Safety and Protective Effectiveness of Two Strains of Lactobacillus with Probiotic Features in an Experimental Model of Salmonellosis
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Bacteria
2.3. Pre-treatment of Mice with Lactobacillus Strains
2.4. Pathogen Challenge
2.5. Intestinal Secretory Immunoglobulin Type A (sIgA)
2.6. Histological and Morphometric Analysis of Mice Organs
2.7. Salmonella Typhimurium Translocation
2.8. Total RNA Isolation
2.9. Reverse Transcription
2.10. Real-Time RT-qPCR Analysis
2.11. Statistical Analysis
3. Results
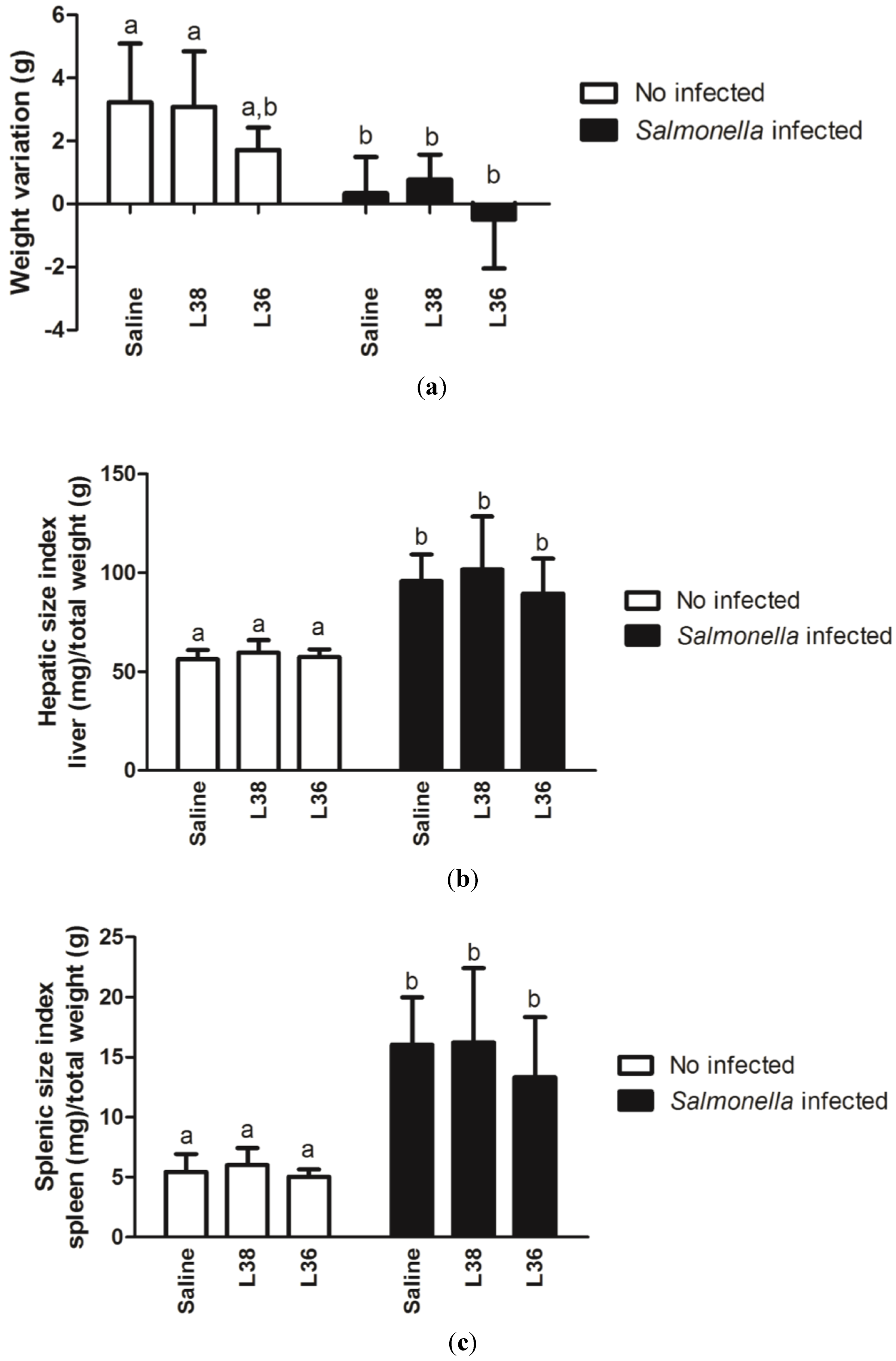
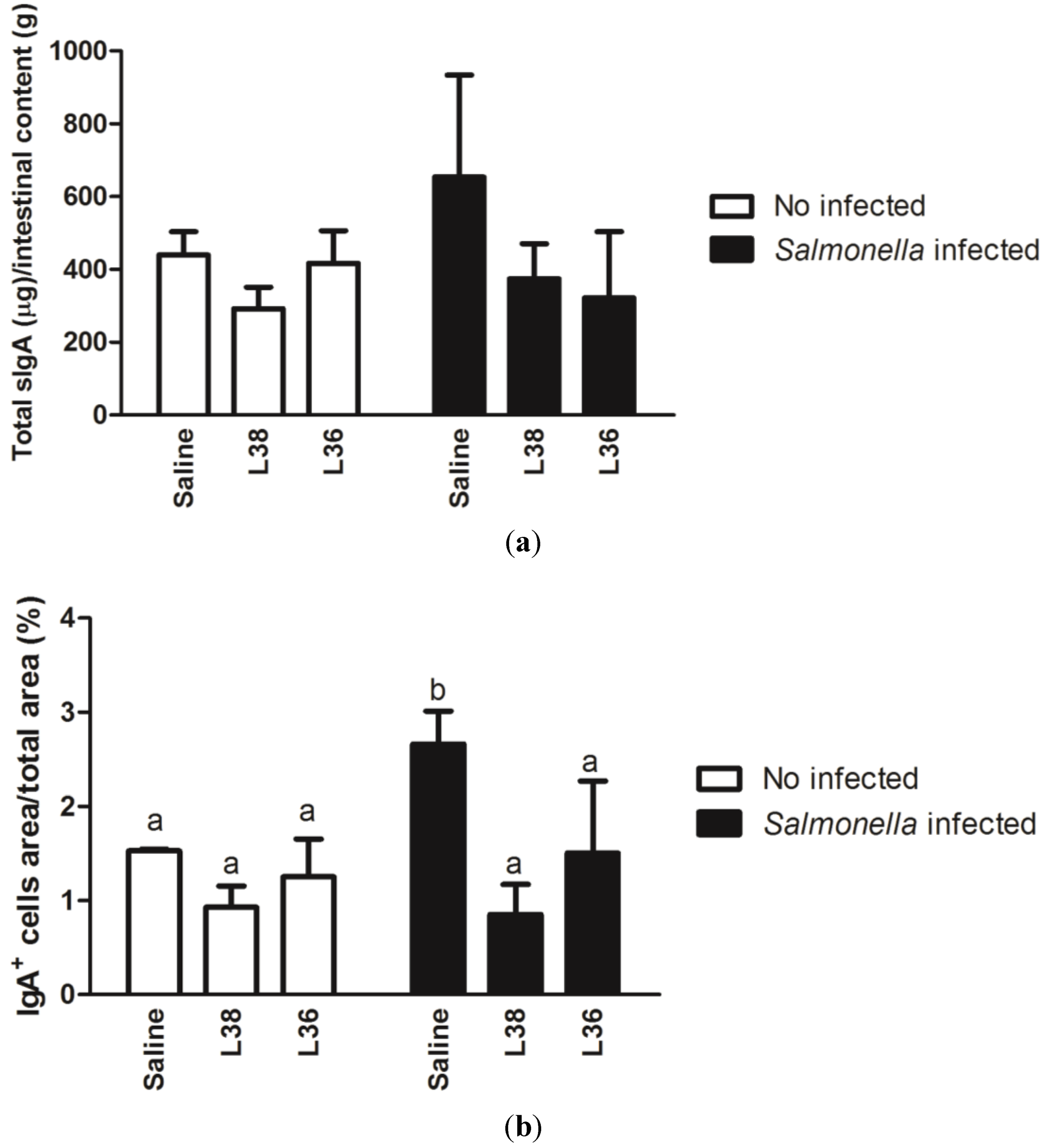
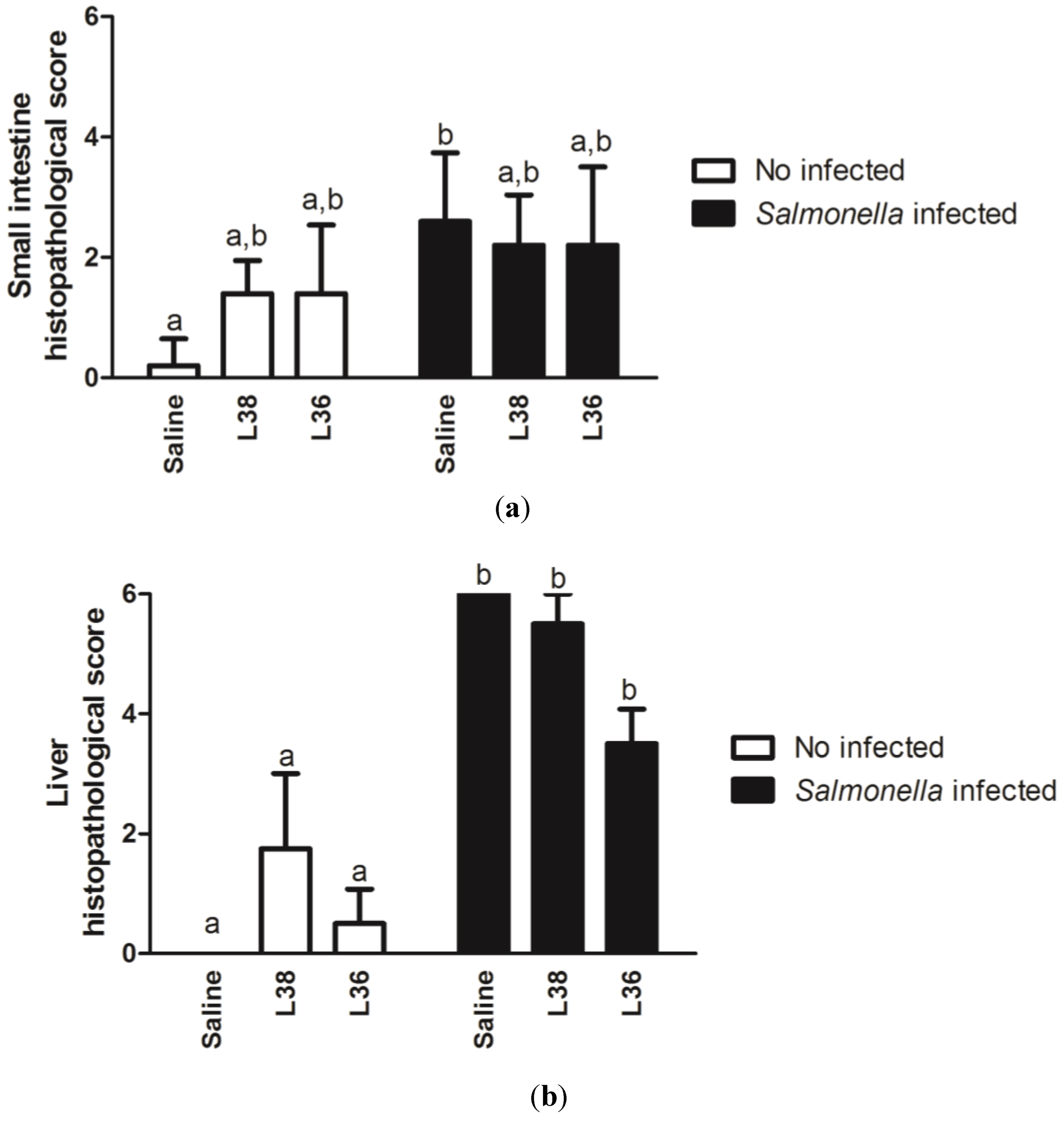


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agricultural Organization/World Health Organization. Report of a Joint FAO/WHO Expert Consultation on Guidelines for the Evaluation of Probiotics in Food; FAO/WHO of the United Nations: London, ON, Canada, 2002. [Google Scholar]
- European Union. Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: http://europa.eu/legislation_summaries/food_safety/animal_nutrition/l12037d_en.htm (accessed on 20 August 2012).
- U.S. Food and Drug Administration. The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals; New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209; U.S. Food and Drug Administration: Rockville, MD, USA, 2012. [Google Scholar]
- U.S. Food and Drug Administration. Draft Guidance for Industry #213, New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209; U.S. Food and Drug Administration: Rockville, MD, USA, 2012. [Google Scholar]
- Fagundes, C.T.; Souza, D.G.; Nicoli, J.R.; Teixeira, M.M. Control of host inflammatory responsiveness by indigenous microbiota reveals an adaptive component of the innate immune system. Microbes Infect. 2011, 13, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Cho, G.-S.; Holzapfel, W.H.; Galvez, A. Safety of Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 341–360. [Google Scholar]
- Jouany, J.P.; Morgavi, D.P. Use of “natural” products as alternatives to antibiotic feed additives in ruminant production. Animal 2007, 1, 1443–1466. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Higgins, S.E.; Wolfenden, A.D.; Henderson, S.N.; Torres-Rodriguez, A.; Vicente, J.L.; Hargis, B.M.; Tellez, G. Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella Enteritidis in neonatal broilers. Poult. Sci. 2010, 89, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Brashears, M.M.; Galyean, M.L.; Loneragan, G.H.; Mann, J.E.; Killinger-Mann, K. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J. Food Prot. 2003, 66, 748–754. [Google Scholar] [PubMed]
- Tabe, E.S.; Oloya, J.; Doetkott, D.K.; Bauer, M.L.; Gibbs, P.S.; Khaitsa, M.L. Comparative effect of direct-fed microbials on fecal shedding of Escherichia coli O157:H7 and Salmonella in naturally infected feedlot cattle. J. Food Prot. 2008, 71, 539–544. [Google Scholar] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel. Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Maassen, C.B.; Van Holten-Neelen, C.; Balk, F.; Den Bak-Glashouwer, M.J.; Leer, R.J.; Laman, J.D.; Boersma, W.J.; Claassen, E. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 2000, 18, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Frøkiær, H.; Pestka, J. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 2002, 168, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Perdigón, G.; Galdeano, M.C.; Valdez, J.C.; Medici, M. Interaction of lactic acid bacteria with the gut immune system. In Eur. J. Clin. Nutr.; 2002; Volume 564, pp. 21–26. [Google Scholar]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73 (Suppl. 2), 444S–450S. [Google Scholar]
- Gackowska, L.; Michalkiewicz, J.; Krotkiewski, M.; Helmin-Basa, A.; Kubiszewska, I.; Dzierzanowska, D. Combined effect of different lactic acid bacteria strains on the mode of cytokines pattern expression in human peripheral blood mononuclear cells. J. Physiol. Pharmacol. 2006, 57, 13–21. [Google Scholar]
- Winkler, P.; Ghadimi, D.; Schrezenmeir, J.; Kraehenbuhl, J. Molecular and cellular basis of microflora—Host interactions. J. Nutr. 2007, 137 (Suppl. 2), 756S–772S. [Google Scholar]
- Cross, M.L. Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol. Med. Microbiol. 2002, 34, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Filho-Lima, J.V.M.; Vieira, E.C.; Nicoli, J.R. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli combinations against experimental infections with Shigella flexneri and Salmonella enteritidis subsp. Typhimurium in gnotobiotic mice. J. Appl. Microbiol. 2000, 88, 365–370. [Google Scholar]
- Silva, A.M.; Barbosa, F.H.F.; Duarte, R.; Vieira, L.Q.; Arantes, R.M.E.; Nicoli, J.R. Effect of Bifidobacterium longum ingestion on experimental salmonellosis in mice. J. Appl. Microbiol. 2004, 97, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.Q.; Santos, L.M.; Neumann, E.; Silva, A.P.; Moura, L.N.; Nicoli, J.R. Probiotics protect mice against experimental infections. J. Clin. Gastroenterol. 2008, 42 (Suppl. 3), S168–S169. [Google Scholar] [CrossRef]
- Martins, F.S.; Silva, A.A.; Vieira, A.T.; Barbosa, F.H.F.; Arantes, R.M.E.; Teixeira, M.M.; Nicoli, J.R. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch. Microbiol. 2009, 191, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Perdigón, G.; Alvarez, S.; Macias, M.E.N.; Roux, M.E.; Ruiz Holgado, A.P. The oral administration of lactic acid bacteria increases the mucosal intestinal immunity in response to enteropathogens. J. Food. Prot. 1990, 53, 404–410. [Google Scholar]
- Martins, F.S.; Elian, S.D.A.; Vieira, A.T.; Tiago, F.C.P.; Martins, A.K.S.; Silva, F.C.P.; Souza, E.L.S.; Sousa, L.P.; Araújo, H.R.C.; Pimenta, P.F.; et al. Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune response and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int. J. Med. Microbiol. 2011, 301, 359–364. [Google Scholar] [CrossRef]
- Khuntia, A.; Chaudhary, L.C. Performance of male crossbred calves as influenced by substitution of grain by wheat bran and the addition of lactic acid bacteria to diet. Asian-Aust. J. Anim. Sci. 2002, 15, 188–194. [Google Scholar]
- Peterson, R.E.; Klopfenstein, T.J.; Erickson, G.E.; Folmer, J.; Hinkley, S; Moxley, R.A.; Smith, D.R. Effect of Lactobacillus acidophilus strain NP51 on Escherichia coli O157:H7 fecal shedding and finishing performance in beef feedlot cattle. J. Food. Prot. 2007, 70, 287–291. [Google Scholar]
- Steinberg, R.S.; Lima, M.T.; Gomes de Oliveira, N.L.; Miyoshi, A.; Nicoli, J.R.; Neumann, E.; Nunes, A.C. Effect of intestinal colonization by two Lactobacillus strains on the immune responses of gnotobiotic mice. Benef. Microbes 2014. [CrossRef]
- Colégio Brasileiro de Experimentação Animal (COBEA). Legislação e Ética. Available online: www.cobea.org.br (accessed on 1 March 2009).
- Arantes, R.M.; Nogueira, A.M. Distribution of enteroglucagon and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res. 1997, 290, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Castor, M.G.M.; Rezende, B.; Bernardes, P.T.T.; Vieira, T.A.; Arantes, R.M.E.; Souza, D.G.; Silva, T.A.; Teixeira, M.M.; Pinho, V. PI3K controls leukocyte recruitment, tissue injury and lethality in a model of graft-versus-host disease in mice. J. Leukoc. Biol. 2011, 89, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, A.; Overbergh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Opinion of the scientific committee on a request from EFSA related to a generic approach to the safety assessment by EFSA of microorganisms used in food/feed and the production of food/feed additives. EFSA J. 2005, 226, 1–12. [Google Scholar]
- Zhou, J.S.; Shu, Q.; Rutherfurd, K.J.; Prasad, J.; Birtles, M.J.; Gopal, P.K.; Gill, H.S. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol. 2000, 56, 87–96. [Google Scholar]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.A.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Levings, M.K.; Bacchetta, R.; Schulz, U.; Roncarolo, M.G. The Role of IL10 and TGF-β in the differentiation and effector function of T regulatory cells. Int. Arch. Allergy Immunol. 2002, 129, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Zaph, C.; Du, Y.; Saenz, S.A.; Nair, M.G.; Perrigoue, J.G.; Taylor, B.C.; Troy, A.E.; Kobuley, D.E.; Kastelein, R.A.; Cua, D.J.; et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J. Exp. Med. 2008, 205, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Isolauri, E. Role of intestinal flora in the development of allergy. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Mortensen, R.R.; Kudsk, J.; Gill, H.S. Dietary intake of Lactobacillus rhamnosus HNOO1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med. Microbiol. Immunol. 2002, 191, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Younes, J.A.; Van Der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Kagnoff, M.F. Cytokines in host defense against Salmonella. Microbes Infect. 2001, 3, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Mittrücker, H.; Kaufmann, S.H.E. Immune response to infection with Salmonella Typhimurium in mice. J. Leukoc. Biol. 2000, 67, 457–463. [Google Scholar]
- Hess, J.; Ladel, C.; Miko, D.; Kaufmann, S.H.E. Salmonella typhimurium aroA2 infection in gene-targeted mice. J. Immunol. 1996, 156, 3321–3326. [Google Scholar] [PubMed]
- Schulz, S.M.; Köhler, G.; Holscher, C.; Iwakura, Y.; Alber., G. IL17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 2008, 20, 1129–1138. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Gresnigt, M.S.; Kullberg, B.J.; Van der Meer, J.W.M.; Joosten, L.A.B.; Netea, M.G. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 2009, 42, 776–787. [Google Scholar]
- Mead, G.C. Prospects for ‘competitive exclusion’ treatment to control salmonellas and other foodborne pathogens in poultry. Vet. J. 2000, 159, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602–1609. [Google Scholar]
- Foligne, B.; Zoumpopoulou, G.; Dewulf, J.; Younes, A.B.; Chareyre, F.; Sirard, J.; Pot, B.; Grangette, C. A key role of dendritic cells in probiotic functionality. PLoS One 2007, 2, e313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamadzadeh, M.; Pfeiler, E.A.; Brown, J.B.; Zadeh, M.; Gramarossa, M.; Managlia, E.; Bere, P.; Sarraj, B.; Khan, M.W.; Pakanati, K.C.; et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl. Acad. Sci. USA 2011, 108, 4623–4630. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Finamore, A.; Nuccitelli, S.; Carnevali, P.; Brigidi, P.; Vitali, B.; Nobili, F.; Rami, R.; Garaguso, I.; Mengheri, E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of γδT and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel Dis. 2009, 15, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Di Giacinto, C.; Marinaro, M.; Sanchez, M.; Strober, W.; Boirivant, M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL10 and IL10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 2005, 174, 3237–3246. [Google Scholar]
- Park, S.R.; Kim, H.A.; Chun, S.K.; Park, J.B.; Kim, P.H. Mechanisms underlying the effects of LPS and activation-induced cytidine deaminase on IgA isotype expression. Mol. Cells 2005, 19, 445–451. [Google Scholar] [PubMed]
- Corthésy, B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J. Immunol. 2007, 178, 27–32. [Google Scholar]
- Michetti, P.; Porta, N.; Mahan, M.J.; Slauch, J.M.; Mekalanos, J.J.; Blum, A.L.; Kraehenbuhl, J.P.; Neutra, M.R. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella Typhimurium. Gastroenterology 1994, 107, 915–923. [Google Scholar] [PubMed]
- Galdeano, C.M.; De Moreno De Leblanc, A.; Vinderola, G.; Bonet, M.E.B.; Perdigón, G. Proposed model: Mechanisms of immunomodulation induced by probiotic bacteria. Clin. Vaccine Immunol. 2007, 14, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Yadav, H.; Sinha, P.R.; Naito, Y.; Marotta, F. Dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei has a protective effect against Salmonella enteritidis infection in mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 1025–1033. [Google Scholar]
- Jain, S.; Yadav, H.; Sinha, P.R. Probiotic Dahi containing Lactobacillus casei protects against Salmonella enteritidis infection and modulates immune response in mice. J. Med. Food 2009, 12, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting microbial pathogens at epithelial borders. Immunol. Invest. 2010, 39, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Vaerman, J.P.; Dehennin, J.P.; Bord, S.; Brassart, D.; Pochart, P.; Desjeux, J.F.; Rambaud, J.C. Effect of intrajejunal perfusion and chronic ingestion of Lactobacillus johnsonii strain La1 on serum concentrations and jejunal secretions of immunoglobulins and serum proteins in healthy humans. Gastroenterol. Clin. Biol. 1997, 21, 293–298. [Google Scholar] [PubMed]
- Spanhaak, S.; Havenaar, R.; Schaafsma, G. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur. J. Clin. Nutr. 1998, 52, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Dogi, C.A.; Galdeano, C.M.; Perdigón, G. Gut immune stimulation by nonpathogenic Gram(+) and Gram(-) bacteria. Comparison with a probiotic strain. Cytokine 2008, 41, 223–231. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Steinberg, R.S.; Silva, L.C.S.; Souza, T.C.; Lima, M.T.; De Oliveira, N.L.G.; Vieira, L.Q.; Arantes, R.M.E.; Miyoshi, A.; Nicoli, J.R.; Neumann, E.; et al. Safety and Protective Effectiveness of Two Strains of Lactobacillus with Probiotic Features in an Experimental Model of Salmonellosis. Int. J. Environ. Res. Public Health 2014, 11, 8755-8776. https://doi.org/10.3390/ijerph110908755
Steinberg RS, Silva LCS, Souza TC, Lima MT, De Oliveira NLG, Vieira LQ, Arantes RME, Miyoshi A, Nicoli JR, Neumann E, et al. Safety and Protective Effectiveness of Two Strains of Lactobacillus with Probiotic Features in an Experimental Model of Salmonellosis. International Journal of Environmental Research and Public Health. 2014; 11(9):8755-8776. https://doi.org/10.3390/ijerph110908755
Chicago/Turabian StyleSteinberg, Raphael S., Lilian C. S. Silva, Tássia C. Souza, Maurício T. Lima, Nayara L. G. De Oliveira, Leda Q. Vieira, Rosa M. E. Arantes, Anderson Miyoshi, Jacques R. Nicoli, Elisabeth Neumann, and et al. 2014. "Safety and Protective Effectiveness of Two Strains of Lactobacillus with Probiotic Features in an Experimental Model of Salmonellosis" International Journal of Environmental Research and Public Health 11, no. 9: 8755-8776. https://doi.org/10.3390/ijerph110908755



