Association of Sand Dust Particles with Pulmonary Function and Respiratory Symptoms in Adult Patients with Asthma in Western Japan Using Light Detection and Ranging: A Panel Study
Abstract
:1. Introduction
2. Experimental Section
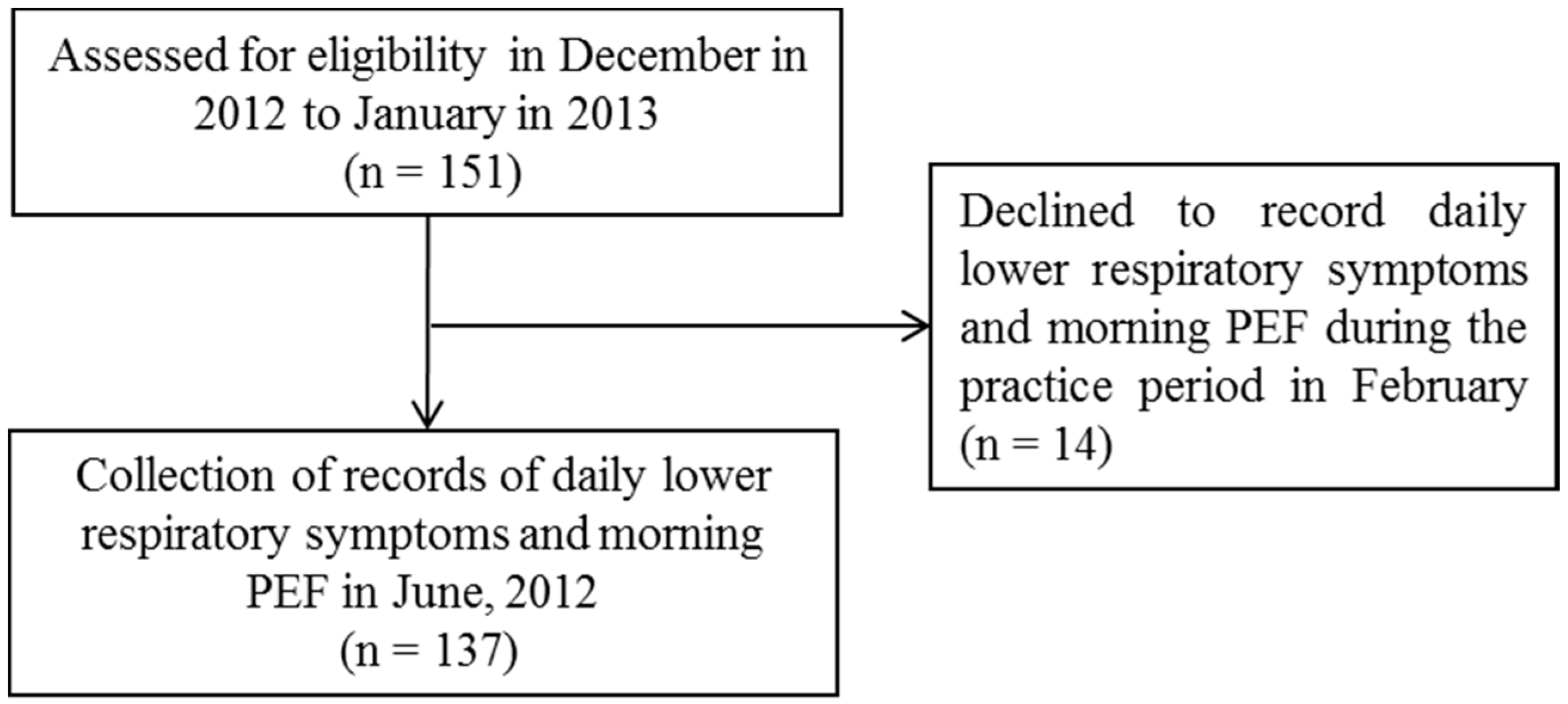
2.1. Study Design
2.2. Definition of the Heavy Dust Days and Monitoring of Air Pollutants
2.3. Recording the Daily Lower Respiratory Tract Symptoms and Morning PEF
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
| Characteristic | Mean/Number (%) |
|---|---|
| Number of patients | 137 |
| Age (years) | 63.5 ± 15.4 |
| Sex (male/female) | 58/79 |
| Smoking status | |
| Never | 92 (67.1) |
| Former | 38 (27.7) |
| Current | 7 (5.2) |
| Pulmonary function | |
| FVC (L) | 2.94 ± 0.70 |
| FEV1 (L) | 2.09 ± 0.60 |
| %FEV1 (%) | 100.4 ± 24.7 |
| Allergic rhinitis and/or chronic sinusitis | 60 (43.8) |
| Atopic disposition | 74 (54.0) |
| Treatment step | |
| Step 1 | 1 (0.7) |
| Step 2 | 14 (10.2) |
| Step 3 | 29 (21.2) |
| Step 4 | 87 (63.5) |
| Step 5 | 6 (4.4) |
3.2. Sand Dust Particle and Aerosolized Air Pollutant Levels


3.3. Lower Respiratory Symptoms and Peak Expiratory Flow
| Meteorological Exposure | ||||
|---|---|---|---|---|
| Sand Dust Particles | Aerosolized Air Pollutants | SPM | PM2.5 | |
| IQR | 0.02 km−1 | 0.06 km−1 | 12.8 μg/m3 | 13.6 μg/m3 |
| Change in the symptoms score | 0.04 | 0.02 | 0.06 | 0.07 |
| 95% CI | 0.03, 0.05 | −0.01, 0.04 | 0.04, 0.08 | 0.04, 0.08 |
| P value | <0.001 | 0.085 | <0.001 | <0.001 |
| Change in the PEF (L/min) | 0.01 | −0.17 | −0.13 | −0.20 |
| 95% CI | −0.62, 0.11 | −0.67, 0.33 | −0.60, 0.35 | −0.80, 0.41 |
| P value | 0.946 | 0.507 | 0.588 | 0.526 |
| Lag Time (Days) | Change in the Symptom Score | 95% CI | P Value |
| Lag 0 | 0.15 | 0.09, 0.20 | <0.001 |
| Lag 0–1 | 0.12 | 0.07, 0.16 | <0.001 |
| Lag 0–2 | 0.11 | 0.07, 0.15 | <0.001 |
| Lag 0–3 | 0.11 | 0.07, 0.14 | <0.001 |
| Lag 0–4 | 0.08 | 0.05, 0.12 | <0.001 |
| Lag 0–5 | 0.06 | 0.02, 0.09 | 0.003 |
| Lag Time (Days) | Change in the PEF (L/min) | 95% CI | P Value |
| Lag 0 | −0.18 | −1.61, 1.25 | 0.805 |
| Lag 0–1 | −0.21 | −1.42, 0.99 | 0.729 |
| Lag 0–2 | 0.11 | −0.95, 1.17 | 0.836 |
| Lag 0–3 | 0.18 | −0.82, 1.17 | 0.730 |
| Lag 0–4 | 0.09 | −0.86, 1.03 | 0.856 |
| Lag 0–5 | 0.36 | −0.56, 1.27 | 0.444 |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goudie, A.S. Desert dust and human health disorders. Environ. Int. 2014, 63, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ramanathan, V.; Li, F.; Kim, D. Dust plumes over the Pacific, Indian, and Atlantic Oceans: Climatology and radiative impact. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Tanaka, T.Y.; Chiba, M. A numerical study of the concentrations of dust source regions to the global dust budget. Glob. Planet. Chang. 2006, 52, 88–104. [Google Scholar] [CrossRef]
- Taylor, D.A. Dust in the wind. Environ. Health Perspect. 2002, 110, A80–A87. [Google Scholar] [CrossRef] [PubMed]
- Duce, R.A.; Unni, C.K.; Ray, B.J.; Prospero, J.M.; Merrill, J.T. Long-range atmospheric transport of soil dust from Asia to the tropical North Pacific: Tempora variability. Science 1980, 209, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Uno, I.; Eguchi, K.; Yumimoto, K.; Takemura, T.; Shimizu, A.; Uematsu, M.; Liu, Z.; Wang, Z.; Hara, Y.; Sugimoto, N. Asian dust transported one full circuit around the globe. Nat. Geosci. 2009, 2, 557–560. [Google Scholar] [CrossRef]
- Zaady, E.; Offer, Z.Y.; Shachak, M. The content and contributions of deposited Aeolian organic matter in a dry land ecosystem of the Negev Desert, Israel. Atmos. Environ. 2001, 35, 769–776. [Google Scholar] [CrossRef]
- Hioki, T.; Nakanishi, S.; Mukai, H.; Murano, K. Analysis of long-lange transported and local air pollution with trace metal concentration ratio and lead isotope ratio in precipitation. J. Jpn. Soc. Atmos. Environ. 2008, 43, 100–111. [Google Scholar]
- Wuebbles, D.J.; Lei, H.; Lin, J. Intercontinental transport of aerosols and photochemical oxidants from Asia and its consequences. Environ. Pollut. 2007, 150, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cho, S.H.; Chun, Y.; Lagarde, F.; Pershagen, G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ. Res. 2002, 90, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, K.T.; Ito, I.; Al-Delaimy, W.K.; Adachi, Y.; Mathews, W.C.; Ramsdell, J.W.; Toyama Asian Desert Dust and Asthma Study Team. Desert-dust exposure is associated with increased risk of asthma hospitalization in children. Am. J. Respir. Crit. Care Med. 2010, 182, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chen, C.S.; Lin, C.L. The threat of Asian dust storms on asthma patients: A population-based study in Taiwan. Glob. Public Health 2014, 9, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nitta, H.; Odajima, H. The effects of weather, air pollutants, and Asian dust on hospitalization for asthma in Fukuoka. Environ. Health Prev. Med. 2010, 15, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Choung, J.T.; Yu, J.; Kim do, K.; Koh, Y.Y. Acute effects of Asian dust events on respiratory symptoms and peak expiratory flow in children with mild asthma. J. Korean Med. Sci. 2008, 23, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lim, Y.H.; Kyung, S.Y.; An, C.H.; Lee, S.P.; Jeong, S.H.; Ju, Y.S. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology 2005, 10, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Lee, C.H. Characteristics of dust aerosols inferred from LIDAR depolarization measurements at two wavelengths. Appl. Opt. 2006, 45, 7468–7474. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Sugimoto, N.; Matsui, I.; Arao, K.; Uno, I.; Maruyama, T.; Kagawa, N.; Aoki, K.; Uchiyama, A.; Yamazaki, A. Continuous observations of Asian dust and other aerosol by polarization LIDARS in China and Japan during ACE-Asia. J. Geophys. Res. 2004, 109. [Google Scholar] [CrossRef]
- Murayama, T.; Sugimoto, T.; Uno, I.; Aoki, K.; Hagiwara, N.; Liu, Z.; Matsui, I.; Sakai, T.; Shibata, T.; Arao, K.; et al. Ground-based network observation of Asian dust events of April 1998 in East Asia. J. Geophys. Res. 2001, 106, 18345–18349. [Google Scholar] [CrossRef]
- Weinmayr, G.; Romeo, E.; de Sario, M.; Weiland, S.K.; Forastiere, F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2006 (Revision); Available online: http://www.ginasthma.org/documents/5/documents_variants/31 (accessed on 15 July 2015).
- Ueda, K.; Shimizu, A.; Inoue, K. Exposure assessment by Light Detection and Ranging for Asian dust aerosol in epidemiological studies to investigate its health effects. Earosoru Kenkyu 2014, 29, 230–236. [Google Scholar]
- MDEJ Real-time DSS Information (LIDAR DSS Observation Data Page). Available online: http://soramame.taiki.go.jp/dss/kosa/ (accessed on 15 July 2013).
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data, 2nd ed.; Springer Verlag: New York, NY, USA, 2009. [Google Scholar]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley: New York, NY, USA, 1987. [Google Scholar]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2012 (Revision); Available online: http://www.ginasthma.org/documents/5/documents_variants/37 (accessed on 15 July 2015).
- Maestrelli, P.; Canova, C.; Scapellato, M.L.; Visentin, A.; Tessari, R.; Bartolucci, G.B.; Simonato, L.; Lotti, M. Personal exposure to particulate matter is associated with worse health perception in adult asthma. J. Invest. Allergol. Clin. Immunol. 2011, 21, 120–128. [Google Scholar]
- Chan-Yeung, M.; Chang, J.H.; Manfreda, J.; Ferguson, A.; Becker, A. Changes in peak flow, symptom score, and the use of medications during acute exacerbations of asthma. Am. J. Respir. Crit. Care Med. 1996, 154, 889–893. [Google Scholar] [CrossRef] [PubMed]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Lagorio, S.; Forastiere, F.; Pistelli, R.; Iavarone, I.; Michelozzi, P.; Fano, V.; Marconi, A.; Ziemacki, G.; Ostro, B.D. Air pollution and lung function among susceptible adult subjects: A panel study. Environ. Health 2006, 5. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Shimizu, A.; Nitta, H.; Inoue, K. Long-range transported Asian Dust and emergency ambulance dispatches. Inhal. Toxicol. 2012, 24, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Min, P.K.; Kim, C.W.; Yun, Y.J.; Chang, J.H.; Chu, J.K.; Lee, K.E.; Han, J.Y.; Park, J.W.; Hong, C.S. Effect of yellow sand on respirator symptoms and diurnal variation of peak expiratory flow in patients with bronchial asthma. J. Asthma Allergy Clin. Immunol. 2001, 21, 1179–1186. [Google Scholar]
- Higashi, T.; Kambayashi, Y.; Ohkura, N.; Fujimura, M.; Nakai, S.; Honda, Y.; Saijoh, K.; Hayakawa, K.; Kobayashi, F.; Michigami, Y.; et al. Effects of Asian dust daily dough occurrence in patients with chronic cough: A panel study. Atmos. Environ. 2014, 92, 506–513. [Google Scholar] [CrossRef]
- Chen, H.; Gould, M.K.; Blanc, P.D.; Miller, D.P.; Kamath, T.V.; Lee, J.H.; Sullivan, S.; TENOR Study Group. Asthma control, severity, and quality of life: Quantifying the effect of uncontrolled disease. J. Allergy Clin. Immunol. 2007, 120, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wiesch, D.G.; Meyers, D.A.; Bleecker, E.R. Genetics of asthma. J. Allergy. Clin. Immunol. 1999, 104, 895–901. [Google Scholar] [CrossRef]
- Holgate, S.T.; Sandström, T.; Frew, A.J.; Stenfors, N.; Nördenhall, C.; Salvi, S.; Blomberg, A.; Helleday, R.; Söderberg, M. Health effects of acute exposure to air pollution. Part I: Healthy and asthmatic subjects exposed to diesel exhaust. Res. Rep. Health. Eff. Inst. 2003, 112, 1–30. [Google Scholar] [PubMed]
- Sierra-Vargas, M.P.; Guzman-Grenfell, A.M.; Blanco-Jimenez, S.; Sepulveda-Sanchez, J.D.; Bernabe-Cabanillas, R.M.; Cardenas-Gonzalez, B.; Ceballos, G.; Hicks, J.J. Airborne particulate matter PM2.5 from Mexico City affects the generation of reactive oxygen species by blood neutrophils from asthmatics: An in vitro approach. J. Occup. Med. Toxicol. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Mitschik, S.; Schierl, R.; Nowak, D.; Jörres, R.A. Effects of particulate matter on cytokine production in vitro: A comparative analysis of published studies. Inhal. Toxicol. 2008, 20, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; McCreanor, J.E.; Cullinan, P.; Chung, K.F.; Ohman-Strickland, P.; Han, I.K.; Järup, L.; Nieuwenhuijsen, M.J. Health effects of real-world exposure to diesel exhaust in persons with asthma. Res. Rep. Health. Eff. Inst. 2009, 138, 5–109. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Noma, H.; Kurai, J.; Shimizu, A.; Sano, H.; Kato, K.; Mikami, M.; Ueda, Y.; Tatsukawa, T.; Ohga, H.; et al. Association of Sand Dust Particles with Pulmonary Function and Respiratory Symptoms in Adult Patients with Asthma in Western Japan Using Light Detection and Ranging: A Panel Study. Int. J. Environ. Res. Public Health 2015, 12, 13038-13052. https://doi.org/10.3390/ijerph121013038
Watanabe M, Noma H, Kurai J, Shimizu A, Sano H, Kato K, Mikami M, Ueda Y, Tatsukawa T, Ohga H, et al. Association of Sand Dust Particles with Pulmonary Function and Respiratory Symptoms in Adult Patients with Asthma in Western Japan Using Light Detection and Ranging: A Panel Study. International Journal of Environmental Research and Public Health. 2015; 12(10):13038-13052. https://doi.org/10.3390/ijerph121013038
Chicago/Turabian StyleWatanabe, Masanari, Hisashi Noma, Jun Kurai, Atsushi Shimizu, Hiroyuki Sano, Kazuhiro Kato, Masaaki Mikami, Yasuto Ueda, Toshiyuki Tatsukawa, Hideki Ohga, and et al. 2015. "Association of Sand Dust Particles with Pulmonary Function and Respiratory Symptoms in Adult Patients with Asthma in Western Japan Using Light Detection and Ranging: A Panel Study" International Journal of Environmental Research and Public Health 12, no. 10: 13038-13052. https://doi.org/10.3390/ijerph121013038






