Strong Associations Between the Pesticide Hexachlorocyclohexane and Type 2 Diabetes in Saudi Adults
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Measurements
Pesticide Analysis
2.3. Statistical Analysis
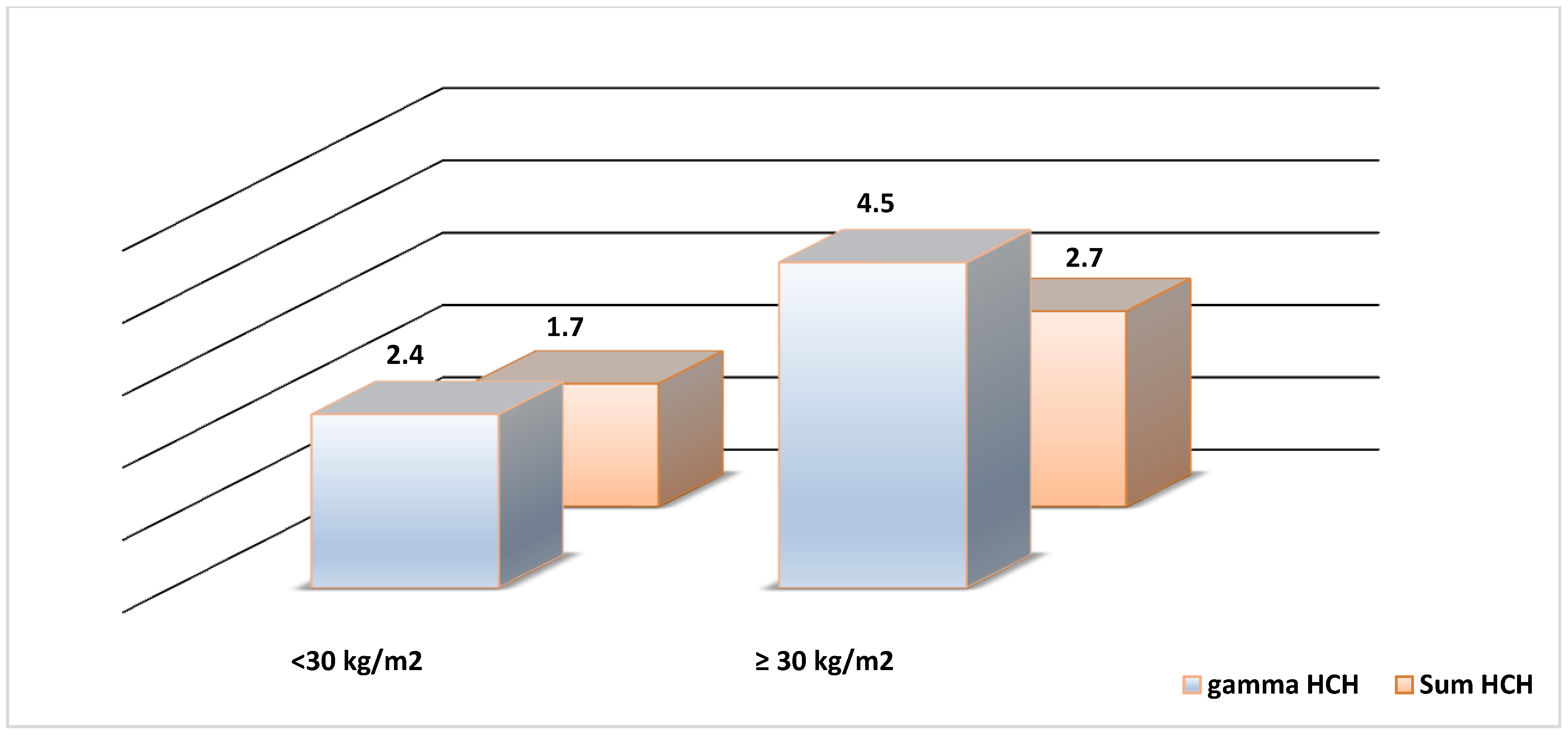
3. Results and Discussion
| Parameters | Control | Diabetes | P |
|---|---|---|---|
| N | 144 | 136 | |
| Gender (M/F) | 49/95 | 60/76 | 0.08 |
| Age (years) | 37.4 ± 6.6 | 43.0 ± 6.8 | <0.001 |
| BMI (kg/m2) | 29.3 ± 5.9 | 32.2 ± 5.3 | <0.001 |
| Waist (cm) | 92.6 ± 16.4 | 94.2 ± 24.7 | 0.11 |
| Hips (cm) | 100.4 ± 27.7 | 106.3 ± 28.6 | 0.09 |
| Sagittal Abdominal Diameter (cm) | 22.4 ± 5.1 | 23.4 ± 7.0 | 0.30 |
| Systolic BP (mmHg) | 111.9 ± 1.3 | 122.6 ± 10.3 | <0.001 |
| Diastolic BP (mmHg) | 73.9 ± 7.5 | 79.1 ± 7.4 | <0.001 |
| Total Cholesterol (mmol/L) | 5.0 ± 1.1 | 5.1 ± 1.1 | 0.56 |
| Glucose (mmol/L) | 5.2 ± 0.79 | 11.1 ± 4.0 | <0.001 |
| HDL-Cholesterol (mmol/L) | 2.4 ± 0.51 | 0.88 ± 0.32 | <0.001 |
| HbA1c (mmol/L) | 5.1 ± 0.50 | 8.0 ± 1.6 | <0.001 |
| Triglycerides (mmol/L) | 1.2 ± 0.86 | 2.1 ± 0.96 | <0.001 |
| Insulin (IU/mL) | 15.3 ± 4.3 | 32.0 ± 6.5 | <0.001 |
| HOMA-IR | 1.6 ± 0.54 | 5.2 ± 2.3 | <0.001 |
| Leptin (ng/mL) | 18.3 ± 5.3 | 26.5 ± 7.3 | <0.001 |
| Resistin (ng/mL) | 3.4 ± 0.86 | 4.5 ± 0.95 | 0.006 |
| Alpha-HCH (ng/mL) * | 0.28 ± 0.05 | 0.41 ± 0.06 | 0.15 |
| Beta-HCH (ng/mL) * | 0.62 ± 0.09 | 0.75 ± 0.09 | 0.35 |
| Gamma-HCH (ng/mL) * | 2.8 ± 0.49 | 7.3 ± 0.84 | <0.001 |
| Sum HCH(ng/mL) | 3.8 ± 0.51 | 8.8 ± 0.88 | <0.001 |
| Parameters | α-HCH (ng/mL) | β-HCH (ng/mL) | γ-HCH (ng/mL) | Sum HCH (ng/mL) |
|---|---|---|---|---|
| Age (years) | −0.009 | 0.01 | 0.13 | 0.14 |
| BMI (kg/m2) | −0.04 | 0.008 | 0.07 | 0.07 |
| Waist (cm) | 0.09 | −0.08 | −0.01 | 0.04 |
| Hips (cm) | −0.09 | −0.08 | −0.05 | −0.05 |
| SAD (cm) | 0.04 | −0.04 | 0.006 | 0.005 |
| Systolic BP (mmHg) | 0.11 | 0.09 | 0.19 ** | 0.23 ** |
| Diastolic BP (mmHg) | 0.04 | 0.09 | 0.13 | 0.16 |
| Total Cholesterol (mmol/L) | −0.02 | 0.04 | 0.01 | 0.005 |
| Glucose (mmol/L) | 0.08 | 0.09 | 0.23 ** | 0.26 ** |
| HDL (mmol/L) | −0.07 | 0.06 | −0.14 | −0.13 * |
| LDL(mmol/L | 0.07 | −0.01 | 0.07 | 0.08 |
| Triglycerides (mmol/L) | 0.06 | 0.007 | 0.15 ** | 0.16 ** |
| Insulin (IU/mL) | −0.03 | 0.09 | 0.09 | 0.09 |
| HOMA-IR | 0.04 | 0.39 ** | 0.16 | 0.22 ** |
| HbA1c (%) | 0.06 | 0.05 | -- | -- |
| Adiponectin (μg/mL) | −0.03 | −0.06 | 0.05 | −0.01 |
| Leptin (ng/mL) | 0.02 | 0.04 | −0.02 | −0.01 |
| Resistin (ng/mL) | −0.02 | 0.02 | 0.12 | 0.12 |
| Parameters | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| α-HCH (ng/mL) | 1.7 (1.0, 3.0) * | 1.9 (0.92, 4.2) | 1.6 (0.86, 2.9) | 1.7 (0.72, 4.3) |
| Female | 1.4 (0.687, 2.9) | 1.6 (0.59, 4.5) | 1.3 (0.57, 3.0) | 1.5 (0.35, 6.2) |
| Male | 2.1 (0.85, 4.9) | 2.3 (0.72, 7.2) | 2.1 (0.79, 5.2) | 2.1 (0.60, 7.2) |
| β-HCH (ng/mL) | 1.6 (1.0,2.7) * | 1.1 (0.58, 2.2) | 1.6 (0.94, 2.9) | 0.96 (0.43, 2.1) |
| Female | 1.5 (0.81, 2.8) | 1.1 (0.47, 2.7) | 1.3 (0.62, 2.9) | 1.1 (0.31, 3.6) |
| Male | 1.8 (0.83, 4.1) | 1.0 (0.38, 3.0) | 2.2 (0.90, 5.4) | 1.0 (0.32, 3.3) |
| γ-HCH (ng/mL) | 1.8 (1.2, 2.9) * | 2.0 (1.1, 3.9) * | 1.8 (1.1, 3.2) * | 1.8 (0.86, 3.9) |
| Female | 2.1 (1.1, 3.8) * | 2.8 (1.2, 6.6) * | 2.9 (1.3, 5.8) * | 3.9 (1.1, 6.8) * |
| Male | 1.4 (0.67, 3.1) | 1.3 (0.49, 3.7) | 1.3 (0.56, 2.9) | 1.2 (0.40, 3.6) |
| Sum HCH (ng/mL) | 2.8 (1.6, 4.4) * | 2.8 (1.4, 5.4) * | 3.2 (1.8, 5.5) * | 2.7 (1.3, 6.0) * |
| Female | 2.4 (1.3, 4.6) * | 3.2 (1.3, 7.8) * | 3.3 (1.9, 7.6) * | 4.3 (2.1, 8.3) * |
| Male | 3.2 (1.4, 7.0) * | 2.6 (0.90, 7.4) | 3.0 (1.2, 7.2) * | 2. 1(0.72, 7.2) |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guariguata , L. By the numbers: New estimates from the IDF diabetes atlas update for 2012. Diabetes Res. Clin. Pract. 2012, 98, 524–525. [Google Scholar]
- Alzaid, A.A. Time to declare war on diabetes. Ann. Saudi. Med. 1997, 17, 154–155. [Google Scholar] [PubMed]
- Fatani, H.H.; Mira, S.A.; el-Zubier, A.G. Prevalence of diabetes mellitus in rural Saudi Arabia. Diabetes Care 1987, 10, 180–183. [Google Scholar] [CrossRef] [PubMed]
- El-Hazmi, M.; Warsy, A.; Al-Swailem, A.; Sulaimani, R. Diabetes mellitus as a health problem in Saudi Arabia. East Mediterr. Health J. 1998, 4, 58–67. [Google Scholar]
- Gillies, C.L.; Abrams, K.R.; Lambert, P.C.; Cooper, N.J.; Sutton, A.J.; Hsu, R.T.; Khunti, K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007, 334. [Google Scholar] [CrossRef]
- Wilson, A.; Gyi, A.A. The status and perspective of diabetes health education in China: Inspiration from Australia. Int. J. Nurs. Pract. 2010, 16, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Obesity: genes, brain, gut, and environment. Nutrition 2010, 26, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Tuomi, T. Type 1 and type 2 diabetes: What do they have in common? Diabetes 2005, 54, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Eichler, E.E.; Flint, J.; Gibson, G.; Kong, A.; Leal, S.M.; Moore, J.H.; Nadeau, J.H. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 2010, 11, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.J.; Bhattacharya, J.; Butte, A.J. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health A. 2006, 69, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.J.; Frithsen, I.L.; Diaz, V.A.; Koopman, R.J.; Simpson, W.M., Jr.; Mainous, A.G. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2007, 103, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, I.K.; Song, K.; Steffes, M.; Toscano, W.; Baker, B.A.; Jacobs, D.R., Jr. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care 2006, 29, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Philibert, A.; Schwartz, H.; Mergler, D. An exploratory study of diabetes in a first nation community with respect to serum concentrations of p,p'-DDE and PCBs and fish consumption. Int. J. Environ. Res. Public Health 2009, 6, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Persky, V.; Piorkowski, J.; Turyk, M.; Freels, S.; Chatterton, R.; Dimos, J.; Bradlow, H.L.; Chary, L.K.; Burse, V.; Unterman, T.; et al. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ. Res. 2011, 111, 817–824. [Google Scholar]
- Kutz, F.W.; Wood, P.H.; Bottimore, D.P. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev. Environ. Contam. Toxicol. 1991, 120, 1–82. [Google Scholar] [PubMed]
- Lucena, R.A.; Allam, M.F.; Jimenez, S.S.; Villarejo, M.L. A review of environmental exposure to persistent organochlorine residuals during the last fifty years. Curr. Drug. Saf. 2007, 2, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Breivik, K.; Pacyna, J.M.; Munch, J. Use of alpha-, beta- and gamma-hexachlorocyclohexane in Europe, 1970–1996. Sci. Total. Environ. 1999, 239, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, J.; Abhilash, P.C.; Li, Y.F.; Lal, R.; Forter, M.; Torres, J.; Singh, N.; Yunus, M.; Tian, C.; Schaffer, A.; Weber, R. Hexachlorocyclohexane (HCH) as new Stockholm Convention POPs—A global perspective on the management of Lindane and its waste isomers. Environ. Sci. Pollut. Res. Int. 2011, 18, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Othman, A; Abd-Alrahman, S.H.; Al-Daghri, N.M. DDT and its metabolites are linked to increased risk of type 2 diabetes among Saudi adults: a cross-sectional study. Environ. Sci. Pollut. R. 2014, 2014. [Google Scholar] [CrossRef]
- Airaksinen, R.; Rantakokko, P.; Eriksson, J.G.; Blomstedt, P.; Kajantie, E.; Kiviranta, H. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care 2011, 34, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Gasull, M.; Pumarega, J.; Tellez-Plaza, M.; Castell, C.; Tresserras, R.; Lee, D.H.; Porta, M. Blood concentrations of persistent organic pollutants and prediabetes and diabetes in the general population of Catalonia. Environ. Sci. Technol. 2012, 46, 7799–7810. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lind, P.M.; Jacobs, D.R., Jr.; Salihovic, S.; van Bavel, B.; Lind, L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care 2011, 34, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Bertrand, K.A.; Choi, A.L.; Hu, F.B.; Laden, F.; Grandjean, P.; Sun, Q. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses’ health study and meta-analysis. Environ. Health Perspect. 2013, 121, 153–161. [Google Scholar] [PubMed]
- Cox, S.; Niskar, A.S.; Narayan, K.M.; Marcus, M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: Hispanic health and nutrition examination survey, 1982-1984. Environ. Health Perspect. 2007, 115, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.J.; Matheson, E.M. Biomarkers of pesticide exposure and diabetes in the 1999–2004 national health and nutrition examination survey. Environ. Int. 2010, 36, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Son, H.K.; Kim, S.A.; Kang, J.H.; Chang, Y.S.; Park, S.K.; Lee, S.K.; Jacobs, D.R., Jr.; Lee, D.H. Strong associations between low-dose organochlorine pesticides and type 2 diabetes in Korea. Environ. Int. 2010, 36, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, J.P.; Pumarega, J.; Gasull, M.; Fernandez, M.F.; Martin-Olmedo, P.; Molina-Molina, J.M.; Fernandez-Rodriguez, M.; Porta, M.; Olea, N. Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ. Res. 2013, 122, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yoon, KH; Lee, JH; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Zimmet, P.; Son, H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 368, 1681–1688. [Google Scholar]
- Sexton, K.; Salinas, J.J.; McDonald, T.J.; Gowen, R.M.; Miller, R.P.; McCormick, J.B.; Fisher-Hoch, S.P. Biomarkers of maternal and fetal exposure to organochlorine pesticides measured in pregnant Hispanic women from Brownsville, Texas. Int. J. Environ. Res. Public Health 2013, 10, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Available online: http://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=754&tid=138 (accessed on 20 August 2014).
- Drummond, L.; Gillanders, E.M.; Wilson, H.K. Plasma gamma-hexachlorocyclohexane concentrations in forestry workers exposed to lindane. Br. J. Ind. Med. 1988, 45, 493–497. [Google Scholar] [PubMed]
- Jung, D.; Becher, H.; Edler, L.; Flesch-Janys, D.; Gurn, P.; Konietzko, J.; Manz, A.; Papke, O. Elimination of beta-hexachlorocyclohexane in occupationally exposed persons. J. of Toxicol. and Environ. Health 1997, 51, 23–34. [Google Scholar]
- Nigam, S.K.; Karnik, A.B.; Majumder, S.K.; Visweswariah, K.; Raju, G.S.; Bai, K.M.; Lakkad, B.C.; Thakore, K.N.; Chatterjee, B.B. Serum hexachlorocyclohexane residues in workers engaged at a HCH manufacturing plant. Int. Arch. Occup. Environ. Health 1986, 57, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Angerer, J.; Heinrich, R.; Lehnert, G. Occupational exposure to hexachlorocyclohexane. I. Body burden of HCH-isomers. Int. Arch. Occup. Environ. Health 1980, 47, 119–127. [Google Scholar]
- Al-Saleh, I.; Echeverria-Quevedo, A.; Al-Dgaither, S.; Faris, R. Residue levels of organochlorinated insecticides in breast milk: a preliminary report from Al-Kharj, Saudi Arabia. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 37–50. [Google Scholar]
- Radikova, Z.; Koska, J.; Ksinantova, L.; Imrich, R.; Kocan, A.; Petrik, J. Increased frequency of diabetes and other forms of dysglycemia in the population of specific areas of eastern Slovakia chronically exposed to contamination with polychlorinated biphenyls (PCB). Organohal. Comp. 2004, 66, 3547–3551. [Google Scholar]
- Vasiliu, O.; Cameron, L.; Gardiner, J.; Deguire, P.; Karmaus, W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 2006, 17, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am. J. of Cardiol. 1999, 83, 25–29. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Gamal, B.A. Effect of diazinon, an organophosphate insecticide, on plasma lipid constituents in experimental animals. J. Biochem. Mol. Biol. 2003, 36, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Rezg, R.; Mornagui, B.; Benahmed, M.; Chouchane, S.G.; Belhajhmida, N.; Abdeladhim, M.; Kamoun, A.; El-fazaa, S.; Gharbi, N. Malathion exposure modulates hypothalamic gene expression and induces dyslipedemia in Wistar rats. Food Chem. Toxicol. 2010, 48, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Ogunbode, A.M.; Ladipo, M.; Ajayi, I.O.; Fatiregun, A.A. Obesity: an emerging disease. Niger. J. Clin. Pract. 2011, 14, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Alokail, S; Al-Daghri, N; Abdulkareem, A; Draz, H.M.; Yakout, S.M.; Alnaami, A.M.; Sabico, S.; Alenad, A.M.; Chrousos, G.P. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Remillard, R.B.; Bunce, N.J. Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ. Health Perspect. 2002, 110, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.J.; Wagner, M.A.; Madhukar, B.V. Potential involvement of calcium, CaM kinase II, and MAP kinases in PCB-stimulated insulin release from RINm5F cells. Toxicol. Appl. Pharmacol. 1999, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Srinivasan, S.; Anandatheerthavarada, H.K.; Avadhani, N.G. Dioxin-mediated tumor progression through activation of mitochondria-to-nucleus stress signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 186–191. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Othman, A.; Yakout, S.; Abd-Alrahman, S.H.; Al-Daghri, N.M. Strong Associations Between the Pesticide Hexachlorocyclohexane and Type 2 Diabetes in Saudi Adults. Int. J. Environ. Res. Public Health 2014, 11, 8984-8995. https://doi.org/10.3390/ijerph110908984
Al-Othman A, Yakout S, Abd-Alrahman SH, Al-Daghri NM. Strong Associations Between the Pesticide Hexachlorocyclohexane and Type 2 Diabetes in Saudi Adults. International Journal of Environmental Research and Public Health. 2014; 11(9):8984-8995. https://doi.org/10.3390/ijerph110908984
Chicago/Turabian StyleAl-Othman, Abdulaziz, Sobhy Yakout, Sherif H. Abd-Alrahman, and Nasser M. Al-Daghri. 2014. "Strong Associations Between the Pesticide Hexachlorocyclohexane and Type 2 Diabetes in Saudi Adults" International Journal of Environmental Research and Public Health 11, no. 9: 8984-8995. https://doi.org/10.3390/ijerph110908984






