Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Preparation of Stock Solutions
2.2. Fish Maintenance
2.3. Experimental Design
2.4. Hormone Measurements
2.5. mRNA Expression of Selected Genes
2.6. Statistical Analysis of Data
| Name | Sequence of Forward (FP) and Reverse Primers (RP) | Genbank Accession |
|---|---|---|
| β-actin | FP 5′-CGAGCAGGAGATGGGAACC-3′ RP 5′-CAACGGAAACGCTCATTGC-3′ | AF057040 |
| tsh-β | FP 5′-GCAGATCCTCACTTCACCTACC-3′ RP 5′-GCACAGGTTTGGAGCATCTCA-3′ | AY135147 |
| dio1 | FP 5′ -GTTCAAACAGCTTGTCAAGGACT-3′ RP 5′ - AGCAAGCCTCTCCTCCAAGTT-3′ | BC076008 |
| dio2 | FP 5′-GCATAGGCAGTCGCTCATTT-3′ RP 5′-TGTGGTCTCTCATCCAACCA-3′ | NM212789 |
| sclc5a5 | FP 5′-GGTGGCATGAAGGCTGTAAT-3′ RP 5′-GATACGGCATCCATTGTTGG-3′ | NM001089391 |
| tg | FP 5′-CCAGCCGAAAGGATAGAGTTG-3′ RP 5′-ATGCTGCCGTGGAATAGGA-3′ | XM001335283 |
| ttr | FP 5′-CGGGTGGAGTTTGACACTTT-3′ RP 5′-GCTCAGAAGGAGAGCCAGTA-3′ | BC081488 |
| tr-α | FP 5′-CTATGAACAGCACATCCGACAAG-3′ RP 5′-CACACCACACACGGCTCATC-3′ | NM131396 |
| tr-β | FP 5′-TGGGAGATGATACGGGTTGT-3′ RP 5′-ATAGGTGCCGATCCAATGTC-3′ | NM131340 |
3. Results
3.1. Developmental Parameters of Zebrafish Larvae
| BPAF (µg/L) | SC | 5 | 50 | 500 |
|---|---|---|---|---|
| 168 hpf Survival (%) | 86.2 ± 2.1 | 83.5 ± 1.8 | 87.2 ± 0.8 | 85.7 ± 2.8 |
| 168 hpf Length (mm) | 3.62 ± 0.08 | 3.60 ± 0.10 | 3.55 ± 0.05 | 3.58 ± 0.06 |
| 168 hpf Weight (mg) | 0.41 ± 0.02 | 0.40 ± 0.03 | 0.38 ± 0.02 | 0.37 ± 0.04 |
| 72 hpf Hatchability (%) | 80.5 ± 2.0 | 78.2 ± 1.5 | 44.3 ± 1.8 * | 40.7 ± 1.6 * |
| 96 hpf Hatchability (%) | 92.4 ± 1.2 | 91.5 ± 1.3 | 90.8 ± 0.8 | 93.2 ± 1.1 |
3.2. Concentration of Hormones in Zebrafish Larvae
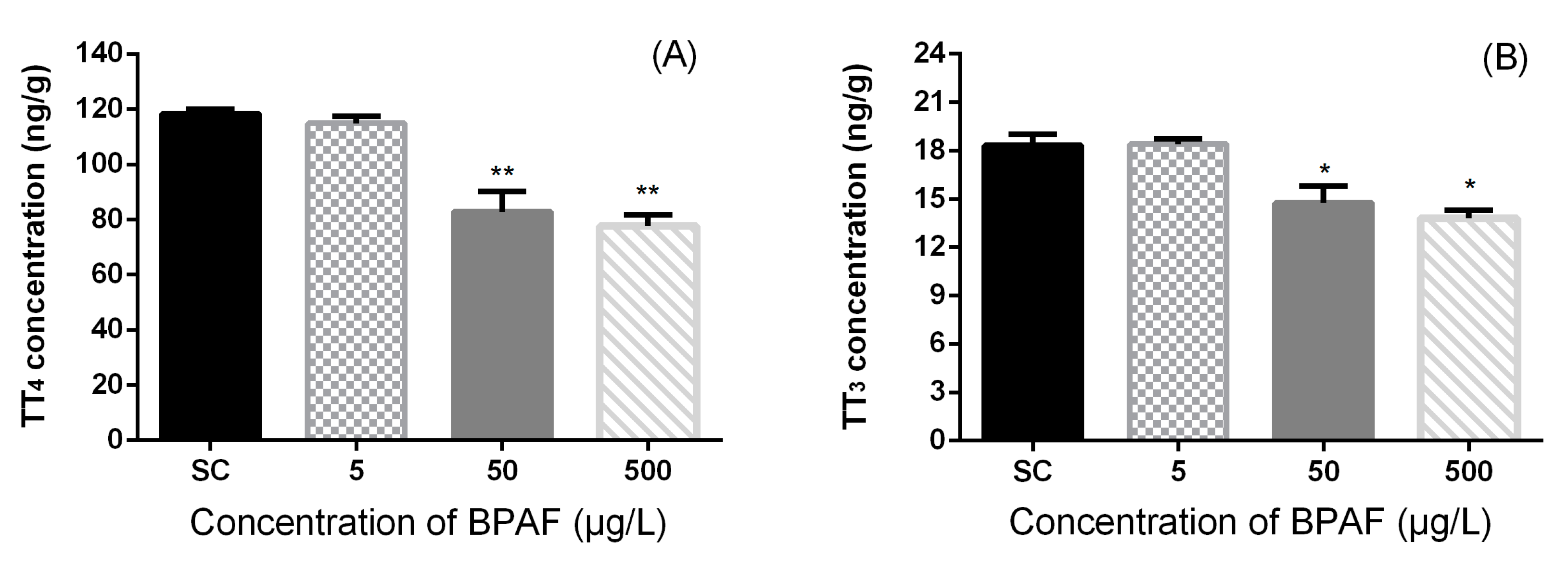
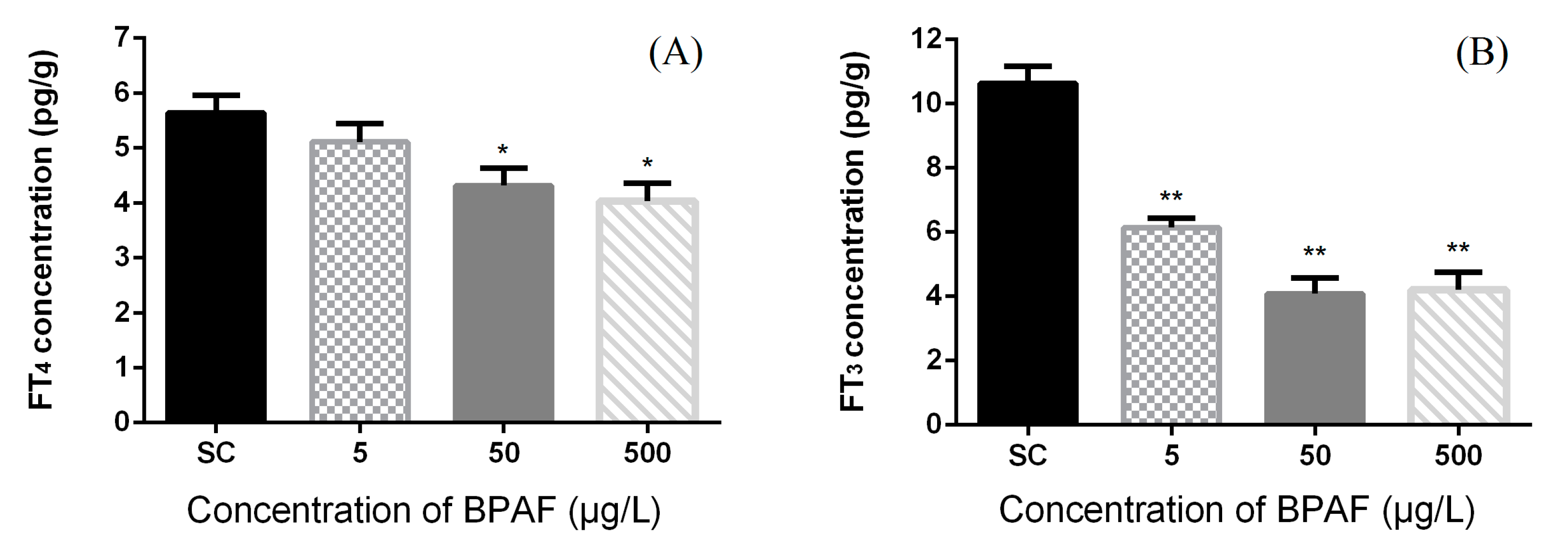
3.3. Effects of BPAF on HPT Gene Expression Levels in Zebrafish Larvae
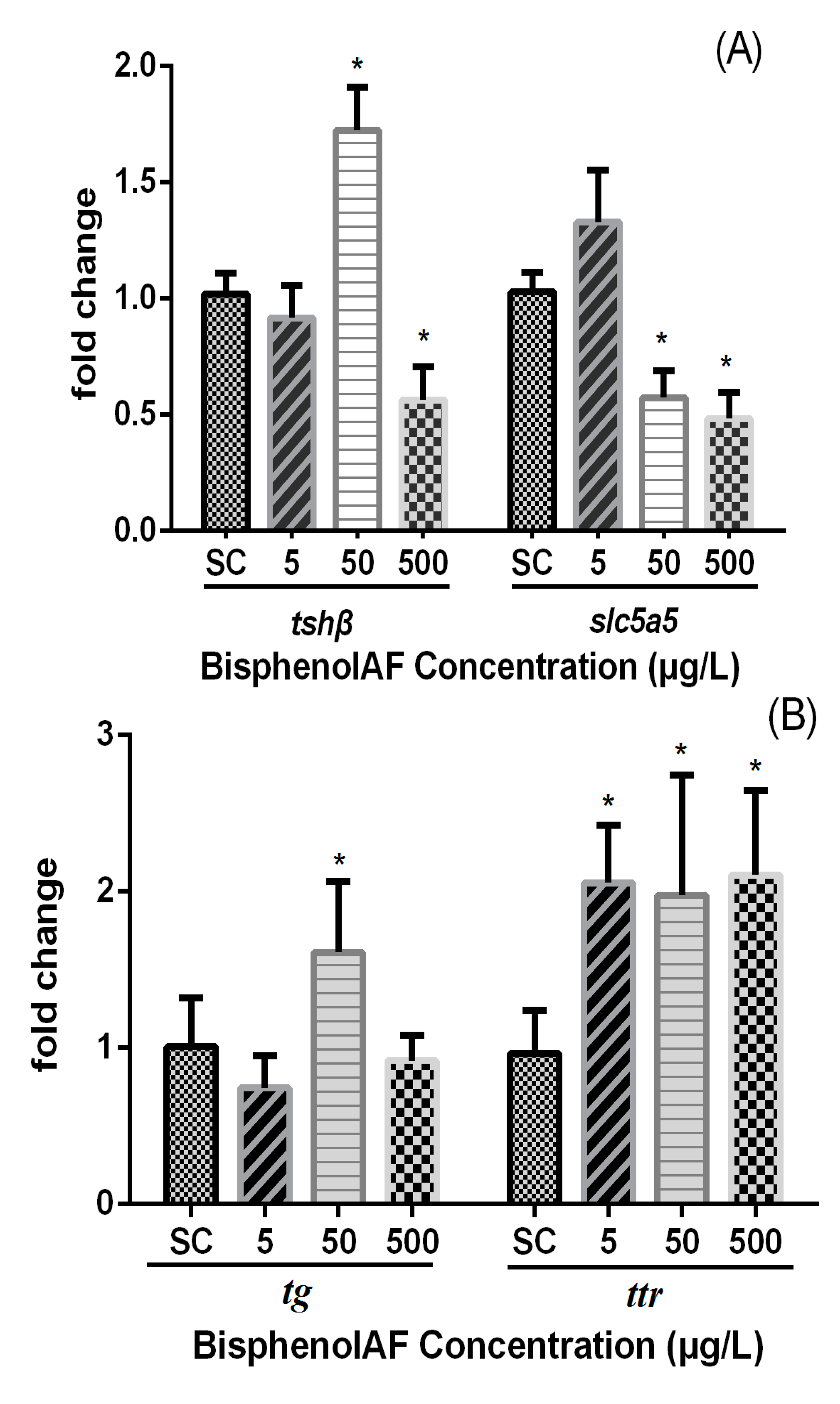
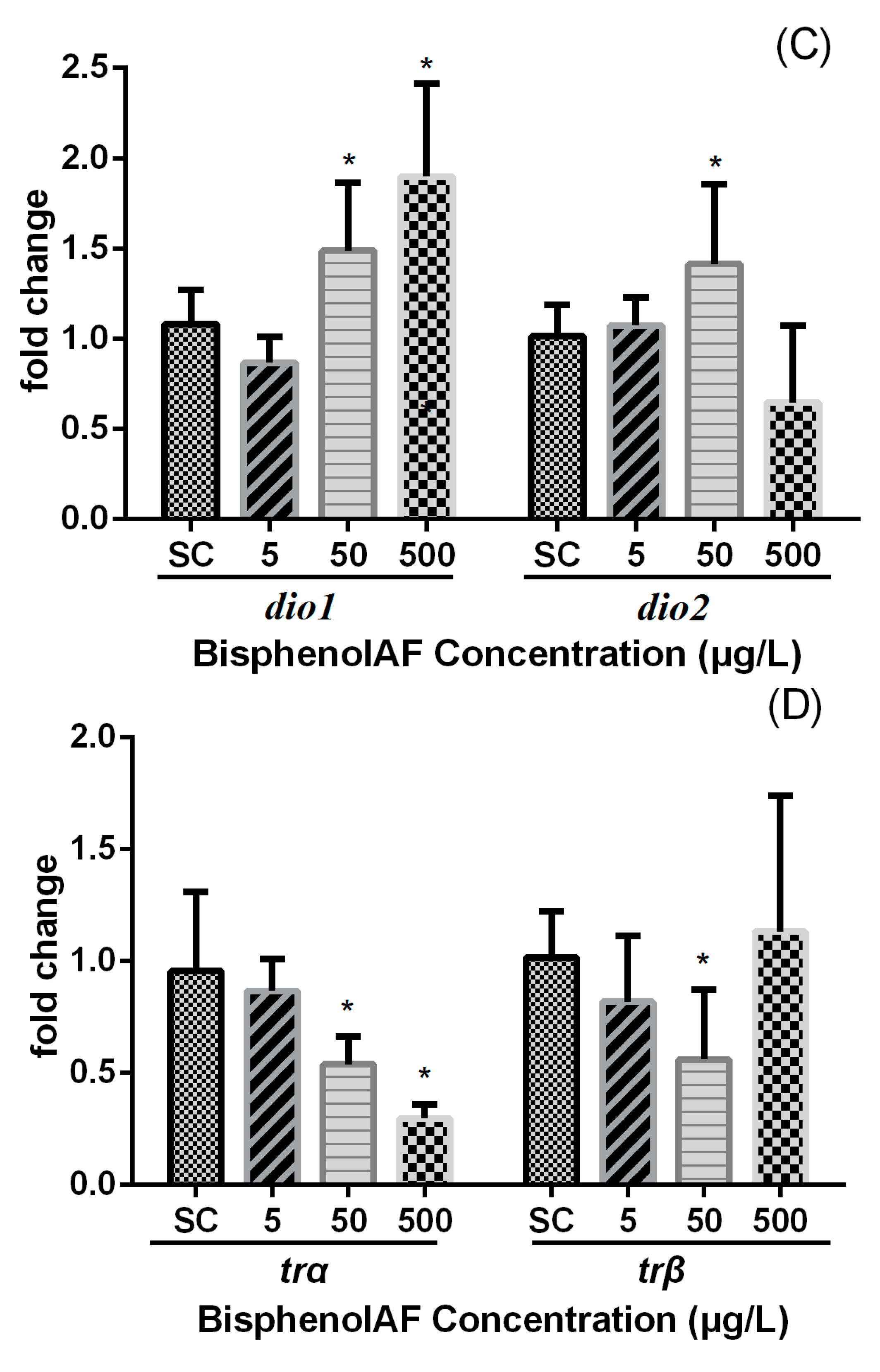
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiao, Z.; Zheng, S.; Li, M.; Zhang, J.; Feng, Y.; Yin, J.; Shao, B. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 2015, 128, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol. 2012, 46, 13136–13143. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Song, M.; Zeng, L.; Wang, T.; Liu, R.; Ruan, T.; Jiang, G. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ. Pollut. 2014, 186, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, L.; Zhang, J.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, J.; Yang, Y.; Zhou, N.; Zhang, J.; Shao, B.; Wu, Y. Determination of bisphenol AF (BPAF) in tissues, serum, urine and feces of orally dosed rats by ultra-high-pressure liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B 2012, 901, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Burns, K.A.; Arao, Y.; Luh, C.J.; Korach, K.S. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ. Health Persp. 2012, 120, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luh, C.J.; Burns, K.A.; Arao, Y.; Jiang, Z. Endocrine-Disrupting Chemicals (EDCs): In vitro Mechanism of Estrogenic Activation and Differential Effects on ER Target Genes. Environ. Health Persp. 2013, 121, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, J.; Gao, W.; Yu, J.; Han, X.; Zhang, J.; Shao, B. Bisphenol AF-induced endogenous transcription is mediated by ERα and ERK1/2 activation in human breast cancer cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J.; Mokra, K.; Bąk, A. Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol. in Vitro 2015, 29, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Waidyanatha, S.; Mathews, J.M.; Patel, P.R.; Black, S.R.; Snyder, R.W.; Fennell, T.R. Disposition of bisphenol AF, a bisphenol A analogue, in hepatocytes in vitro and in male and female Harlan Sprague-Dawley rats and B6C3F1/N mice following oral and intravenous administration. Xenobiotica 2015, 29, 1–9. [Google Scholar]
- Nakano, K.; Nishio, M.; Kobayashi, N.; Hiradate, Y.; Hoshino, Y.; Sato, E.; Tanemura, K. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote 2015, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Einarsdottir, I.E.; Silva, N.; Power, D.M.; Smaradottir, H.; Bjornsson, B.T. Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat. Embryol. 2006, 211, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.; Moller, G.; de Angelis, M.H.; Adamski, J. Zebrafish and steroids: What do we know and what do we need to know? J. Steroid Biochem. 2013, 137, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Raldúa, D.; Thienpont, B.; Babin, P.J. Zebrafish eleutheroembryos as an alternative system for screening chemicals disrupting the mammalian thyroid gland morphogenesis and function. Reprod. Toxicol. 2012, 33, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Elsalini, O.A.; Rohr, K.B. Phenylthiourea disrupts thyroid function in developing zebrafish. Dev. Genes Evol. 2003, 212, 593–598. [Google Scholar] [PubMed]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kloas, W.; Lutz, I. Amphibians as model to study endocrine disrupters. J. Chromatogr. A 2006, 1130, 16–27. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; Decallonne, B.R.; Van der Geyten, S.; Darras, V.M.; Bouillon, R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: Potential thyroid-related health effects. Eur. J. Endocrinol. 2006, 155, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.; Valverde-R, C. Thyroid hormone deiodination in fish. Thyroid 2005, 15, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Huang, G.Y.; Ying, G.G.; Liu, S.S.; Jiang, Y.X.; Liu, S. Progesterone and norgestrel alter transcriptional expression of genes along the hypothalamic-pituitary-thyroid axis in zebrafish embryos-larvae. Comp. Biochem. Physiol. C 2015, 167, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lo, L.J.; Chan, W.K. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish, Danio rerio. Mol. Cell Endocrinol. 2002, 5, 187–195. [Google Scholar]
- Zoeller, R.T.; Tyl, R.W.; Tan, S.W. Current and potential rodent screens and tests for thyroid toxicants. Crit. Rev. Toxicol. 2007, 37, 55–95. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 3rd ed.; University of Oregon Press: Oregon, OR, USA, 1995. [Google Scholar]
- Stout, M.D. Chemical Information Profile for Bisphenol AF. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bisphenolaf_093008_508.pdf (accessed on 4 August 2015).
- Tian, H.; Li, Y.; Wang, W.; Wu, P.; Ru, S. Exposure to monocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success of male guppies (Poecilia reticulata). Toxicol. Appl. Pharm. 2012, 263, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.W.; Tang, T.L.; Tang, W.H. Toxic effects of bisphenol AF on zebrafish embryos and larvae. Res. Environ. Sci. 2015, 28, 41–48. (in Chinese). [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Sudo, R.; Okamura, A.; Kuroki, M.; Tsukamoto, K. Changes in the role of the thyroid axis during metamorphosis of the Japanese eel, Anguilla japonica. J. Exp. Zool. Part A 2014, 321, 357–364. [Google Scholar]
- Chen, Q.; Yu, L.; Yang, L.; Zhou, B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat. Toxicol. 2012, 110–111, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, P.; Zhan, X. Changes in plasma thyroid hormones and cortisol levels in crucian carp (Carassius auratus) exposed to the extracted microcystins. Chemosphere 2008, 74, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Huang, Z.; Chen, L.; Feng, C.; Li, B.; Li, T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.M.; Paiva, M.S.; Haggblom, M.H.; Cooper, K.R.; White, L.A. Embryonic exposure to tetrabromobisphenol A and its metabolites, bisphenol A and tetrabromobisphenol A dimethyl ether disrupts normal zebrafish (Danio rerio) development and matrix metalloproteinase expression. Aquat. Toxicol. 2010, 100, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Maniyar, R.A.; Ahmed, R.N.; David, M. Monocrotophos: Toxicity evaluation and respiratory responses of Cyprinus carpio (Linnaeus). Rec. Res. Sci. Tech. 2011, 3, 51–54. [Google Scholar]
- Van der Geyten, S.; Byamungu, N.; Reyns, G.E.; Kühn, E.R.; Darras, V.M. Iodothyronine deiodinases and the control of plasma and tissue thyroid hormone levels in hyperthyroid tilapia (Oreochromis niloticus). J. Endocrinol. 2005, 184, 467–479. [Google Scholar]
- Coimbra, A.M.; Reis-Henriques, M.A.; Darras, V.M. Circulating thyroid hormone levels and iodothyronine deiodinase activities in Nile tilapia (Oreochromis niloticus) following dietary exposure to Endosulfan and Arochlor 1254. Comp. Biochem. Phys. C 2005, 141, 8–14. [Google Scholar]
- MacKenzie, D.S.; Jones, R.A.; Miller, T.C. Thyrotropin in teleost fish. Gen. Comp. Endocr. 2009, 161, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, H.; Wang, W.; Ru, S. Exposure to monocrotophos pesticide causes disruption of the hypothalamic-pituitary-thyroid axis in adult male goldfish (Carassius auratus). Gen. Comp. Endocr. 2013, 193, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Deng, J.; Shi, X.; Liu, C.; Yu, K.; Zhou, B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010, 97, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chan, K.M. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kim, S.; Kho, Y.; Paek, D.; Sakong, J.; Ha, J.; Kim, S.; Choi, K. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Environ. Int. 2012, 45, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Porazzi, P.; Calebiro, D.; Benato, F.; Tiso, N.; Persani, L. Thyroid gland development and function in the zebrafish model. Mol. Cell Endocrinol. 2009, 312, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, J.; Zhao, Y.; Zhu, G. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon. Environ. Toxicol. Pharm. 2011, 32, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Dickey, J.T.; Schultz, I.R.; Swanson, P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J. Endocrinol. 2009, 202, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Walpita, C.N.; Van der Geyten, S.; Rurangwa, E.; Darras, V.M. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen. Comp. Endocr. 2007, 152, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.; Infante, C.; Rebordinos, L.; Cañavate, J.P. Molecular characterization, gene expression and transcriptional regulation of thyroid hormone receptors in Senegalese sole. Gen. Comp. Endocr. 2009, 160, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Seoka, M.; Miyashita, S.; Kumai, H.; Ohta, H. Characterization of transthyretin in the Pacific bluefin tuna, Thunnus orientalis. Zool. Sci. 2006, 23, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Power, D.M.; Elias, N.P.; Richardson, S.J.; Mendes, J.; Soares, C.M.; Santos, C.R. Evolution of the thyroid hormone-binding protein, transthyretin. Gen. Comp. Endocr. 2000, 119, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; van Zanden, J.J.; Luijks, E.A.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Dickey, J.T.; Schultz, I.R.; Swanson, P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ. Health Persp. 2008, 116, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Yang, Y.; Chen, Y.; Tang, W.; Wang, F.; Diao, X. Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF. Int. J. Environ. Res. Public Health 2015, 12, 13069-13084. https://doi.org/10.3390/ijerph121013069
Tang T, Yang Y, Chen Y, Tang W, Wang F, Diao X. Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF. International Journal of Environmental Research and Public Health. 2015; 12(10):13069-13084. https://doi.org/10.3390/ijerph121013069
Chicago/Turabian StyleTang, Tianle, Yang Yang, Yawen Chen, Wenhao Tang, Fuqiang Wang, and Xiaoping Diao. 2015. "Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF" International Journal of Environmental Research and Public Health 12, no. 10: 13069-13084. https://doi.org/10.3390/ijerph121013069






