Hypotension and Environmental Noise: A Replication Study
Abstract
:1. Introduction
2. Methods
2.1. Area, Study Design and Sampling
2.2. Sound Exposure Assessment
2.3. Air Pollution Exposure Assessment
2.4. Main Health Outcome Measures
2.5. Confounding and Moderation
2.6. Statistical Analysis
| Categorical variables | Reported | Reported | Test Statistic | Hypotension | Hypotension | Chi-Square- |
|---|---|---|---|---|---|---|
| Hypotension: No | Hypotension: Yes | Chi-Square-Statistic | Medication: No | Medication: Yes | Statistic | |
| n (%) | n (%) | p value | n (%) | n (%) | p value | |
| Total | 693 (86) | 114 (14) | 724 (90) | 79 (10) | ||
| Gender | <0.001 | <0.001 | ||||
| Female | 336 (48.5) | 93 (81.6) | 366 (50.6) | 62 (78.5) | ||
| Male | 357 (51.5) | 21 (18.4) | 358 (49.4) | 17 (21.5) | ||
| Health status | <0.001 | < 0.001 | ||||
| very good/good | 395 (57) | 40 (35.4) | 417 (57.6) | 18 (23.1) | ||
| less than good | 298 (43) | 73 (64.6) | 307 (42.4) | 60 (76.9) | ||
| Educational level | 0.652 | 0.537 | ||||
| Basic | 174 (25.4) | 26 (22.8) | 179 (24.9) | 21 (26.9) | ||
| Skilled labour | 227 (33.1) | 41 (36) | 236 (32.9) | 29 (37.2) | ||
| Vocational | 152 (22.2) | 29 (25.4) | 163 (22.7) | 18 (23.1) | ||
| A-level | 133 (19.4) | 18 (15.8) | 140 (19.5) | 10 (12.8) | ||
| Family history of hypertension | 0.033 | 0.880 | ||||
| Yes | 223 (32.3) | 49 (43) | 204 (28.2) | 24 (30.4) | ||
| No | 468 (67.7) | 65 (57) | 313 (43.3) | 32 (40.5) | ||
| Area of valley | 0.935 | |||||
| East | 197 (28.5) | 31 (27.2) | 204 (28.2) | 24 (30.4) | 0.615 | |
| bottom | 299 (43.2) | 49 (43) | 240 (33.2) | 29 (36.7) | ||
| West | 196 (28.3) | 34 (29.8) | 483 (66.8) | 50 (63.3) | ||
| Antihypertensive treatment | <0.001 | 0.005 | ||||
| No | 567 (82.1) | 112 (99.1) | 604 (83.4) | 75 (96.2) | ||
| Yes | 124 (17.9) | 1 (0.9) | 120 (16.6) | 3 (3.8) | ||
| Continuous variables | Reported | Reported | Ranksum Test p Value | Hypotension | Hypotension | Ranksum Test p Value |
| Hypotension: No | Hypotension: Yes | Medication: No | Medication: Yes | |||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||
| Age | 0.109 | 0.050 | ||||
| median (IQR) | 44 (34, 57.5) | 40 (33, 53.8) | 43 (34, 57) | 50 (37.5, 59.5) | ||
| Total sound level: dBA, Ldn+ | 0.183 | 0.870 | ||||
| median (IQR) | 57.7 (54.5, 61.3) | 58.3 (54.9, 62.6) | 57.7 (54.6, 61.4) | 57.3 (54.1, 61.8) | ||
| Rail sound level: dBA, Ldn+ | 0.227 | 0.887 | ||||
| median (IQR) | 54.3 (51.7, 58.7) | 54.5 (52.1, 61.5) | 54.4 (51.7, 59) | 53.6 (51.7, 60.2) | ||
| Highway sound level: dBA, Ldn+ | 0.653 | 0.786 | ||||
| median (IQR) | 53.8 (50, 56.3) | 54 (49.9, 56.7) | 53.8 (50, 56.3) | 53.9 (49.3, 55.7) | ||
| Distance to main road | 0.024 | 0.484 | ||||
| median (IQR) | 435.7 (197.1, 1172.7) | 306.1 (162.6, 1132.2) | 428.5 (185.1, 1172.7) | 380 (178.4, 1140) | ||
| Annoyance by local road | 0.118 | 0.012 | ||||
| median (IQR) | 3 (1, 6) | 4 (2, 6) | 3 (1, 6) | 5 (2, 7) | ||
| NO2: annual average, µg/m³ | 0.343 | 0.698 | ||||
| median (IQR) | 33.9 (32, 35.7) | 34.1 (32.4, 36.2) | 33.9 (32.1, 35.8) | 33.8 (31.9, 35.7) | ||
| Noise sensitivity | 0.006 | 0.006 | ||||
| median (IQR) | 5 (2, 8) | 6 (3, 8) | 5 (2, 8) | 6 (3, 9) | ||
| Weather sensitivity | <0.001 | <0.001 | ||||
| median (IQR) | 3 (1, 5) | 5 (3, 8) | 3 (1, 5) | 6 (3, 8) | ||
| GHQ score * | <0.001 | <0.001 | ||||
| median (IQR) | 21 (18, 26) | 24.5 (20.8, 31) | 21 (18, 26) | 26 (21, 32) | ||
| Sleep score * | <0.001 | <0.001 | ||||
| median (IQR) | 6 (3, 10) | 9 (4, 13) | 6 (3, 10) | 10 (5, 14) |
3. Results
3.1. Sample Characteristics by Health Outcome
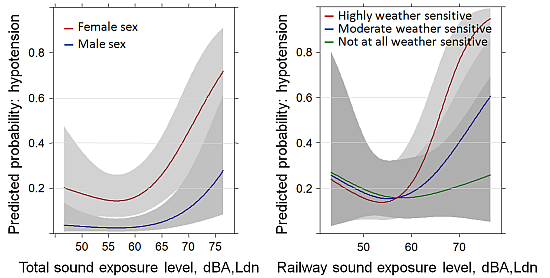
3.2. Hypotension Past Year: Full Sample (N = 748)
| Factor | Total Sound Exposure | Railway Sound Exposure | Highway Sound Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chi-Square | d.f. | p Value | Chi-Square | d.f. | p Value | Chi-Square | d.f. | p Value | |
| Sound level as Ldn | 24.25 | 8 | 0.0021 | 23.88 | 8 | 0.0024 | 10.36 | 8 | 0.2407 |
| Nonlinear component sound level | 16.24 | 4 | 0.0027 | 14.02 | 4 | 0.0072 | 4.26 | 4 | 0.3717 |
| Distance to main road | 10.32 | 3 | 0.0160 | 11.36 | 3 | 0.0099 | 7.15 | 3 | 0.0671 |
| Annoyance by local roads | 8.25 | 3 | 0.0412 | 7.72 | 3 | 0.0521 | 2.48 | 3 | 0.4786 |
| Sex | 32.33 | 2 | <0.0001 | 31.85 | 2 | <0.0001 | 30.18 | 2 | <0.0001 |
| Age | 14.82 | 2 | 0.0006 | 14.30 | 2 | 0.0008 | 12.73 | 2 | 0.0017 |
| Educational level | 4.08 | 3 | 0.2532 | 4.25 | 3 | 0.2354 | 3.87 | 3 | 0.2754 |
| Family history of hypertension | 4.96 | 1 | 0.0259 | 4.83 | 1 | 0.0279 | 3.48 | 1 | 0.0620 |
| Region (west-bottom-east) | 4.19 | 2 | 0.1230 | 4.29 | 2 | 0.1171 | 1.44 | 2 | 0.4862 |
| Antihypertensive treatment | 15.74 | 1 | 0.0001 | 14.67 | 1 | 0.0001 | 14.31 | 1 | 0.0002 |
| Weather sensitivity | 29.84 | 4 | <0.0001 | 28.18 | 4 | <0.0001 | 24.56 | 4 | 0.0001 |
| Health status | 12.99 | 2 | 0.0015 | 13.57 | 2 | 0.0011 | 11.93 | 2 | 0.0026 |
| Sleep score | 2.95 | 1 | 0.0858 | 2.64 | 1 | 0.1044 | 4.56 | 1 | 0.0328 |
| Age × sex | 14.52 | 1 | 0.0001 | 14.02 | 1 | 0.0002 | 12.20 | 1 | 0.0005 |
| Weather sensitivity × health | 6.89 | 1 | 0.0087 | 7.25 | 1 | 0.0071 | 6.41 | 1 | 0.0114 |
| Sound level × weather sensitivity | 7.93 | 2 | 0.0190 | 5.76 | 2 | 0.0561 | 4.32 | 2 | 0.1153 |
| Nonlinear Interaction | 6.60 | 1 | 0.0102 | 3.73 | 1 | 0.0533 | 4.22 | 1 | 0.0400 |
| Sound level × distance main road | 8.18 | 2 | 0.0167 | 8.99 | 2 | 0.0112 | 5.46 | 2 | 0.0651 |
| Nonlinear Interaction | 7.81 | 1 | 0.0052 | 8.71 | 1 | 0.0032 | 0.00 | 1 | 0.9447 |
| Sound level × annoyance local roads | 6.94 | 2 | 0.0312 | 6.07 | 2 | 0.0480 | 0.99 | 2 | 0.6091 |
| Nonlinear Interaction | 2.94 | 1 | 0.0863 | 0.64 | 1 | 0.4244 | 0.70 | 1 | 0.4016 |
| TOTAL nonlinear | 16.24 | 4 | 0.0027 | 14.02 | 4 | 0.0072 | 4.26 | 4 | 0.3717 |
| TOTAL interaction | 32.99 | 8 | 0.0001 | 32.74 | 8 | 0.0001 | 25.17 | 8 | 0.0015 |
| TOTAL nonlinear+interaction | 35.22 | 9 | 0.0001 | 34.53 | 9 | 0.0001 | 25.74 | 9 | 0.0022 |
| TOTAL | 95.11 | 24 | <0.0001 | 96.39 | 24 | <0.0001 | 94.59 | 24 | <0.0001 |


3.3. Hypotension Past Year: Reduced Sample (N = 528) Including Body Mass Index
| Factor | Total Sound Exposure | Railway Sound Exposure | Highway Sound Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chi-Square | d.f. | p Value | Chi-Square | d.f. | p Value | Chi-Square | d.f. | p Value | |
| Sound level as Ldn | 24.00 | 8 | 0.0023 | 23.53 | 8 | 0.0027 | 9.86 | 8 | 0.2750 |
| Nonlinear component sound level | 14.72 | 4 | 0.0053 | 12.57 | 4 | 0.0136 | 2.85 | 4 | 0.5837 |
| Distance to main road | 8.01 | 3 | 0.0458 | 8.68 | 3 | 0.0338 | 6.97 | 3 | 0.0729 |
| Annoyance by local roads | 8.17 | 3 | 0.0427 | 7.61 | 3 | 0.0547 | 2.18 | 3 | 0.5355 |
| Sex | 18.68 | 2 | 0.0001 | 18.22 | 2 | 0.0001 | 16.65 | 2 | 0.0002 |
| Age | 5.95 | 2 | 0.0510 | 5.06 | 2 | 0.0795 | 3.86 | 2 | 0.1448 |
| Educational level | 5.88 | 3 | 0.1178 | 6.28 | 3 | 0.0987 | 4.43 | 3 | 0.2183 |
| Family history of hypertension | 5.14 | 1 | 0.0234 | 4.98 | 1 | 0.0256 | 3.39 | 1 | 0.0656 |
| Region (west-bottom-east) | 3.44 | 2 | 0.1788 | 3.75 | 2 | 0.1536 | 0.45 | 2 | 0.7994 |
| Weather sensitivity | 21.66 | 4 | 0.0002 | 20.46 | 4 | 0.0004 | 17.31 | 4 | 0.0017 |
| Health status | 5.75 | 2 | 0.0564 | 5.77 | 2 | 0.0557 | 5.54 | 2 | 0.0625 |
| Sleep score | 1.44 | 1 | 0.2308 | 1.30 | 1 | 0.2541 | 2.55 | 1 | 0.1101 |
| BMI | 5.20 | 1 | 0.0226 | 5.35 | 1 | 0.0208 | 5.62 | 1 | 0.0177 |
| Antihypertensive treatment | 12.90 | 1 | 0.0003 | 11.60 | 1 | 0.0007 | 10.80 | 1 | 0.0010 |
| Age × sex | 5.93 | 1 | 0.0149 | 5.05 | 1 | 0.0246 | 3.86 | 1 | 0.0494 |
| Weather sensitivity × health | 3.61 | 1 | 0.0575 | 3.62 | 1 | 0.0570 | 3.78 | 1 | 0.0519 |
| Sound level × weather sensitivity | 7.13 | 2 | 0.0283 | 5.90 | 2 | 0.0522 | 2.75 | 2 | 0.2532 |
| Nonlinear Interaction | 4.83 | 1 | 0.0279 | 2.92 | 1 | 0.0873 | 2.73 | 1 | 0.0986 |
| Sound level × distance main road | 5.88 | 2 | 0.0528 | 6.23 | 2 | 0.0444 | 5.54 | 2 | 0.0628 |
| Nonlinear Interaction | 5.70 | 1 | 0.0170 | 6.08 | 1 | 0.0137 | 0.00 | 1 | 0.9821 |
| Sound level × annoyance local roads | 7.01 | 2 | 0.0300 | 6.36 | 2 | 0.0415 | 0.62 | 2 | 0.7348 |
| Nonlinear Interaction | 3.42 | 1 | 0.0643 | 0.87 | 1 | 0.3505 | 0.61 | 1 | 0.4343 |
| TOTAL nonlinear | 14.72 | 4 | 0.0053 | 12.57 | 4 | 0.0136 | 2.85 | 4 | 0.5837 |
| TOTAL interaction | 20.49 | 8 | 0.0086 | 20.07 | 8 | 0.0101 | 13.82 | 8 | 0.0867 |
| TOTAL nonlinear+interaction | 23.85 | 9 | 0.0045 | 22.98 | 9 | 0.0062 | 15.25 | 9 | 0.0842 |
| TOTAL | 69.02 | 25 | <0.0001 | 69.62 | 25 | <0.0001 | 69.56 | 25 | <0.0001 |

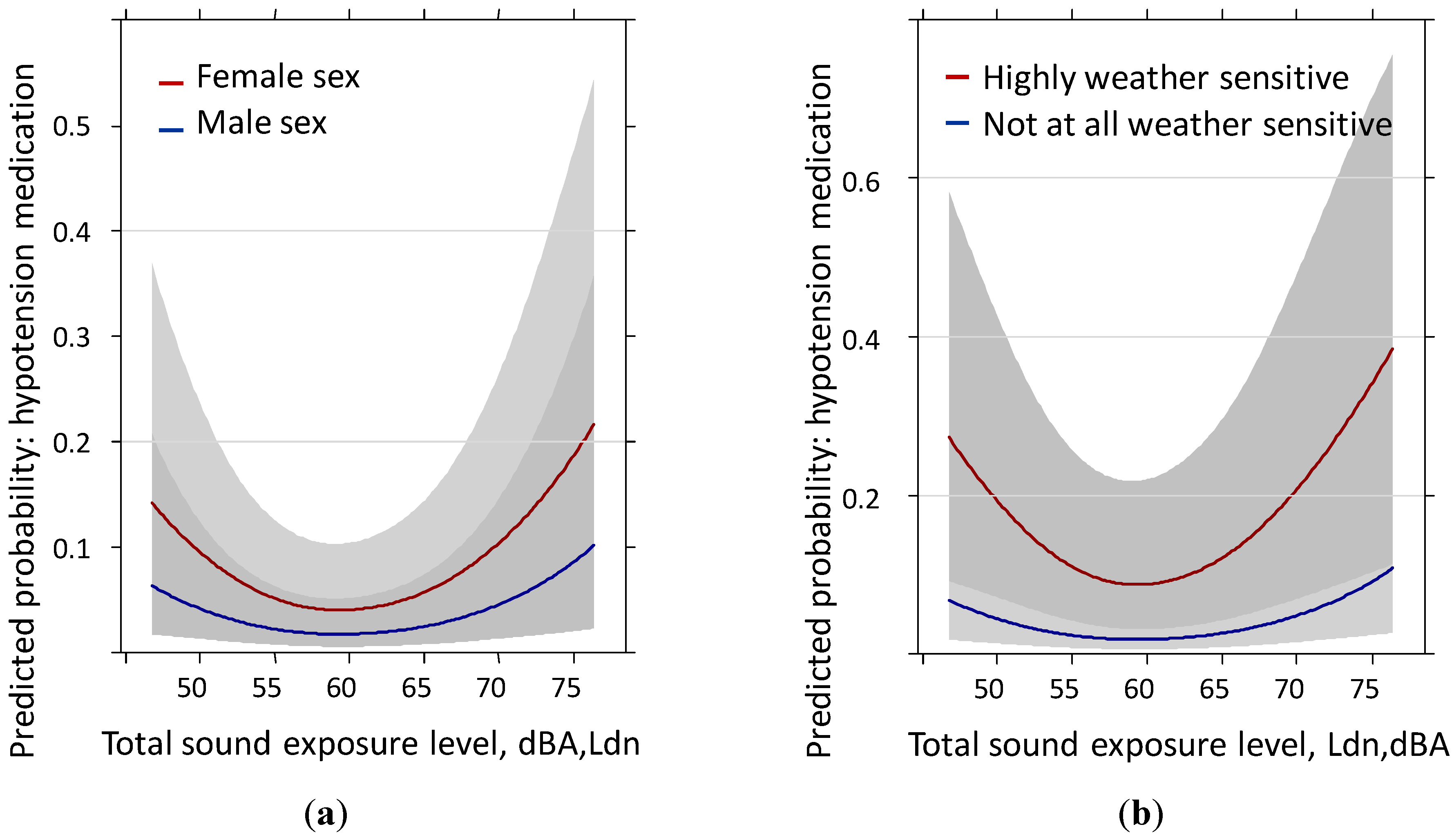
3.4. Hypotension Medication Past Year: Reduced Sample (N = 528) Including Body Mass Index
| Factor | Total Sound Exposure | Railway Sound Exposure | Highway Sound Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Wald Chi-Square | d.f. | p Value | Wald Chi-Square | d.f. | p Value | Wald Chi-Square | d.f. | p Value | |
| Sound level as Ldn | 10.49 | 2 | 0.0053 | 7.97 | 2 | 0.0186 | 5.09 | 2 | 0.0786 |
| Nonlinear component sound level | 10.11 | 1 | 0.0015 | 6.92 | 1 | 0.0085 | 3.93 | 1 | 0.0474 |
| Distance to main road | 0.00 | 1 | 0.9733 | 0.02 | 1 | 0.8912 | 0.25 | 1 | 0.6177 |
| Annoyance by local roads | 0.62 | 1 | 0.4314 | 0.55 | 1 | 0.4599 | 0.46 | 1 | 0.4956 |
| Sex | 6.43 | 2 | 0.0402 | 6.42 | 2 | 0.0403 | 7.02 | 2 | 0.0299 |
| Age | 10.58 | 2 | 0.0050 | 10.23 | 2 | 0.0060 | 9.22 | 2 | 0.0099 |
| Educational Level | 5.91 | 3 | 0.1160 | 5.74 | 3 | 0.1252 | 6.11 | 3 | 0.1063 |
| Family History of Hypertension | 0.06 | 1 | 0.8142 | 0.07 | 1 | 0.7918 | 0.02 | 1 | 0.8789 |
| Region (west-bottom-east) | 0.30 | 2 | 0.8610 | 0.16 | 2 | 0.9216 | 0.86 | 2 | 0.6499 |
| BMI | 7.15 | 1 | 0.0075 | 7.53 | 1 | 0.0061 | 6.64 | 1 | 0.0100 |
| Antihypertensive Treatment | 12.57 | 1 | 0.0004 | 12.45 | 1 | 0.0004 | 12.78 | 1 | 0.0004 |
| Weather Sensitivity | 9.89 | 1 | 0.0017 | 9.75 | 1 | 0.0018 | 9.41 | 1 | 0.0022 |
| Health status | 8.86 | 1 | 0.0029 | 9.18 | 1 | 0.0024 | 10.28 | 1 | 0.0013 |
| Sleep Score | 1.68 | 1 | 0.1945 | 1.49 | 1 | 0.2228 | 2.58 | 1 | 0.1080 |
| Age × sex | 0.45 | 1 | 0.5026 | 0.49 | 1 | 0.4855 | 0.19 | 1 | 0.6631 |
| TOTAL nonlinear + interaction | 10.42 | 2 | 0.0055 | 7.28 | 2 | 0.0262 | 4.25 | 2 | 0.1196 |
| TOTAL | 65.09 | 18 | <0.0001 | 64.52 | 18 | <0.0001 | 62.56 | 18 | <0.0001 |
| Sound Source and Health Outcome | Increase in Odds Ratio (95% CI) at Different Sound Levels | ||
|---|---|---|---|
| 55–65 Ldn, dBA | 60–70 Ldn, dBA | 65–75 Ldn, dBA | |
| Full sample | |||
| Total sound: reported hypotension | 2.01 (1.23–3.30) | 3.00 (1.42–6.32) | 3.31 (1.40–7.84) |
| Railway sound: reported hypotension | 2.22 (1.36–3.62) | 2.84 (1.43–5.67) | 2.98 (1.43–6.24) |
| Highway sound: reported hypotension | 0.63 (0.15–2.60) | 0.61 (0.14–2.73) | 0.61 (0.14–2.73) |
| Total sound: hypotension medication | 1.08 (0.73–1.59) | 2.00 (1.09–3.70) | 2.35 (1.16–4.74) |
| Railway sound: hypotension medication | 1.43 (0.95–2.15) | 1.95 (1.09–3.51) | 2.08 (1.11–3.88) |
| Highway sound: hypotension medication | 1.63 (0.49–5.44) | 1.72 (0.48–6.10) | 1.72 (0.48–6.10) |
| Reduced sample (incl. BMI) | |||
| Total sound: reported hypotension | 2.05 (1.26–3.34) | 4.28 (2.04–9.01) | 5.57 (2.22–13.94) |
| Railway sound: reported hypotension | 2.44 (1.53–3.89) | 3.99 (1.96–8.13) | 4.64 (2.06–10.44) |
| Highway sound: reported hypotension | 1.13 (0.30–4.24) | 1.13 (0.28–4.57) | 1.13 (0.28–4.57) |
| Total sound: hypotension medication | 1.11 (0.71–1.74) | 2.74 (1.36–5.52) | 3.79 (1.60–86) |
| Railway sound: hypotension medication | 1.59 (1.01–2.49) | 2.58 (1.30–5.11) | 2.99 (1.38–6.50) |
| Highway sound: hypotension medication | 2.57 (0.66–10.00) | 2.80 (0.66–11.80) | 2.80 (0.66–11.80) |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Owens, P.; O’Brien, E. Hypotension: A forgotten illness? Blood Press. Monit. 1997, 2, 3–14. [Google Scholar]
- De Buyzere, M.; Clement, D.L.; Duprez, D. Chronic low blood pressure: A review. Cardiovasc. Drugs Ther. 1998, 12, 29–35. [Google Scholar] [CrossRef]
- Pilgrim, J.A.; Stansfeld, S.; Marmot, M. Low blood pressure, low mood? BMJ 1992, 304, 75–78. [Google Scholar] [CrossRef]
- Wessely, S.; Nickson, J.; Cox, B. Symptoms of low blood pressure: A population study. BMJ 1990, 301, 362–365. [Google Scholar] [CrossRef]
- Rosengren, A.; Tibblin, G.; Wilhelmsen, L. Low systolic blood pressure and self perceived wellbeing in middle aged men. BMJ 1993, 306, 243–246. [Google Scholar] [CrossRef]
- Akahoshi, M.; Hida, A.; Imaizumi, M.; Soda, M.; Maeda, R.; Ichimaru, S.; Nakashima, E.; Seto, S.; Yano, K. Basic characteristics of chronic hypotension cases: A longitudinal follow-up study from 1958 through 1999. Hypertens. Res. 2006, 29, 1–7. [Google Scholar] [CrossRef]
- Lucas, K.E.; Rowe, P.C.; Coresh, J.; Klag, M.J.; Meoni, L.A.; Ford, D.E. Prospective association between hypotension and idiopathic chronic fatigue. J. Hypertens. 2004, 22, 691–695. [Google Scholar] [CrossRef]
- Hildrum, B.; Romild, U.; Holmen, J. Anxiety and depression lowers blood pressure: 22-year follow-up of the population based HUNT study, Norway. BMC Public Health 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Hildrum, B.; Mykletun, A.; Holmen, J.; Dahl, A.A. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. Br. J. Psychiatry 2008, 193, 108–113. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Stordal, E.; Bjelland, I.; Dahl, A.A.; Holmen, J. Association of low blood pressure with anxiety and depression: The Nord-Trøndelag Health Study. J. Epidemiol. Community Health 2007, 61, 53–58. [Google Scholar] [CrossRef]
- Duschek, S.; Meinhardt, J.; Schandry, R. Reduced cortical activity due to chronic low blood pressure: An EEG study. Biol. Psychol. 2006, 72, 241–250. [Google Scholar] [CrossRef]
- Thomas, D.J.; Bannister, R. Preservation of autoregulation of cerebral blood flow in autonomic failure. J. Neurol. Sci. 1980, 44, 205–212. [Google Scholar] [CrossRef]
- Aasvang, G.M.; Øverland, B.; Ursin, R.; Moum, T. A field study of effects of road traffic and railway noise on polysomnographic sleep parameters. J. Acoust. Soc. Am. 2011, 129, 3716–3726. [Google Scholar] [CrossRef]
- Dinkel, R.H.; Görtler, E.; Heinemann, L.; Potthoff, P. Die Sterblichkeit von Personen mit, zu niedrigem Blutdruck (Hypotension) in einem Beobachtungszeitraum von 16 Jahren. J. Public Health (Bangkok). 1996, 4, 225–233. [Google Scholar] [CrossRef]
- Robbins, J.M.; Korda, H.; Shapiro, M.F. Treatment for a nondisease: The case of low blood pressure. Soc. Sci. Med. 1982, 16, 27–33. [Google Scholar] [CrossRef]
- Rose, K.M.; Eigenbrodt, M.L.; Biga, R.L.; Couper, D.J.; Light, K.C.; Sharrett, A.R.; Heiss, G. Orthostatic hypotension predicts mortality in middle-aged adults: The Atherosclerosis Risk In Communities (ARIC) Study. Circulation 2006, 114, 630–636. [Google Scholar] [CrossRef]
- Kennelly, S.P.; Lawlor, B.A.; Kenny, R.A. Review: Blood pressure and dementia—A comprehensive review. Ther. Adv. Neurol. Disord. 2009, 2, 241–260. [Google Scholar] [CrossRef]
- Frewen, J.; Savva, G.M.; Boyle, G.; Finucane, C.; Kenny, R.A. Cognitive performance in orthostatic hypotension: Findings from a nationally representative sample. J. Am. Geriatr. Soc. 2014, 62, 117–122. [Google Scholar] [CrossRef]
- Xin, W.; Lin, Z.; Mi, S. Orthostatic hypotension and mortality risk: A meta-analysis of cohort studies. Heart 2013, 100, 406–413. [Google Scholar] [CrossRef]
- Fedorowski, A.; Stavenow, L.; Hedblad, B.; Berglund, G.; Nilsson, P.M.; Melander, O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur. Heart J. 2010, 31, 85–91. [Google Scholar] [CrossRef]
- Eigenbrodt, M.L.; Rose, K.M.; Couper, D.J.; Arnett, D.K.; Smith, R.; Jones, D. Orthostatic hypotension as a risk factor for stroke: The atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000, 31, 2307–2313. [Google Scholar] [CrossRef]
- Gangavati, A.; Hajjar, I.; Quach, L.; Jones, R.N.; Kiely, D.K.; Gagnon, P.; Lipsitz, L.A. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: The maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J. Am. Geriatr. Soc. 2011, 59, 383–389. [Google Scholar] [CrossRef]
- Ricci, F.; Radico, F.; Romanello, M.; Tatasciore, A.; Di Nicola, M.; Zimarino, M.; De Caterina, R. Morbidity and mortality related to orthostatic hypotension: Results of a meta-analysis of non-randomized observational studies. Eur. Heart J. 2013, 34. [Google Scholar] [CrossRef]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef]
- Van Kempen, E.; Babisch, W. The quantitative relationship between road traffic noise and hypertension: A meta-analysis. J. Hypertens. 2012, 30, 1075–1086. [Google Scholar] [CrossRef]
- Babisch, W.; van Kamp, I. Exposure-response relationship of the association between aircraft noise and the risk of hypertension. Noise Health 2009, 11, 161–168. [Google Scholar] [CrossRef]
- Andrén, L.; Hansson, L.; Björkman, M.; Jonsson, A.; Borg, K.O. Hemodynamic and hormonal changes induced by noise. Acta Med. Scand. Suppl. 1979, 625, 13–18. [Google Scholar]
- Andrén, L.; Hansson, L.; Björkman, M. Haemodynamic effects of noise exposure before and after beta 1-selective and non-selective beta-adrenoceptor blockade in patients with essential hypertension. Clin. Sci. (Lond). 1981, 61 (Suppl. 7), 89s–91s. [Google Scholar]
- Sakamoto, H.; Hayashi, F.; Sugiura, S.; Tsujikawa, M. Psycho-circulatory responses caused by listening to music, and exposure to fluctuating noise or steady noise. J. Sound Vib. 2002, 250, 23–29. [Google Scholar] [CrossRef]
- Cartwright, L.B.; Thompson, R.N. The effects of broadband noise on the cardiovascular system on normal resting adults. Am. Ind. Hyg. Assoc. J. 1975, 36, 653–658. [Google Scholar] [CrossRef]
- Parrot, J.; Petiot, J.C.; Lobreau, J.P.; Smolik, H.J. Cardiovascular effects of impulse noise, road traffic noise, and intermittent pink noise at LAeq = 75 dB, as a function of sex, age, and level of anxiety: A comparative study. Int. Arch. Occup. Environ. Health 1992, 63, 477–484. [Google Scholar] [CrossRef]
- Ising, H.; Dienel, D.; Günther, T.; Markert, B. Health effects of traffic noise. Int. Arch. Occup. Environ. Health 1980, 47, 179–190. [Google Scholar] [CrossRef]
- Neus, H. Auswirkungen des Lärms auf den Blutdruck. Zschrift Lärmb 1981, 28, 105–110. [Google Scholar]
- Ising, H.; Günther, T.; Melchert, H. Nachweis und Wirkungsmechanismen der blutdrucksteigernden Wirkung von Arbeitslärm. Zbl. Arbeitsmed. 1980, 30, 194–203. [Google Scholar]
- Ising, H. Stressreaktionen und Gesundheitsrisiko bei Verkehrslärmbelastung; WaBoLu-Ber: Berlin, Germany, 1983. [Google Scholar]
- Tomei, G.; Fioravanti, M.; Cerratti, D.; Sancini, A.; Tomao, E.; Rosati, M.V.; Vacca, D.; Palitti, T.; Di Famiani, M.; Giubilati, R.; De Sio, S.; Tomei, F. Occupational exposure to noise and the cardiovascular system: A meta-analysis. Sci. Total Environ. 2010, 408, 681–689. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Lai, Y.-A.; Hsieh, H.-H.; Lai, J.-S.; Liu, C.-S. Effects of environmental noise exposure on ambulatory blood pressure in young adults. Environ. Res. 2009, 109, 900–905. [Google Scholar] [CrossRef]
- Andrén, L.; Hansson, L.; Eggertsen, R.; Hedner, T.; Karlberg, B.E. Circulatory effects of noise. Acta Med. Scand. 1983, 213, 31–35. [Google Scholar]
- Green, M.S.; Peled, I.; Harari, G.; Luz, J.; Akselrod, S.; Norymberg, M.; Melamed, S. Association of silent ST-segment depression on one-hour ambulatory ECGs with exposure to industrial noise among blue-collar workers in Israel examined at different levels of ambient temperature—The CORDIS Study. Public Health Rev. 1991, 19, 277–293. [Google Scholar]
- Ising, H.; Gunther, T. Wirkungen mehrstündiger Lärmbelastungen auf Wohlbefinden, Körperfunktionen und Leistung des Menschen. Z. Lärmbekämpfung 1983, 30, 11–15. [Google Scholar]
- Lercher, P.; Widmann, U. Association and moderation of self-reported hypotension with traffic noise exposure: A neglected relationship. Noise Health 2013, 15, 205–216. [Google Scholar] [CrossRef]
- Peng, R.D.; Dominici, F.; Zeger, S.L. Reproducible epidemiologic research. Am. J. Epidemiol. 2006, 163, 783–789. [Google Scholar] [CrossRef]
- Jasny, B.R.; Chin, G.; Chong, L.; Vignieri, S. Again, and again, and again …. Science 2011, 334. [Google Scholar] [CrossRef]
- Simons, D.J. The value of direct replication. Perspect. Psychol. Sci. 2014, 9, 76–80. [Google Scholar] [CrossRef]
- Stroebe, W.; Strack, F. The alleged crisis and the illusion of exact replication. Perspect. Psychol. Sci. 2014, 9, 59–71. [Google Scholar] [CrossRef]
- Wotawa, G.; Seibert, P.; Kromp-Kolb, H.; Michaela-Maria, H. Verkehrsbedingte Stickoxid-Belastung im Inntal: Einfluss Meteorologischer und Topographischer Faktoren; Institut für Meteorologie und Physik, Universität für Bodenkultur Wien: Wien, Austria, 2000; p. 28. [Google Scholar]
- Thudium, J. The air and noise situation in the alpine transit valleys of Fréjus, Mont-Blanc, Gotthard and Brenner. J. Alp. Res. | Rev. Géograph. Alp. 2009, 43–51. [Google Scholar]
- Heimann, D.; Schafer, K.; Emeis, S.; Suppan, P.; Obleitner, F.; Uhrner, U. Combined evaluations of meteorological parameters, traffic noise and air pollution in an Alpine valley. Meteorol. Zeitschrift 2010, 19, 47–61. [Google Scholar] [CrossRef]
- Thudium, J.; Kocsis, O.; Scherer, S.; Göldi-Kunz, B. Immissionsklima und Ausbreitungsmodellierung im Unterinntal; Chur und Bruck: Zürich, Switzerland, 2000. [Google Scholar]
- Thudium, J. Empirical Modelling of Air Pollution in the Proximity of Roads. In 14th International Symposium «Transport and Air Pollution»; Eichlseder, H., Ed.; Verlag der Technischen Universität Graz: Graz, Austria, 2005. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Harrell, F.E., Jr. RMS: Regression Modelling Strategies. Available online: http://cran.r-project.org/web/packages/rms/rms.pdf (accessed on 15 June 2014).
- Selvin, S. Statistical Analysis of Epidemiologic Data, 3rd ed.; Oxford University Press: New York, NY, USA, 2004; p. 488. [Google Scholar]
- Greenland, S.; Rothman, K. Concepts of Interaction. In Modern Epidemiology; Rothman, K.J., Greenland, S., Eds.; Lippincott-Raven: New York, NY, USA, 1998; pp. 329–342. [Google Scholar]
- Harrell, F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- Royston, P.; Sauerbrei, W. Interactions. In Multivariable Model—Building: A Pragmatic Approach to Regression Anaylsis Based on Fractional Polynomials for Modelling Continuous Variables; John Wiley & Sons Ltd.: Chichester, UK, 2008; p. 322. [Google Scholar]
- Pennig, S.; Quehl, J.; Mueller, U.; Rolny, V.; Maass, H.; Basner, M.; Elmenhorst, E.-M. Annoyance and self-reported sleep disturbance due to night-time railway noise examined in the field. J. Acoust. Soc. Am. 2012, 132, 3109–3117. [Google Scholar] [CrossRef]
- Aasvang, G.M.; Moum, T.; Engdahl, B. Self-reported sleep disturbances due to railway noise: Exposure-response relationships for nighttime equivalent and maximum noise levels. J. Acoust. Soc. Am. 2008, 124, 257–268. [Google Scholar] [CrossRef]
- Passchier-Vermeer, W.; Vos, H.; Janssen, S.A.; Miedema, H.M.E. Sleep and Traffic Noise; Summary Report 2007-D-20012/A; TNO: Delft, The Netherlands, 2007. [Google Scholar]
- Griefahn, B.; Bröde, P.; Marks, A.; Basner, M. Autonomic arousals related to traffic noise during sleep. Sleep 2008, 31, 569–577. [Google Scholar]
- Basner, M.; Müller, U.; Elmenhorst, E.-M. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep 2011, 34, 11–23. [Google Scholar]
- Smith, M.G.; Croy, I.; Ögren, M.; Waye, K.P. On the influence of freight trains on humans: A laboratory investigation of the impact of nocturnal low frequency vibration and noise on sleep and heart rate. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Croy, I.; Smith, M.G.; Waye, K.P. Effects of train noise and vibration on human heart rate during sleep: An experimental study. BMJ Open 2013, 3. [Google Scholar] [CrossRef]
- Lercher, P.; Brink, M.; Rüdisser, J.; van Renterghem, T.; Botteldooren, D.; Baulac, M.; Defrance, J. The effects of railway noise on sleep medication intake: Results from the ALPNAP-study. Noise Health 2010, 12, 110–119. [Google Scholar] [CrossRef]
- Roberts, M.; Western, A.; Webber, M. A theory of patterns of passby noise. J. Sound Vib. 2003, 262, 1047–1056. [Google Scholar] [CrossRef]
- Lercher, P.; Bockstael, A.; Dekoninck, L.; De Coensel, B.; Botteldooren, D. The application of a notice-event model to improve classical exposure-annoyance estimation. J. Acoust. Soc. Am. 2012, 131, 3223. [Google Scholar] [CrossRef]
- Lercher, P.; Bockstael, A.; Dekoninck, L.; De Coensel, B.; Botteldooren, D. Can noise from a main road be more annoying than from highway? An environmental health and soundscape approach. In Proceedings of the 42nd International Congress and Exposition on Noise Control Engineering (Inter-Noise 2013), Innsbruck, Austria, 15–18 September 2013; p. 9.
- Convertino, V.A. Gender differences in autonomic functions associated with blood pressure regulation. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1909–R1920. [Google Scholar]
- Kim, A.; Deo, S.H.; Vianna, L.C.; Balanos, G.M.; Hartwich, D.; Fisher, J.P.; Fadel, P.J. Sex differences in carotid baroreflex control of arterial blood pressure in humans: Relative contribution of cardiac output and total vascular conductance. Am. J. Physiol. Circ. Physiol. 2011, 301, H2454–H2465. [Google Scholar]
- Hinojosa-Laborde, C.; Chapa, I.; Lange, D.; Haywood, J.R. Gender differences in sympathetic nervous system regulation. Clin. Exp. Pharmacol. Physiol. 1999, 26, 122–126. [Google Scholar] [CrossRef]
- Arzeno, N.M.; Stenger, M.B.; Lee, S.M.; Ploutz-Snyder, R.; Platts, S.H. Sex differences in blood pressure control during 6° head-down tilt bed rest. Am. J. Physiol. Hear. Circ. Physiol. 2013, 304, H1114–H1123. [Google Scholar]
- Cheng, Y.-C.; Vyas, A.; Hymen, E.; Perlmuter, L.C. Gender differences in orthostatic hypotension. Am. J. Med. Sci. 2011, 342, 221–225. [Google Scholar] [CrossRef]
- Fahrenberg, J. Die Freiburger Beschwerdenliste. Zeitschrift für Klin. Psychol. 1975, 4, 79–100. [Google Scholar]
- Höppe, P. Die Bedeutung von Wetter und Klima in der Umweltmedizin. Umweltmed. Forsch. Prax. 1999, 4, 101–106. [Google Scholar]
- Jendritzky, G. Wirkungen von Wetter und Klima auf die Gesundheit des Menschen. In Handbuch der Umweltmedizin; Wichmann, H., Schlipkoeter, H., Fulgraff, G., Eds.; Ecomed: Landsberg, Germany, 1992; pp. 1–14. [Google Scholar]
- Von Mackensen, S.; Hoeppe, P.; Maarouf, A.; Tourigny, P.; Nowak, D. Prevalence of weather sensitivity in Germany and Canada. Int. J. Biometeorol. 2005, 49, 156–166. [Google Scholar] [CrossRef]
- Lercher, P.; Botteldooren, D.; Widmann, U.; Uhrner, U.; Kammeringer, E. Cardiovascular effects of environmental noise: Research in Austria. Noise Heal. 2011, 13, 234–250. [Google Scholar] [CrossRef] [Green Version]
- Owens, P.E.; Lyons, S.P.; O’Brien, E.T. Arterial hypotension: Prevalence of low blood pressure in the general population using ambulatory blood pressure monitoring. J. Hum. Hypertens. 2000, 14, 243–247. [Google Scholar] [CrossRef]
- Pal, A.; De, S.; Sengupta, P.; Maity, P.; Dhara, P.C. Relationship of body compositional and nutritional parameters with blood pressure in adults. J. Hum. Nutr. Diet. 2013. [Google Scholar] [CrossRef]
- Peterson, H.R.; Rothschild, M.; Weinberg, C.R.; Fell, R.D.; McLeish, K.R.; Pfeifer, M.A. Body fat and the activity of the autonomic nervous system. N. Engl. J. Med. 1988, 318, 1077–1083. [Google Scholar] [CrossRef]
- Scherrer, U.; Randin, D.; Tappy, L.; Vollenweider, P.; Jéquier, E.; Nicod, P. Body fat and sympathetic nerve activity in healthy subjects. Circulation 1994, 89, 2634–2640. [Google Scholar] [CrossRef]
- Shibao, C.; Gamboa, A.; Diedrich, A.; Ertl, A.C.; Chen, K.Y.; Byrne, D.W.; Farley, G.; Paranjape, S.Y.; Davis, S.N.; Biaggioni, I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 2007, 49, 27–33. [Google Scholar] [CrossRef]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp. Physiol. 2008, 93, 715–724. [Google Scholar] [CrossRef]
- Karason, K.; Mølgaard, H.; Wikstrand, J.; Sjöström, L. Heart rate variability in obesity and the effect of weight loss. Am. J. Cardiol. 1999, 83, 1242–1247. [Google Scholar] [CrossRef]
- Maser, R.E.; Lenhard, M.J. An overview of the effect of weight loss on cardiovascular autonomic function. Curr. Diabetes Rev. 2007, 3, 204–211. [Google Scholar] [CrossRef]
- Rissanen, P.; Franssila-Kallunki, A.; Rissanen, A. Cardiac parasympathetic activity is increased by weight loss in healthy obese women. Obes. Res. 2001, 9, 637–643. [Google Scholar] [CrossRef]
- Ohira, T.; Maruyama, M.; Imano, H.; Kitamura, A.; Kiyama, M.; Okada, T.; Maeda, K.; Yamagishi, K.; Noda, H.; Cui, R.; et al. Risk factors for sudden cardiac death among Japanese: The Circulatory Risk in Communities Study. J. Hypertens. 2012, 30, 1137–1143. [Google Scholar] [CrossRef]
- Robertson, D. The pathophysiology and diagnosis of orthostatic hypotension. Clin. Auton. Res. 2008, 18 (Suppl. 1), 2–7. [Google Scholar] [CrossRef]
- Wu, J.S.; Yang, Y.C.; Lu, F.H.; Wu, C.H.; Chang, C.J. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens. Res. 2008, 31, 897–904. [Google Scholar] [CrossRef]
- Radtke, A.; Lempert, T.; von Brevern, M.; Feldmann, M.; Lezius, F.; Neuhauser, H. Prevalence and complications of orthostatic dizziness in the general population. Clin. Auton. Res. 2011, 21, 161–168. [Google Scholar] [CrossRef]
- Brook, R.D. You are what you breathe: Evidence linking air pollution and blood pressure. Curr. Hypertens. Rep. 2005, 7, 427–434. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S. Particulate matter, air pollution, and blood pressure. J. Am. Soc. Hypertens. 2009, 3, 332–350. [Google Scholar] [CrossRef]
- Baccarelli, A.; Barretta, F.; Dou, C.; Zhang, X.; McCracken, J.P.; Díaz, A.; Bertazzi, P.A.; Schwartz, J.; Wang, S.; Hou, L. Effects of particulate air pollution on blood pressure in a highly exposed population in Beijing, China: A repeated-measure study. Environ. Heal. 2011, 10. [Google Scholar] [CrossRef]
- Fredrikson, M.; Edman, G.; Levander, S.E.; Schalling, D.; Svensson, J.; Tuomisto, M. Electrodermal responsivity in young hypotensive and hypertensive men. Psychophysiology 1990, 27, 649–655. [Google Scholar] [CrossRef]
- Covassin, N.; de Zambotti, M.; Cellini, N.; Sarlo, M.; Stegagno, L. Cardiovascular down-regulation in essential hypotension: relationships with autonomic control and sleep. Psychophysiology 2013, 50, 767–776. [Google Scholar] [CrossRef]
- Covassin, N.; de Zambotti, M.; Cellini, N.; Sarlo, M.; Stegagno, L. Nocturnal cardiovascular activity in essential hypotension: Evidence of differential autonomic regulation. Psychosom. Med. 2012, 74, 952–960. [Google Scholar] [CrossRef]
- Parati, G.; Di Rienzo, M.; Coruzzi, P.; Castiglioni, P. Chronic hypotension and modulation of autonomic cardiovascular regulation. Hypertens. Res. 2009, 32, 931–933. [Google Scholar] [CrossRef]
- Duschek, S.; Dietel, A.; Schandry, R.; Del Paso, G.A.R. Increased baroreflex sensitivity and reduced cardiovascular reactivity in individuals with chronic low blood pressure. Hypertens. Res. 2008, 31, 1873–1878. [Google Scholar] [CrossRef]
- Duschek, S.; Schandry, R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin. Auton. Res. 2007, 17, 69–76. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lercher, P.; Widmann, U.; Thudium, J. Hypotension and Environmental Noise: A Replication Study. Int. J. Environ. Res. Public Health 2014, 11, 8661-8688. https://doi.org/10.3390/ijerph110908661
Lercher P, Widmann U, Thudium J. Hypotension and Environmental Noise: A Replication Study. International Journal of Environmental Research and Public Health. 2014; 11(9):8661-8688. https://doi.org/10.3390/ijerph110908661
Chicago/Turabian StyleLercher, Peter, Ulrich Widmann, and Jürg Thudium. 2014. "Hypotension and Environmental Noise: A Replication Study" International Journal of Environmental Research and Public Health 11, no. 9: 8661-8688. https://doi.org/10.3390/ijerph110908661