Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-a and Microcystins in a Large Shallow Lake, Lake Taihu, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
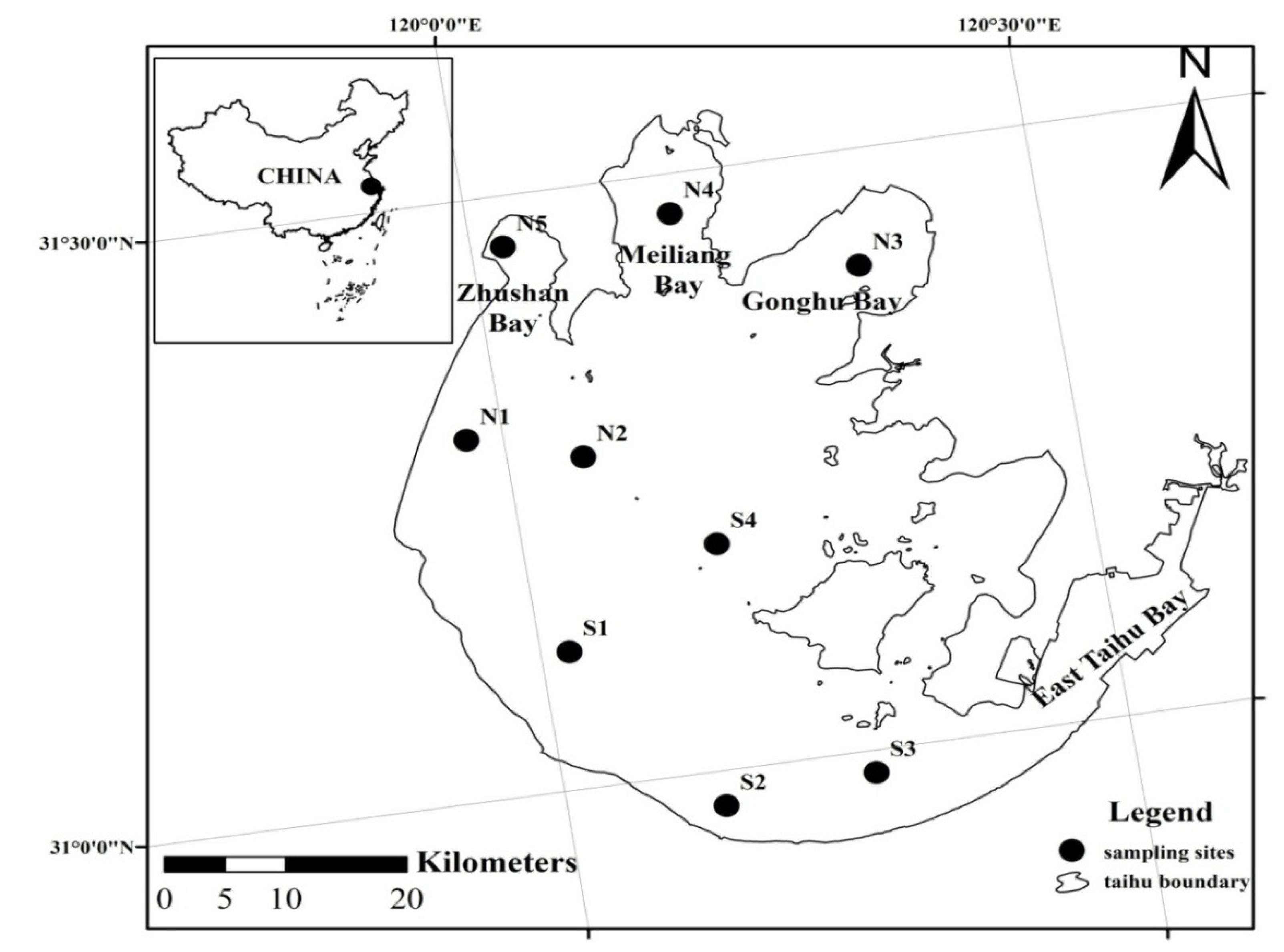
2.2. Sampling and Data Measurements
2.3. Statistical Analysis
3. Results and Discussion
3.1. Temporal Changes in Nutrients and Chl-a in Lake Taihu
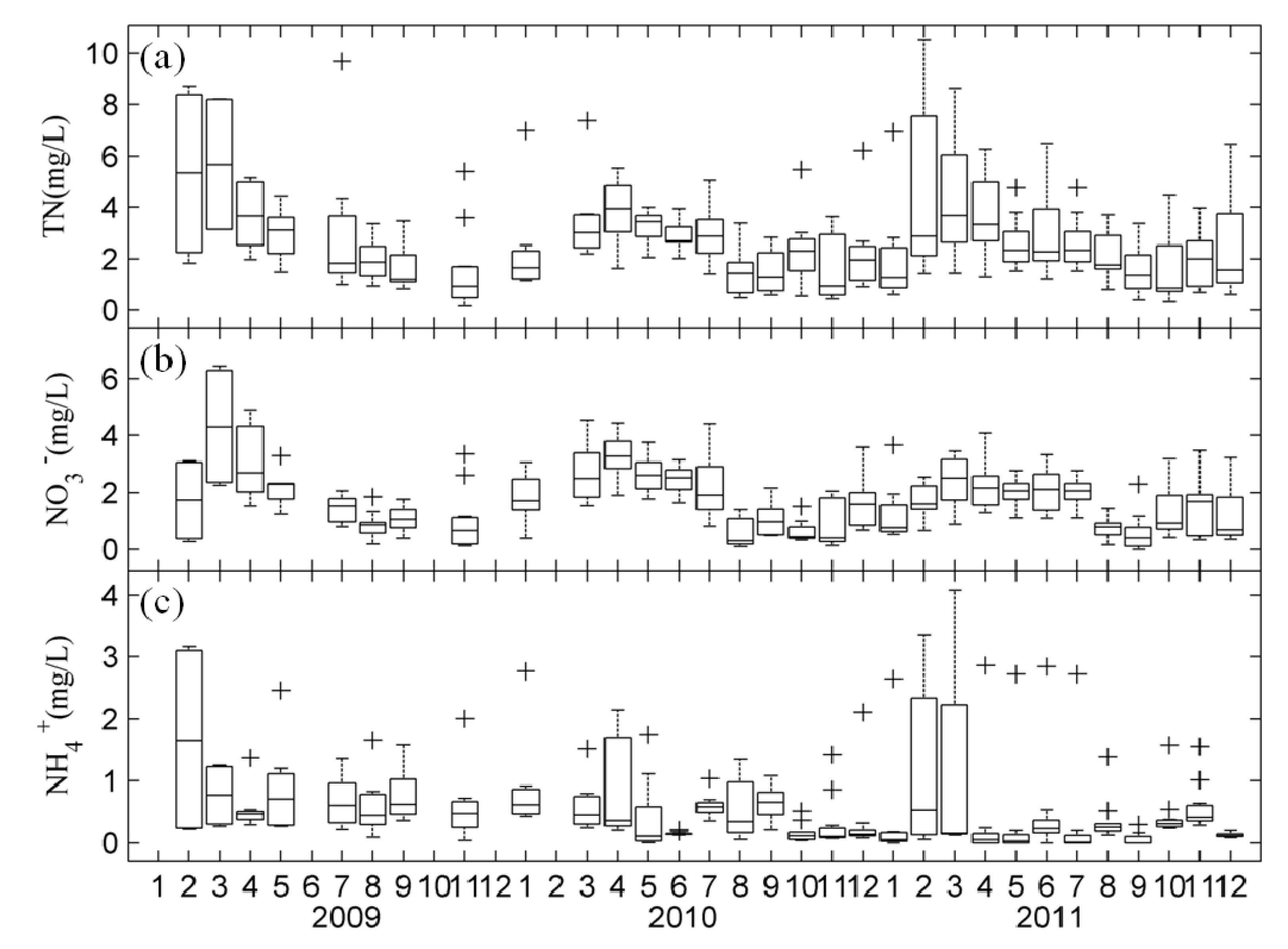
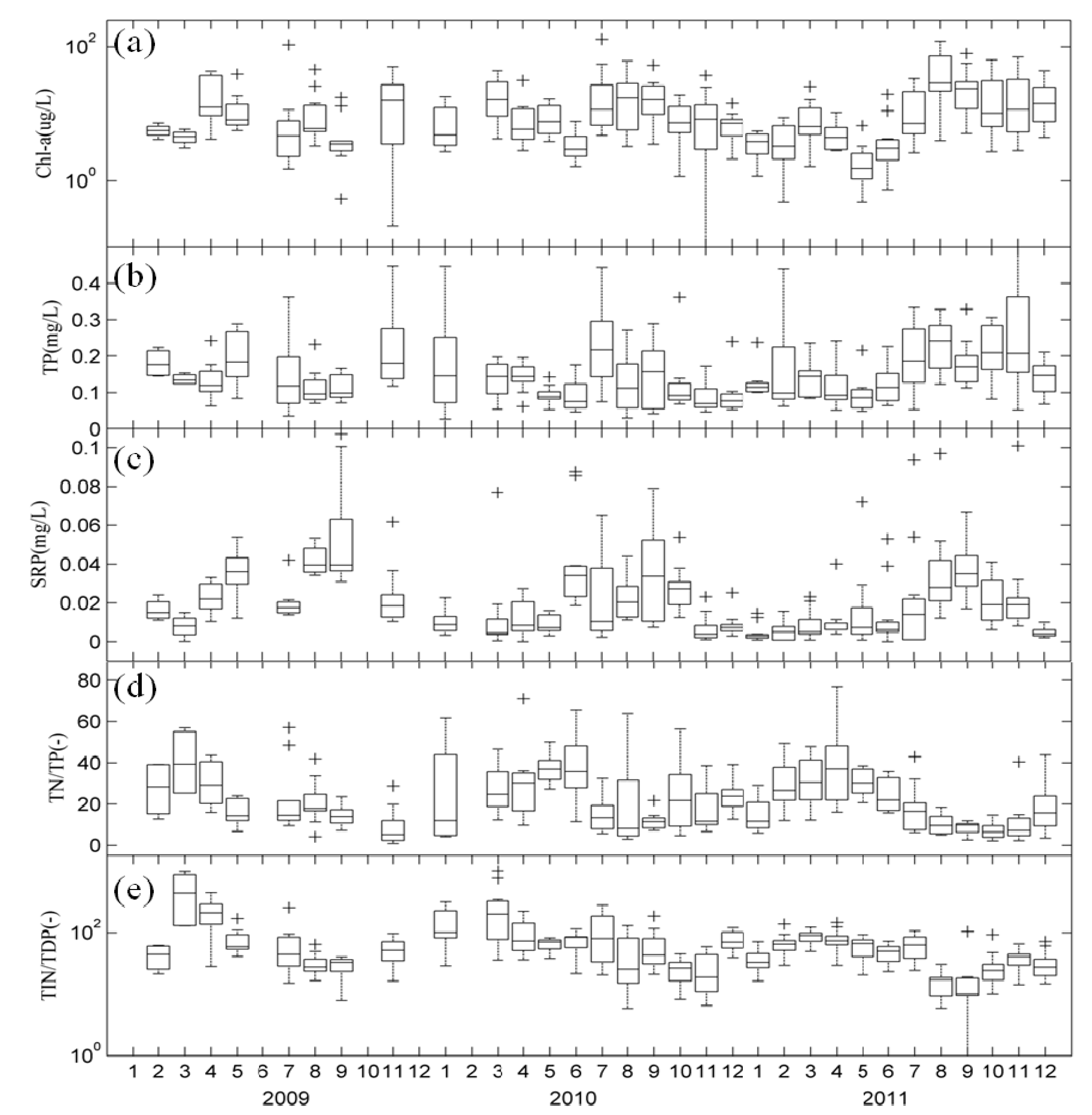
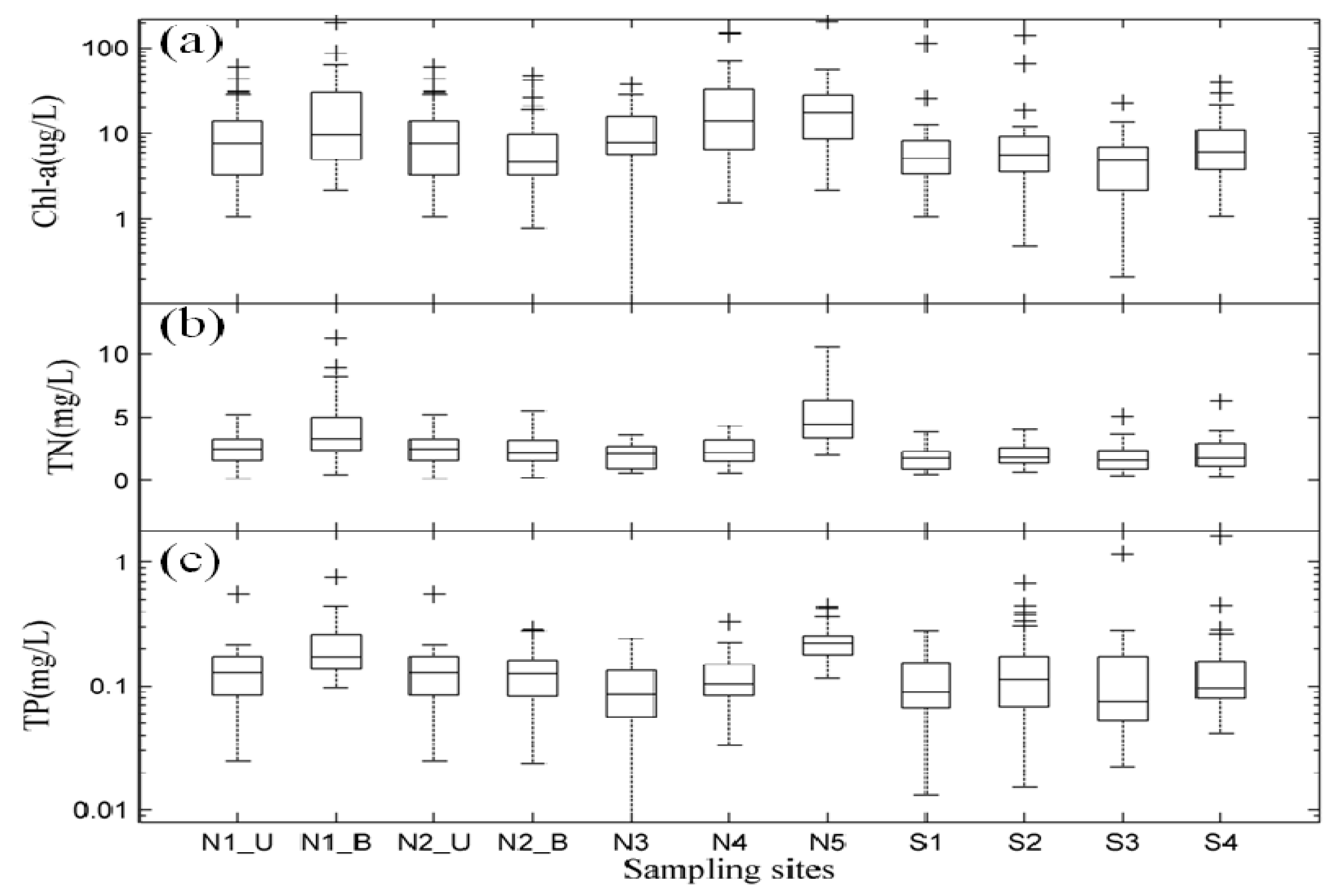
3.2. Spatial Distribution of Major Environmental Factors and Chl-a in Lake Taihu
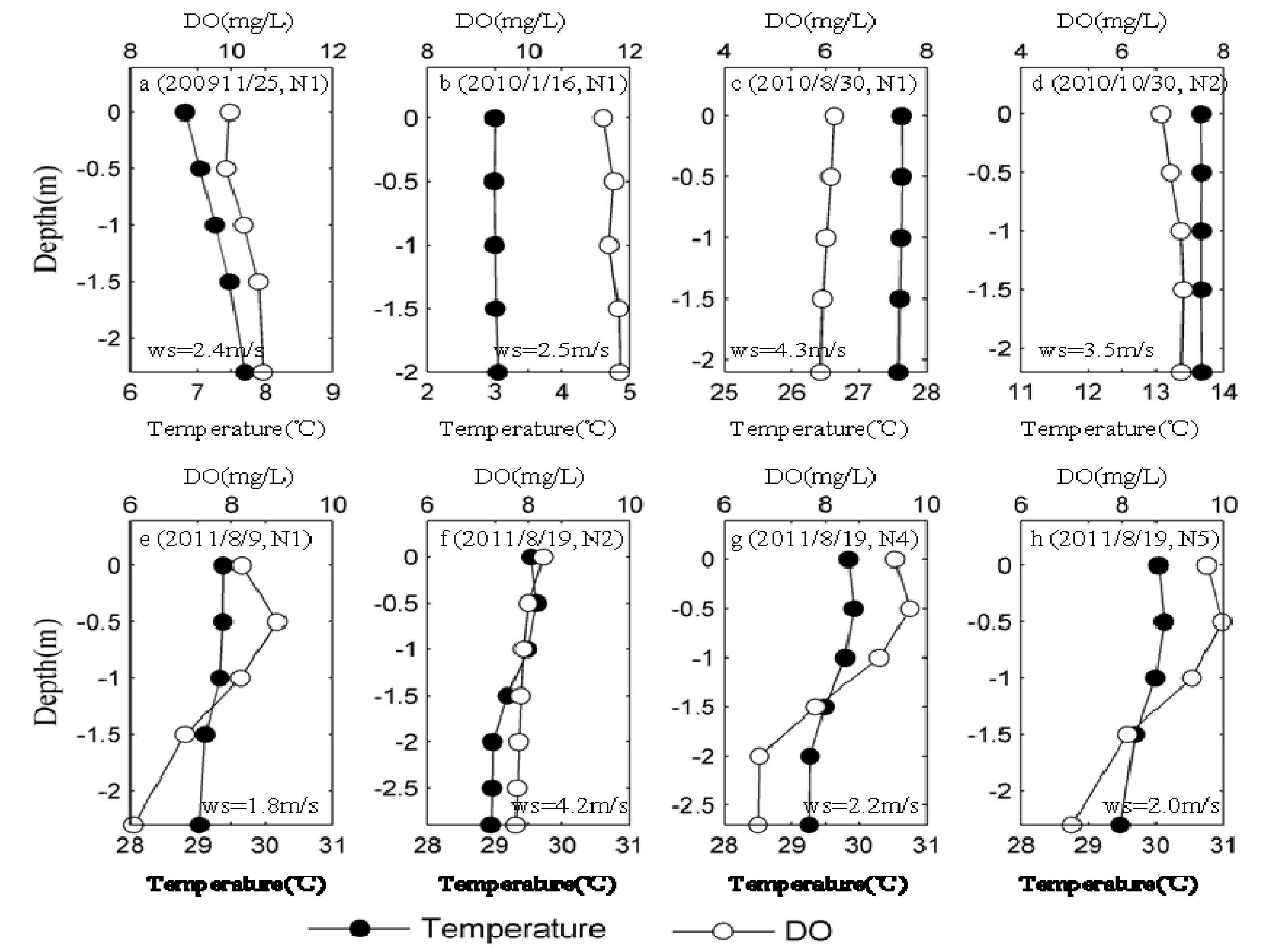
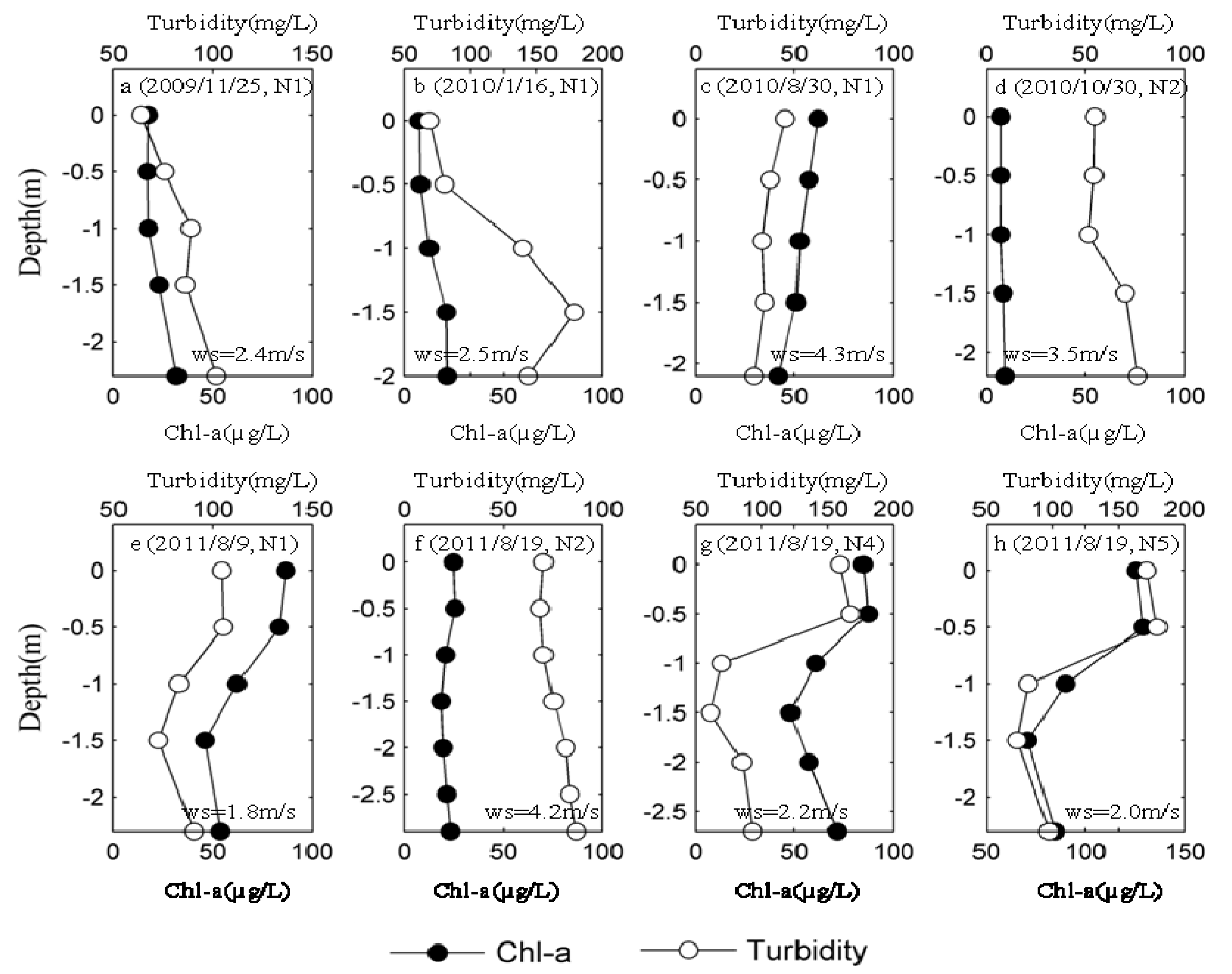
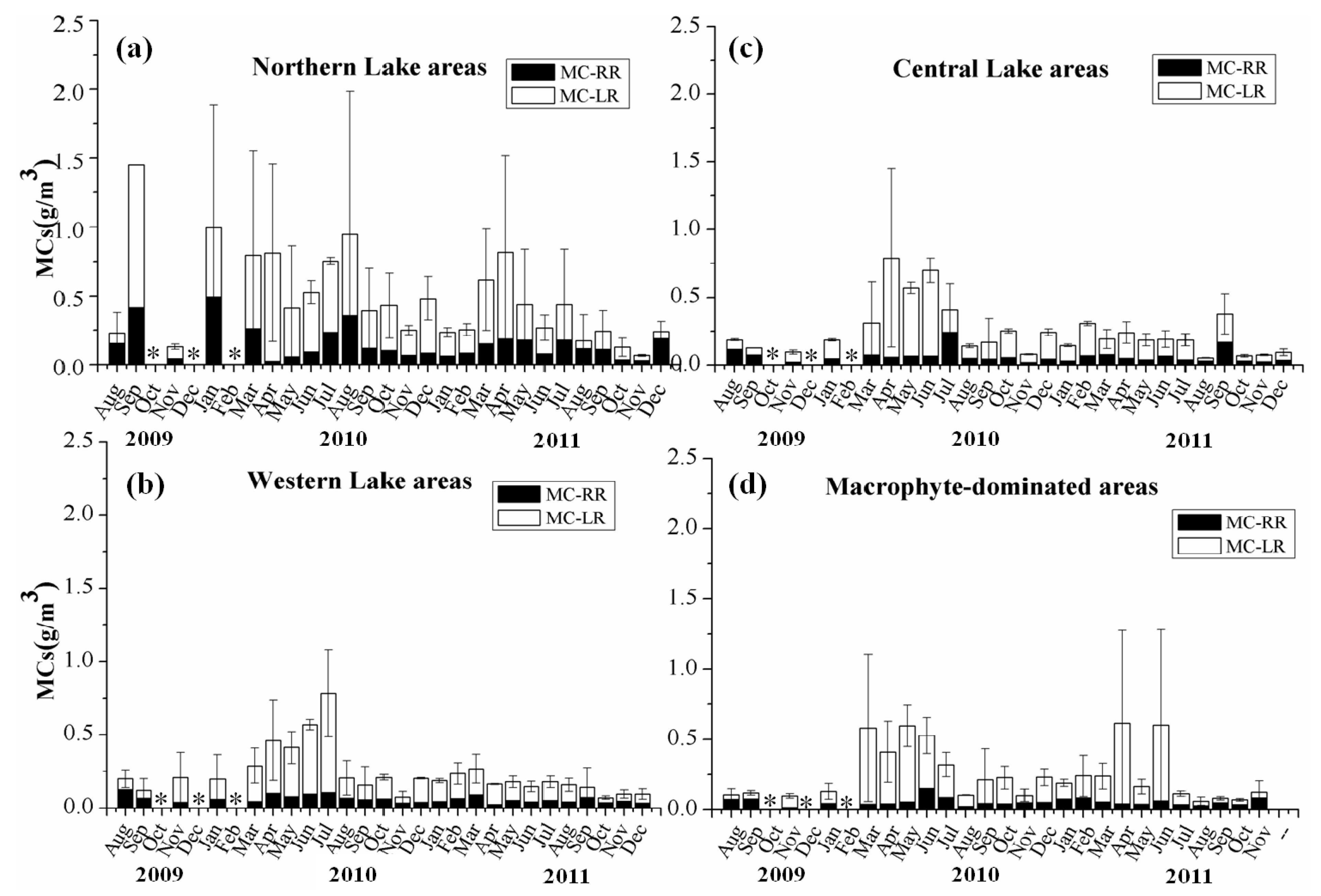
3.3. Spatio-Temporal Distribution of MCs in Lake Taihu
3.4. Correlations between Extracellular MCs and Environmental Factors
| Study Area | Northern Lake Area (n = 27) | Western Lake Area (n = 39) | ||||
| Variables | MC-LR | MC-RR | LR/RR | MC-LR | MC-RR | LR/RR |
| TN | 0.029 | 0.086 | 0.392 * | 0.419 ** | −0.021 | 0.447 ** |
| NO3− | 0.384 | 0.353 | 0.497 ** | 0.303 | −0.084 | 0.300 |
| NH4+ | −0.009 | 0.259 | −0.104 | 0.124 | 0.204 | 0.090 |
| Chl-a | −0.416 * | −0.552 ** | 0.027 | 0.295 | 0.282 | 0.007 |
| TP | −0.322 | −0.262 | −0.093 | 0.540 ** | 0.311 | 0.254 |
| TDP | −0.122 | 0.089 | −0.156 | 0.126 | 0.261 | −0.016 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Huisman, J.; Matthijs, H.C.; Visser, P.P.M. Harmful Cyanobacteria; Springer: Berlin, Germany, 2005. [Google Scholar]
- Padisak, J.; Reynolds, C.S. Shallow lakes: The absolute, the relative, the functional and the pragmatic. Hydrobiologia 2003, 506, 1–11. [Google Scholar] [CrossRef]
- Paerl, H.; Fulton, R., III. Ecology of Harmful Cyanobacteria. In Ecology of Harmful Algae; Springer: Berlin, Germany, 2006; pp. 95–109. [Google Scholar]
- Falconer, I.R. Tumor promotion and liver injury caused by oral consumption of cyanobacteria. Environ. Toxicol. Water Qual. 1991, 6, 177–184. [Google Scholar] [CrossRef]
- Dawson, R. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Mbukwa, E.A.; Msagati, T.A.; Mamba, B.B. Quantitative variations of intracellular microcystin-LR,-RR and -YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season. Int. J. Env. Res. Public Health 2012, 9, 3484–3505. [Google Scholar] [CrossRef]
- Baldia, S.F.; Conaco, M.C.G.; Nishijima, T.; Imanishi, S.; Harada, K.I. Microcystin production during algal bloom occurrence in Laguna de Bay, the Philippines. Fish. Sci. 2003, 69, 110–116. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. Toxic cyanobacteria in water: A guide to their public health consequences,monitoring and management. Spon Press: Abingdon, UK, 1999. [Google Scholar]
- Metcalf, J.S.; Bell, S.G.; Codd, G.A. Colorimetric immuno-protein phosphatase inhibition assay for specific detection of microcystins and nodularins of cyanobacteria. Appl. Environ. Microbiol. 2001, 67, 904–909. [Google Scholar] [CrossRef]
- Mur, R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the Environment; Spon Press: London, UK, 1999. [Google Scholar]
- Park, H.D.; Watanabe, M.F.; Harada, K.I.; Suzuki, M.; Hayashi, H.; Okino, T. Seasonal variations of Microcystis species and toxic heptapeptide microcystins in Lake Suwa. Environ. Toxicol. Water Qual. 1993, 8, 425–435. [Google Scholar] [CrossRef]
- Ward, C.J.; Beattie, K.A.; Lee, E.Y.; Codd, G.A. Colorimetric protein phosphatase inhibition assay of laboratory strains and natural blooms of cyanobacteria: Comparisons with high performance liquid chromatographic analysis for microcystins. FEMS Microbiol. Lett. 1997, 153, 465–473. [Google Scholar] [CrossRef]
- Guo, L. Doing battle with the green monster of Taihu Lake. Science 2007, 317, 1166–1166. [Google Scholar] [CrossRef]
- Yang, L.; Lei, K.; Meng, W.; Fu, G.; Yan, W. Temporal and spatial changes in nutrients and chlorophyll- α in a shallow lake, Lake Chaohu, China: An 11-year investigation. J. Environ. Sci. 2013, 25, 1117–1123. [Google Scholar] [CrossRef]
- Ye, C.; Shen, Z.; Zhang, T.; Fan, M.; Lei, Y.; Zhang, J. Long-term joint effect of nutrients and temperature increase on algal growth in Lake Taihu, China. J. Environ. Sci. 2011, 23, 222–227. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Liu, X.; Zhu, G.; Qin, B.; Shi, Z.; Feng, L. Temporal and spatial variations of chemical oxygen demand in Lake Taihu, China, from 2005 to 2009. Hydrobiologia 2011, 665, 129–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Liu, J.; Qian, X.; Ge, Y. Time-series MODIS image-based retrieval and distribution analysis of total suspended matter concentrations in Lake Taihu (China). Int. J. Env. Res. Public Health 2010, 7, 3545–3560. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Qian, X.; Wang, Q.G.; Qian, Y.; Liu, J.; Ge, Y. Temporal and spatial variability of chlorophyll a concentration in Lake Taihu using MODIS time-series data. Hydrobiologia 2011, 661, 235–250. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, G.; Li, W.; Zhang, Y.; Zhao, L.; Gu, Z. Estimation of the algal-available phosphorus pool in sediments of a large, shallow eutrophic lake (Taihu, China) using profiled SMT fractional analysis. Environ. Pollut. 2013, 173, 216–223. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Li, Z.; Guo, Z.; Burch, M.; Lin, T.-F. Taihu Lake not to Blame for Wuxi’s woes. Science 2008, 319. [Google Scholar] [CrossRef]
- Tan, X.; Kong, F.; Zeng, Q.; Cao, H.; Qian, S.; Zhang, M. Seasonal variation of Microcystis in Lake Taihu and its relationships with environmental factors. J. Environ. Sci. 2009, 21, 892–899. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420. [Google Scholar] [CrossRef]
- Ye, C.; Li, C.-H.; Yu, H.-C.; Song, X.-F.; Zou, G.-Y.; Liu, J. Study on ecological restoration in near-shore zone of a eutrophic lake, Wuli Bay, Taihu Lake. Ecol. Eng. 2011, 37, 1434–1437. [Google Scholar] [CrossRef]
- Zhang, R.; Qian, X.; Yuan, X.; Ye, R.; Xia, B.; Wang, Y. Simulation of water environmental capacity and pollution load reduction using QUAL2K for water environmental management. Int. J. Env. Res. Public Health 2012, 9, 4504–4521. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, C.; Teubner, K.; Dokulil, M. Changes of nutrients and phytoplankton chlorophyll-a in a large shallow lake, Taihu, China: An 8-year investigation. Hydrobiologia 2003, 506, 273–279. [Google Scholar]
- Ke, Z.; Xie, P.; Guo, L. Controlling factors of spring—Summer phytoplankton succession in Lake Taihu (Meiliang Bay, China). Hydrobiologia 2008, 607, 41–49. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Niu, Y.; Shen, H.; Chen, J.; Xie, P.; Yang, X.; Tao, M.; Ma, Z.; Qi, M. Phytoplankton community succession shaping bacterioplankton community composition in Lake Taihu, China. Water Res. 2011, 45, 4169–4182. [Google Scholar]
- Wang, X.; Sun, M.; Wang, J.; Yang, L.; Luo, L.; Li, P.; Kong, F. Microcystis genotype succession and related environmental factors in Lake Taihu during cyanobacterial blooms. Microb. Ecol. 2012, 64, 986–999. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Qin, B.; Hu, W.; Chen, W. The Process and Mechanism of Water Environment Evolvement in Taihu Lake; Science Press: Beijing, China, 2004. [Google Scholar]
- National Environmental Bureau. Standard Methods for the Examination Analysis of Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002; pp. 243–280. [Google Scholar]
- Yang, H.; Xie, P.; Xu, J.; Zheng, L.; Deng, D.; Zhou, Q.; Wu, S. Seasonal variation of microcystin concentration in Lake Chaohu, a shallow subtropical lake in the People’s Republic of China. Bull. Environ. Contam. Toxicol. 2006, 77, 367–374. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.; Peng, L.; Wan, N.; Gan, N.; Zhang, X. Distribution and bioaccumulation of microcystins in water columns: A systematic investigation into the environmental fate and the risks associated with microcystins in Meiliang Bay, Lake Taihu. Water Res. 2007, 41, 2853–2864. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: http://www.lsw.uni-heidelberg.de/users/christlieb/teaching/UKStaSS10/R-refman.pdf (accessed on 8 May 2014).
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of box plots. Am. Stat. 1978, 32, 12–16. [Google Scholar]
- Xie, L.; Xie, P.; Li, S.; Tang, H.; Liu, H. The low TN: TP ratio, a cause or a result of Microcystis blooms? Water Res. 2003, 37, 2073–2080. [Google Scholar] [CrossRef]
- Wang, S.; Qian, X.; Han, B.-P.; Wang, Q.-H.; Ding, Z.-F. Physical limnology of a typical subtropical reservoir in south China. Lake Reserv. Manage. 2011, 27, 149–161. [Google Scholar] [CrossRef]
- Bai, X.; Hu, W.; Hu, Z.; Li, X. Importation of wind-driven drift of mat-like algae bloom into Meiliang Bay of Taihu Lake in 2004 summer. (In Chinese)J. Environ. Sci 2005, 26, 57–60. [Google Scholar]
- Shao, K.; Gao, G.; Qin, B.; Tang, X.; Wang, Y.; Chi, K.; Dai, J. Comparing sediment bacterial communities in the macrophyte-dominated and algae-dominated areas of eutrophic Lake Taihu, China. Can. J. Microbiol. 2011, 57, 263–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B.; Hu, W.; Wang, S.; Chen, Y.; Chen, W. Temporal-spatial variations of euphotic depth of typical lake regions in Lake Taihu and its ecological environmental significance. Sci. China Ser. D 2006, 49, 431–442. [Google Scholar] [CrossRef]
- Dong, X.; Bennion, H.; Battarbee, R.; Yang, X.; Yang, H.; Liu, E. Tracking eutrophication in Taihu Lake using the diatom record: potential and problems. J. Paleolimnol. 2008, 40, 413–429. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.; Vijayaraghavan, R.; Rao, P. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Vézie, C.; Rapala, J.; Vaitomaa, J.; Seitsonen, J.; Sivonen, K. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 2002, 43, 443–454. [Google Scholar] [CrossRef]
- Ohtake, A.; Shirai, M.; Aida, T.; Mori, N.; Harada, K.; Matsuura, K.; Suzuki, M.; Nakano, M. Toxicity of Microcystis species isolated from natural blooms and purification of the toxin. Appl. Environ. Microbiol. 1989, 55, 3202–3207. [Google Scholar]
- Liu, Y.; Xie, P.; Zhang, D.; Wen, Z. Seasonal dynamics of microcystins with associated biotic and abiotic parameters in two bays of Lake Taihu, the third largest freshwater lake in China. Bull. Environ. Contam. Toxicol. 2008, 80, 24–29. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Verspagen, J.M.; Lürling, M.; van Donk, E.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: an experimental test of the carbon—Nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, G.; Chai, W.; Song, G.; Li, R. Congruence between mcy based genetic type and microcystin composition within the populations of toxic Microcystis in a plateau lake, China. Environ. Microbiol. Rep. 2013, 5, 637–647. [Google Scholar]
- Ma, J.R.; Brookes, J.D.; Qin, B.Q.; Paerl, H.W.; Gao, G.; Wu, P.; Zhang, W.; Deng, J.M.; Zhu, G.W.; Zhang, Y.L.; et al. Environmental factors controlling colony formation in blooms of the cyanobacteria Microcystis spp. in Lake Taihu, China. Harmful Algae 2014, 31, 136–142. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ye, R.; Shan, K.; Gao, H.; Zhang, R.; Xiong, W.; Wang, Y.; Qian, X. Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-a and Microcystins in a Large Shallow Lake, Lake Taihu, China. Int. J. Environ. Res. Public Health 2014, 11, 5155-5169. https://doi.org/10.3390/ijerph110505155
Ye R, Shan K, Gao H, Zhang R, Xiong W, Wang Y, Qian X. Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-a and Microcystins in a Large Shallow Lake, Lake Taihu, China. International Journal of Environmental Research and Public Health. 2014; 11(5):5155-5169. https://doi.org/10.3390/ijerph110505155
Chicago/Turabian StyleYe, Rui, Kun Shan, Hailong Gao, Ruibin Zhang, Wen Xiong, Yulei Wang, and Xin Qian. 2014. "Spatio-Temporal Distribution Patterns in Environmental Factors, Chlorophyll-a and Microcystins in a Large Shallow Lake, Lake Taihu, China" International Journal of Environmental Research and Public Health 11, no. 5: 5155-5169. https://doi.org/10.3390/ijerph110505155




