Biodegradation Kinetics of Tetrahydrofuran, Benzene, Toluene, and Ethylbenzene as Multi-substrate by Pseudomonas oleovorans DT4
Abstract
:1. Introduction
2. Experimental Section
2.1. Microbial Strain and Growth Medium
2.2. Kinetic Experiments
2.3. Analytical Methods
2.4. Kinetic Models and Parameter Estimation
3. Results and Discussion
3.1. Determination of the Kinetics Model for Single Substrate
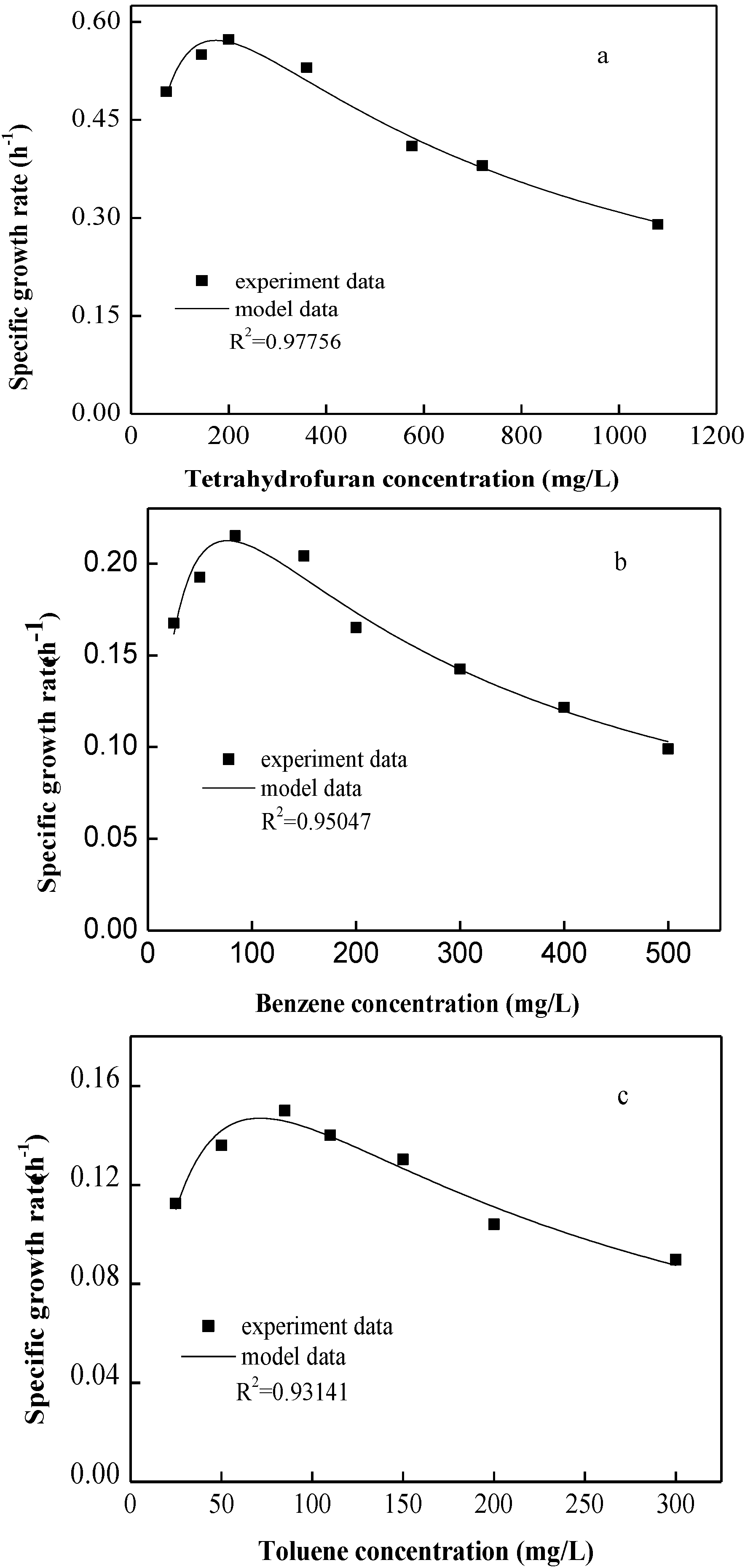
| Substrate | μmax (h−1) | KS (mg/L) | KI (mg/L) | Microorganism | Reference |
|---|---|---|---|---|---|
| Tetrahydrofuran | 1.01 | 65.95 | 455.1 | P. oleovorans DT4 | this study |
| B | 0.39 | 31.83 | 184.0 | P. oleovorans DT4 | this study |
| 0.335 | 3.17 | -- | P. fragi B1 | Chang et al. [30] | |
| 0.44 | 3.36 | -- | P. putida O1 | Oh et al. [21] | |
| 0.73 | 0.12 | -- | P. putida F1 | Reardon et al. [31] | |
| 0.62 | 1.65 | 180 | P. putida F1 | Abuhamed et al. [21] | |
| 0.44 | 27.57 | -- | mixed bacteria | Littlejohns and Daugulis [22] | |
| T | 0.33 | 44.83 | 113.6 | P. oleovorans DT4 | this study |
| 0.437 | 6 | 1980 | P. putida ATCC23973 | Choi et al. [32] | |
| 0.42 | 3.98 | 42.8 | P. putida 54G | Mirpuri and Bryers [33] | |
| 0.86 | 13.8 | -- | P. putida F1 | Reardon et al. [31] | |
| 0.61 | 6.47 | 88 | P. putida F1 | Abuhamed et al. [21] | |
| 0.60 | 34.12 | -- | mixed bacteria | Littlejohns and Daugulis [22] |
3.2. Degradation Kinetic of Tetrahydrofuran with Mixture of Benzene, Toluene, or Ethylbenzene


| Compounds | Ii,j | Microorganism | Reference |
|---|---|---|---|
| Tetrahydrofuran and BTE | ITHF,B2 = −0.38, IB,THF = 35.4 | P. oleovorans DT4 | this study |
| ITHF,T = −0.23, IT,THF = 48.9 | |||
| ITHF,E =−1.5, IE,THF = 65.3 | |||
| BT and Phenol (P) | IT,P = 55, IP,T = 0.01 | P. putida F1 | Reardon et al. [31] |
| IT,B = 5, IB,T = 0.01 | |||
| IB,P = 18.5, IP,B =0.01 | |||
| BTP | IT,P = 0.14, IP,T = 1.03 | P. putida F1 | Abuhamed et al. [21] |
| IT,B = 5.16, IB,T = 0.49 | |||
| IB,P = 0.27, IP,B = 1.08 | |||
| BTE | IT,B = 2 | mixed bacterial | Littlejohns and Daugulis [22] |
| IB,T = −0.4 | |||
| IE,B = 4 | |||
| Phenol (P), Vanillin (V), Oxalic (O) and Formic acid (F) 1 | IP,V = 0.03 | P. putida CECT324 | Martin et al. [36] |
| IV,O = 105 | |||
| BTE | IT,B = 1, IB,T = 0.0023 | P. putida F1 | Trigueros et al. [34] |
| IE,B = 10, IB,E = 0.175 | |||
| IT,E = 0.025, IE,T = 4.5 |
3.3. Degradation Kinetic of the Quaternary Substrate Experiment

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hewitt, C.N. Reactive Hydrocarbons in the Atmosphere; Academic Press: San Diego, CA, USA, 1999; pp. 41–96. [Google Scholar]
- Isaacson, C.; Mohr, T.K.; Field, J.A. Quantitative determination of 1, 4-dioxane and tetrahydrofuran in groundwater by solid phase extraction GC/MS/MS. Environ. Sci. Technol. 2006, 40, 7305–7311. [Google Scholar] [CrossRef] [PubMed]
- Painter, H.; King, E. Biodegradation of water-soluble compounds. In Reactions and Processes; Springer: Berlin, Germany, 1985; pp. 87–120. [Google Scholar]
- Moody, D.E. The effect of tetrahydrofuran on biological systems: Does a hepatotoxic potential exist? Drug Chem. Toxicol. 1991, 14, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, F.; Rodney, B.; Jim, B.; Chris, L.; David, M.; Erik, R.; Joost, V.R.; Marcy, B. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Herbert, R.; Roycroft, J.; Chou, B.; Miller, R.; Renne, R. Carcinogenesis studies of tetrahydrofuran vapors in rats and mice. Toxicol. Sci. 1998, 41, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hermida, S.A.; Possari, E.P.; Souza, D.B.; de Arruda Campos, I.P.; Gomes, O.F.; Mascio, P.D.; Medeiros, M.H.; Loureiro, A.P. 2ʹ-deoxyguanosine, 2ʹ-deoxycytidine, and 2ʹ-deoxyadenosine adducts resulting from the reaction of tetrahydrofuran with DNA bases. Chem. Res. Toxicol. 2006, 19, 927–936. [Google Scholar] [CrossRef]
- Chhabra, R.; Elwell, M.; Chou, B.; Miller, R.; Renne, R. Subchronic toxicity of tetrahydrofuran vapors in rats and mice. Fundam. Appl. Toxicol. 1990, 14, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guan, J.; Tang, P.; Jiao, H.; Lin, C.; Wang, J.; Lu, Z.; Min, H.; Gao, H. Assessment of toxicity of tetrahydrofuran on the microbial community in activated sludge. Bioresour. Technol. 2010, 101, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Painter, H.A.; King, E.F. Ring Test Program 1983–84: Assessment of Biodegradability of Chemicals in Water by Manometric Respirometry; Commission of the European Communities: Luxembourg, 1985; pp. 4–10. [Google Scholar]
- Bernhardt, D.; Diekmann, H. Degradation of dioxane, tetrahydrofuran and other cyclic ethers by an environmental Rhodococcus strain. Appl. Microbiol. Biotechnol. 1991, 36, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lv, Z.; Min, H.; Lv, Z.; Jiao, H. Isolation, identification and characterization of a novel Rhodococcus sp. strain in biodegradation of tetrahydrofuran and its medium optimization using sequential statistics-based experimental designs. Bioresour. Technol. 2009, 100, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Kohlweyer, U.; Thiemer, B.; Schräder, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Parales, R.; Adamus, J.; White, N.; May, H. Degradation of 1, 4-dioxane by an actinomycete in pure culture. Appl. Environ. Microbiol. 1994, 60, 4527–4530. [Google Scholar] [PubMed]
- Vainberg, S.; McClay, K.; Masuda, H.; Root, D.; Condee, C.; Zylstra, G.J.; Steffan, R.J. Biodegradation of ether pollutants by Pseudonocardia sp. strain ENV478. Appl. Environ. Microbiol. 2006, 72, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, K.; Hashimoto, S.; Ito, H.; Edmonds, J.S.; Morita, M. Degradation of 1, 4-dioxane and cyclic ethers by an isolated fungus. Appl. Environ. Microbiol. 2005, 71, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Zhou, Y.Y.; Chen, D.Z.; Jin, X.J. A newly isolated strain capable of effectively degrading tetrahydrofuran and its performance in a continuous flow system. Bioresour. Technol. 2010, 101, 6461–6467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Chen, D.Z.; Zhu, R.Y.; Chen, J.M. Substrate interactions during the biodegradation of BTEX and THF mixtures by Pseudomonas oleovorans DT4. Bioresour. Technol. 2011, 102, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, M.; Dowling, D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int. J. Environ. Res. Public Health 2009, 6, 2226–2247. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.E.; Dwarakanath, V. Chlorinated decreasing solvents: Physical-chemical properties affecting aquifer contamination and remediation. Ground water Monit. Rem. 1999, 19, 102–110. [Google Scholar] [CrossRef]
- Abuhamed, T.; Bayraktar, E.; Mehmetoğlu, T.; Mehmetoğlu, Ü. Kinetics model for growth of Pseudomonas putida F1 during benzene, toluene and phenol biodegradation. Process Biochem. 2004, 39, 983–988. [Google Scholar] [CrossRef]
- Littlejohns, J.V.; Daugulis, A.J. Kinetics and interactions of BTEX compounds during degradation by a bacterial consortium. Process Biochem. 2008, 43, 1068–1076. [Google Scholar] [CrossRef]
- Oh, Y.S.; Shareefdeen, Z.; Baltzis, B.C.; Bartha, R. Interactions between benzene, toluene, and p-xylene (BTX) during their biodegradation. Biotechnol. Bioeng. 1994, 44, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, A.R.; Stensel, H.D. Modeling competitive inhibition effects during biodegradation of BTEX mixtures. Water Res. 1999, 33, 707–714. [Google Scholar] [CrossRef]
- Deeb, R.A.; Alvarez-Cohen, L. Temperature effects and substrate interactions during the aerobic biotransformation of BTEX mixtures by toluene-enriched consortia and Rhodococcus rhodochrous. Biotechnol. Bioeng. 1999, 62, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Hamed, T.A.; Bayraktar, E.; Mehmetoğlu, T.; Mehmetoğlu, Ü. Substrate interactions during the biodegradation of benzene, toluene and phenol mixtures. Process Biochem. 2003, 39, 27–35. [Google Scholar] [CrossRef]
- Shuler, M.L.; Kargi, F. Bioprocess Engineering Basic Concepts, 2nd ed.; Prentice Hall: Bergen County, NJ, USA, 2002. [Google Scholar]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Webb, J.L. Enzyme and Metabolic Inhibitors; Academic Press New York: New York, NY, USA, 1966; Volume 3. [Google Scholar]
- Chang, M.K.; Voice, T.C.; Criddle, C.S. Kinetics of competitive inhibition and cometabolism in the biodegradation of benzene, toluene, and p-xylene by two Pseudomonas isolates. Biotechnol. Bioeng. 1993, 41, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Reardon, K.F.; Mosteller, D.C.; Bull Rogers, J.D. Biodegradation kinetics of benzene, toluene, and phenol as single and mixed substrates for Pseudomonas putida F1. Biotechnol. Bioeng. 2000, 69, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.B.; Lee, J.Y.; Kim, H.S. A novel bioreactor for the biodegradation of inhibitory aromatic solvents: Experimental results and mathematical analysis. Biotechnol. Bioeng. 1992, 40, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Mirpuri, R.; Jones, W.; Bryers, J.D. Toluene degradation kinetics for planktonic and biofilm-grown cells of Pseudomonas putida 54G. Biotechnol. Bioeng. 1997, 53, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, D.E.; Módenes, A.N.; Kroumov, A.D.; Espinoza-Quiñones, F.R. Modeling of biodegradation process of BTEX compounds: Kinetic parameters estimation by using Particle Swarm Global Optimizer. Process Biochem. 2010, 45, 1355–1361. [Google Scholar] [CrossRef]
- Yoon, H.; Klinzing, G.; Blanch, H. Competition for mixed substrates by microbial populations. Biotechnol. Bioeng. 1977, 19, 1193–1210. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Pérez, J.; Fernández, F.; Sánchez, J.; López, J.; Rodríguez, S.M.A. Kinetics study on the biodegradation of synthetic wastewater simulating effluent from an advanced oxidation process using Pseudomonas putida CECT 324. J. Hazard. Mater. 2008, 151, 780–788. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-Z.; Ding, Y.-F.; Zhou, Y.-Y.; Ye, J.-X.; Chen, J.-M. Biodegradation Kinetics of Tetrahydrofuran, Benzene, Toluene, and Ethylbenzene as Multi-substrate by Pseudomonas oleovorans DT4. Int. J. Environ. Res. Public Health 2015, 12, 371-384. https://doi.org/10.3390/ijerph120100371
Chen D-Z, Ding Y-F, Zhou Y-Y, Ye J-X, Chen J-M. Biodegradation Kinetics of Tetrahydrofuran, Benzene, Toluene, and Ethylbenzene as Multi-substrate by Pseudomonas oleovorans DT4. International Journal of Environmental Research and Public Health. 2015; 12(1):371-384. https://doi.org/10.3390/ijerph120100371
Chicago/Turabian StyleChen, Dong-Zhi, Yun-Feng Ding, Yu-Yang Zhou, Jie-Xu Ye, and Jian-Meng Chen. 2015. "Biodegradation Kinetics of Tetrahydrofuran, Benzene, Toluene, and Ethylbenzene as Multi-substrate by Pseudomonas oleovorans DT4" International Journal of Environmental Research and Public Health 12, no. 1: 371-384. https://doi.org/10.3390/ijerph120100371





