | 8.1 (3.6–19.3) | 0.15 |
| Summer | 21 | 9.0 (4.9–21.08) | 16 | 9.3 (4.1–19.3) | 0.30 |
| Winter | 18 | 5.9 (3.5–10.3) | 19 | 7.0 (3.6–15.1) | 0.46 |
| S vs. W * | (p-value) | 0.01 | <0.01 | ||
| NO2 (ppb) | 34 | 7.3 (2.4–14.61) | 36 | 7.5 (4.2–14.0) | 0.69 |
| Summer | 16 | 8.5 (5.4–14.6) | 15 | 8.1 (4.7–14.0) | 0.45 |
| Winter | 18 | 6.2 (2.4–10.7) | 21 | 7.1 (4.2–11.6) | 0.26 |
| S vs. W * | (p-value) | <0.001 | 0.08 |
| Pollutant | n | rs | p-Value |
|---|---|---|---|
| PM2.5 | 42 | −0.42 | 0.01 |
| Summer | 19 | −0.71 | 0.001 |
| Winter | 23 | −0.44 | 0.04 |
| BC | 35 | −0.24 | 0.16 |
| Summer | 16 | 0.10 | 0.70 |
| Winter | 19 | −0.51 | 0.03 |
| NO2 | 36 | −0.36 | 0.03 |
| Summer | 15 | −0.49 | 0.07 |
| Winter | 21 | −0.34 | 0.14 |
3.3. Indoor Environmental Exposures
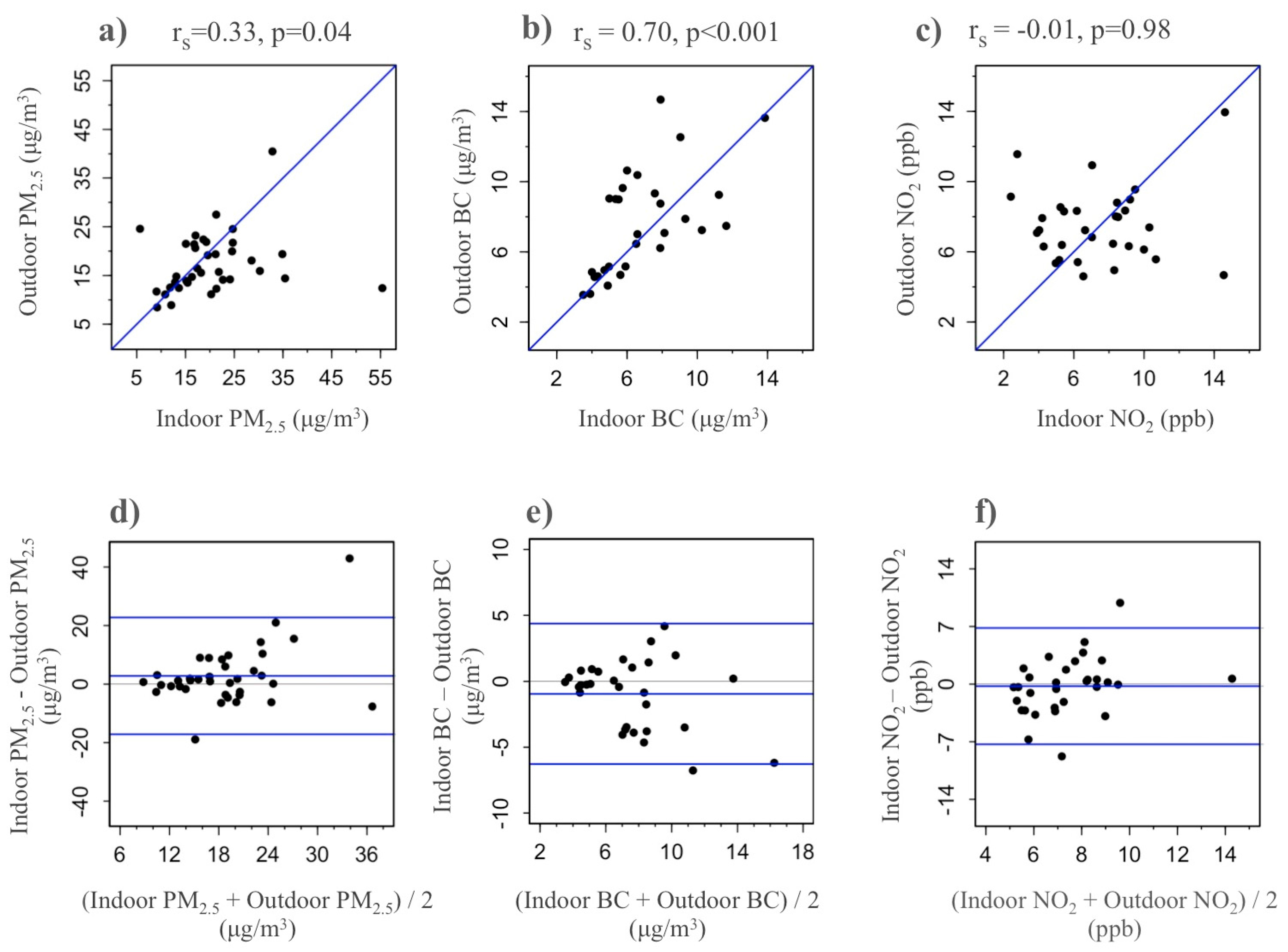
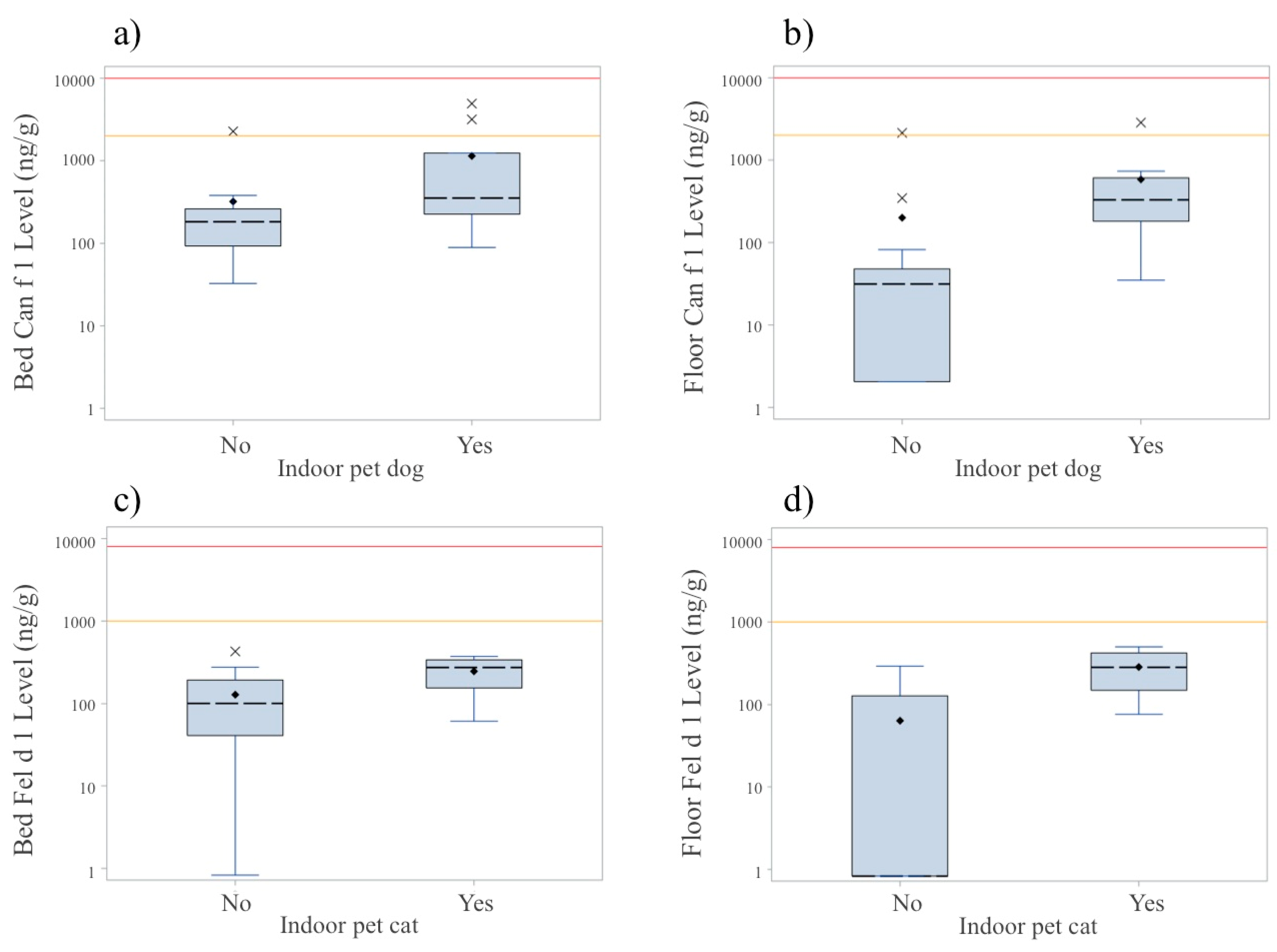
3.4. Allergens
| Allergen | Threshold Categories a | Total Detectable | Concentration | p-Value b | ||
|---|---|---|---|---|---|---|
| LOW | MEDIUM | HIGH | Median (IQR) | |||
| Dog (Can f 1) | (ng/g) | |||||
| Bed | 88% | 12% | 0% | 100% | 194 (107, 380) | <0.01 |
| Floor | 68% | 8% | 0% | 76% | 48 (27, 345) | |
| Cat (Fel d 1) | (ng/g) | |||||
| Bed | 80% | 0% | 0% | 80% | 133 (43, 239) | 0.05 |
| Floor | 48% | 0% | 0% | 48% | 1 (1, 169) | |
| Mouse (Mus m 1) | (ng/g) | |||||
| Bed | 72% | 24% | 0% | 96% | 162 (122, 482) | 0.37 |
| Floor | 76% | 16% | 8% | 100% | 125 (37, 437) | |
| Mite (Der p 1) | (ng/g) | |||||
| Bed | 16% | 60% | 24% | 100% | 5573 (2676, 9539) | <0.001 |
| Floor | 92% | 8% | 0% | 100% | 543 (223, 1153) | |
| Cockroach (Bla g 1) | (U/g) | |||||
| Bed | 40% | 8% | 0% | 48% | 0.02 (0.02, 1.2) | 0.66 |
| Floor | 32% | 8% | 0% | 40% | 0.02 (0.02, 0.8) | |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, G.; Murray, F. Air Pollution and Health in Rapidly Developing Countries; Earthscan: London, UK, 2012. [Google Scholar]
- Kunzli, N.; Kaiser, R.; Medina, S.; Studnicka, M.; Chanel, O.; Filliger, P.; Herry, M.; Horak, F., Jr.; Puybonnieux-Texier, V.; Quenel, P.; et al. Public-health impact of outdoor and traffic-related air pollution: A European assessment. Lancet 2000, 356, 795–801. [Google Scholar] [CrossRef]
- Han, X.; Naeher, L.P. A review of traffic-related air pollution exposure assessment studies in the developing world. Environ. Int. 2006, 32, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S.; Murray, C.J.; Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef]
- Carbajal-Arroyo, L.; Barraza-Villarreal, A.; Durand-Pardo, R.; Moreno-Macias, H.; Espinoza-Lain, R.; Chiarella-Ortigosa, P.; Romieu, I. Impact of traffic flow on the asthma prevalence among school children in Lima, Peru. J. Asthma 2007, 44, 197–202. [Google Scholar] [PubMed]
- Robinson, C.L.; Baumann, L.M.; Romero, K.; Combe, J.M.; Gomez, A.; Gilman, R.H.; Cabrera, L.; Gonzalvez, G.; Hansel, N.N.; Wise, R.A.; et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 2011, 66, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, B.A.; Blangiardo, M.; Briggs, D.J.; Hansell, A.L. Traffic air pollution and other risk factors for respiratory illness in schoolchildren in the niger-delta region of Nigeria. Environ. Health Perspect. 2011, 119, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Taniyama, T. Atmospheric NO2 and CO concentration in Lima, Peru. Environ. Int. 2002, 28, 227–233. [Google Scholar] [CrossRef]
- Baumann, L.M.; Robinson, C.L.; Combe, J.M.; Gomez, A.; Romero, K.; Gilman, R.H.; Cabrera, L.; Hansel, N.N.; Wise, R.A.; Breysse, P.N.; et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J. Allergy Clin. Immunol. 2011, 127, 875–882. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. 40 CFR 50 Appendix L. Available online: https://www.law.cornell.edu/cfr/text/40/part-50/appendix-L (accessed on 31 August 2015).
- Chakrabarti, B.; Fine, P.M.; Delfino, R.; Sioutas, C. Performance evaluation of the active-flow personal dataram PM2.5 mass monitor (thermo anderson pDR-1200) designed for continuous personal exposure measurements. Atmos. Environ. 2004, 38, 3329–3340. [Google Scholar] [CrossRef]
- Palmes, E.D.; Gunnison, A.F.; DiMattio, J.; Tomczyk, C. Personal sampler for nitrogen dioxide. Am. Ind. Hyg. Assoc. J. 1976, 37, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Roadman, M.J.; Scudlark, J.R.; Meisingerb, J.J.; Ullmana, W.J. Validation of ogawa passive samplers for the determination of gaseous ammonia concentrations in agricultural settings. Atmos. Environ. 2003, 37, 2317–2325. [Google Scholar] [CrossRef]
- Korpi, A.; Mantyjarvi, R.; Rautiainen, J.; Kaliste, E.; Kalliokoski, P.; Renstrom, A.; Pasanen, A.L. Detection of mouse and rat urinary aeroallergens with an improved elisa. J. Allergy Clin. Immunol. 2004, 113, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Curtin-Brosnan, J.; Matsui, E.C.; Breysse, P.; McCormack, M.C.; Hansel, N.N.; Tonorezos, E.S.; Eggleston, P.A.; Williams, D.L.; Diette, G.B. Parent report of pests and pets and indoor allergen levels in inner-city homes. Ann. Allergy Asthma Immunol. 2008, 101, 517–523. [Google Scholar] [CrossRef]
- Custovic, A.; Fletcher, A.; Pickering, C.A.; Francis, H.C.; Green, R.; Smith, A.; Chapman, M.; Woodcock, A. Domestic allergens in public places III: House dust mite, cat, dog and cockroach allergens in British hospitals. Clin. Exp. Allergy 1998, 28, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy 2014, 69, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Breysse, P.N.; Diette, G.B.; Matsui, E.C.; Butz, A.M.; Hansel, N.N.; McCormack, M.C. Indoor air pollution and asthma in children. Proc. Am. Thorac. Soc. 2010, 7, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.K.; Clougherty, J.E.; Laden, F.; Levy, J.I. Predictors of concentrations of nitrogen dioxide, fine particulate matter, and particle constituents inside of lower socioeconomic status urban homes. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Levy, J.I.; Yanagisawa, Y.; Spengler, J.D.; Billick, I.H. The boston residential nitrogen dioxide characterization study: Classification and prediction of indoor NO2 exposure. J. Air Waste Manag. Assoc. 1998, 48, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.A.; Mitchell, H.; O’Connor, G.T.; Neas, L.; Lippmann, M.; Kattan, M.; Koenig, J.; Stout, J.W.; Vaughn, B.J.; Wallace, D.; et al. Particle concentrations in inner-city homes of children with asthma: The effect of smoking, cooking, and outdoor pollution. Environ. Health Perspect. 2003, 111, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Breysse, P.N.; Buckley, T.J.; Williams, D.; Beck, C.M.; Jo, S.J.; Merriman, B.; Kanchanaraksa, S.; Swartz, L.J.; Callahan, K.A.; Butz, A.M.; et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city baltimore. Environ. Res. 2005, 98, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kinney, P.L.; Aggarwal, M.; Northridge, M.E.; Janssen, N.A.; Shepard, P. Airborne concentrations of PM2.5 and diesel exhaust particles on harlem sidewalks: A community-based pilot study. Environ. Health Perspect. 2000, 108, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Padro-Martinez, L.T.; Patton, A.P.; Trull, J.B.; Zamore, W.; Brugge, D.; Durant, J.L. Mobile monitoring of particle number concentration and other traffic-related air pollutants in a near-highway neighborhood over the course of a year. Atmos. Environ. 2012, 61, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Pérez, N.; Pey, J.; Cusack, M.; Reche, C.; Querol, X.; Alastuey, A.; Viana, M. Variability of particle number, black carbon, and PM10, PM2.5, and PM1 levels and speciation: Influence of road traffic emissions on urban air quality. Aerosol Sci. Technol. 2010, 44, 487–499. [Google Scholar] [CrossRef]
- Janssen, N.A.; Hoek, G.; Simic-Lawson, M.; Fischer, P.; van Bree, L.; ten Brink, H.; Keuken, M.; Atkinson, R.W.; Anderson, H.R.; Brunekreef, B.; et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Latha, K.; Badarinath, K.; Manikya Reddy, P. Scavenging efficiency of rainfall on black carbon aerosols over an urban environment. Atmos. Sci. Lett. 2005, 6, 148–151. [Google Scholar] [CrossRef]
- Calvo, M.; Fernandez-Caldas, E.; Arellano, P.; Marin, F.; Carnes, J.; Hormaechea, A. Mite allergen exposure, sensitisation and clinical symptoms in Valdivia, Chile. J. Investig. Allergol. Clin. Immunol. 2005, 15, 189–196. [Google Scholar] [PubMed]
- Camara, A.A.; Silva, J.M.; Ferriani, V.P.; Tobias, K.R.; Macedo, I.S.; Padovani, M.A.; Harsi, C.M.; Cardoso, M.R.; Chapman, M.D.; Arruda, E.; et al. Risk factors for wheezing in a subtropical environment: Role of respiratory viruses and allergen sensitization. J. Allergy Clin. Immunol. 2004, 113, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C.; Eggleston, P.A.; Buckley, T.J.; Krishnan, J.A.; Breysse, P.N.; Rand, C.S.; Diette, G.B. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann. Allergy Asthma Immunol. 2006, 97, 514–520. [Google Scholar] [CrossRef]
- Diette, G.B.; Hansel, N.N.; Buckley, T.J.; Curtin-Brosnan, J.; Eggleston, P.A.; Matsui, E.C.; McCormack, M.C.; Williams, D.L.; Breysse, P.N. Home indoor pollutant exposures among inner-city children with and without asthma. Environ. Health Perspect. 2007, 115, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Salo, P.M.; Arbes, S.J., Jr.; Crockett, P.W.; Thorne, P.S.; Cohn, R.D.; Zeldin, D.C. Exposure to multiple indoor allergens in us homes and its relationship to asthma. J. Allergy Clin. Immunol. 2008, 121, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Pollart, S.M.; Mullins, D.E.; Vailes, L.D.; Hayden, M.L.; Platts-Mills, T.A.; Sutherland, W.M.; Chapman, M.D. Identification, quantitation, and purification of cockroach allergens using monoclonal antibodies. J. Allergy Clin. Immunol. 1991, 87, 511–521. [Google Scholar] [CrossRef]
- Pomes, A.; Melen, E.; Vailes, L.D.; Retief, J.D.; Arruda, L.K.; Chapman, M.D. Novel allergen structures with tandem amino acid repeats derived from German and American cockroach. J. Biol. Chem. 1998, 273, 30801–30807. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Underhill, L.J.; Bose, S.; Williams, D.L.; Romero, K.M.; Malpartida, G.; Breysse, P.N.; Klasen, E.M.; Combe, J.M.; Checkley, W.; Hansel, N.N. Association of Roadway Proximity with Indoor Air Pollution in a Peri-Urban Community in Lima, Peru. Int. J. Environ. Res. Public Health 2015, 12, 13466-13481. https://doi.org/10.3390/ijerph121013466
Underhill LJ, Bose S, Williams DL, Romero KM, Malpartida G, Breysse PN, Klasen EM, Combe JM, Checkley W, Hansel NN. Association of Roadway Proximity with Indoor Air Pollution in a Peri-Urban Community in Lima, Peru. International Journal of Environmental Research and Public Health. 2015; 12(10):13466-13481. https://doi.org/10.3390/ijerph121013466
Chicago/Turabian StyleUnderhill, Lindsay J., Sonali Bose, D’Ann L. Williams, Karina M. Romero, Gary Malpartida, Patrick N. Breysse, Elizabeth M. Klasen, Juan M. Combe, William Checkley, and Nadia N. Hansel. 2015. "Association of Roadway Proximity with Indoor Air Pollution in a Peri-Urban Community in Lima, Peru" International Journal of Environmental Research and Public Health 12, no. 10: 13466-13481. https://doi.org/10.3390/ijerph121013466





