Combination of a Fast Cleanup Procedure and a DR-CALUX® Bioassay for Dioxin Surveillance in Taiwanese Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Design

2.2. Procedure of Sample Extraction and Cleanup
2.3. DR-CALUX® Assay of Dioxins
2.4. Statistical Analysis
3. Results
| Soil Characteristics | Location in Taiwan | X2 (p) a | ||||
|---|---|---|---|---|---|---|
| Northern (n = 184) | Central (n = 247) | Southern (n = 132) | Eastern (n = 122) | |||
| Frequency (Number) | ||||||
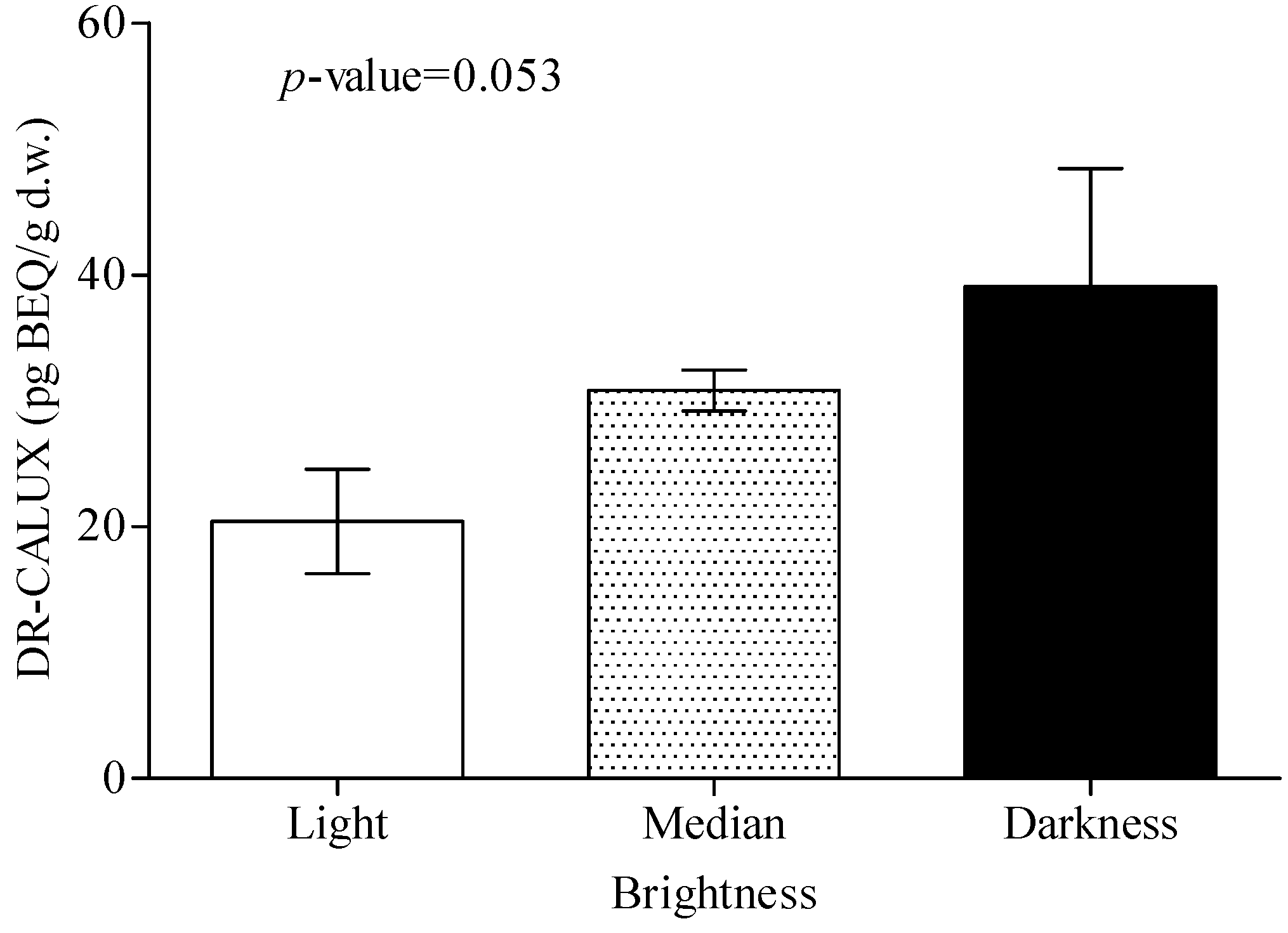
| Soil brightness | 0.696 | |||||
| Light | ||||||
| Yellow brown | 7 | 9 | 8 | 2 | ||
| Medium | ||||||
| Gray | 31 | 39 | 18 | 18 | ||
| Brown | 94 | 143 | 72 | 58 | ||
| Charcoal gray | 43 | 47 | 28 | 39 | ||
| Dark | ||||||
| Black brown | 7 | 8 | 5 | 3 | ||
| Black | 2 | 1 | 1 | 2 | ||
| Sampling depth | 0.036 * | |||||
| 10 cm | 128 | 168 | 107 | 82 | ||
| 15 cm | 56 | 79 | 25 | 40 | ||
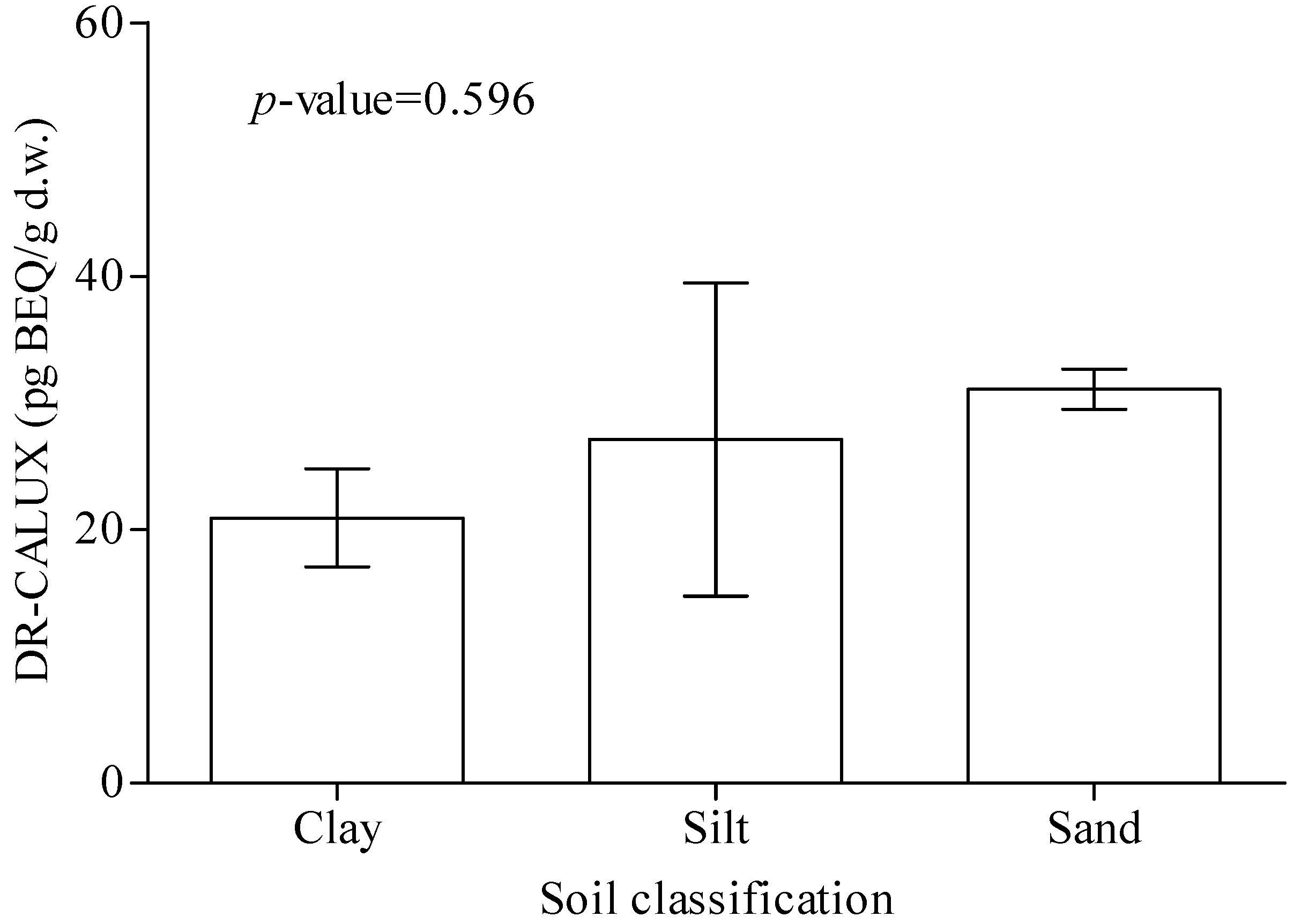
| Soil classification | 0.321 | |||||
| Clay | 4 | 5 | 6 | 1 | ||
| Silt | 2 | 4 | 5 | 2 | ||
| Sand | 178 | 238 | 121 | 119 | ||
| Soil moisture | 0.794 | |||||
| Wet | 70 | 103 | 53 | 45 | ||
| Dry | 114 | 144 | 79 | 77 | ||
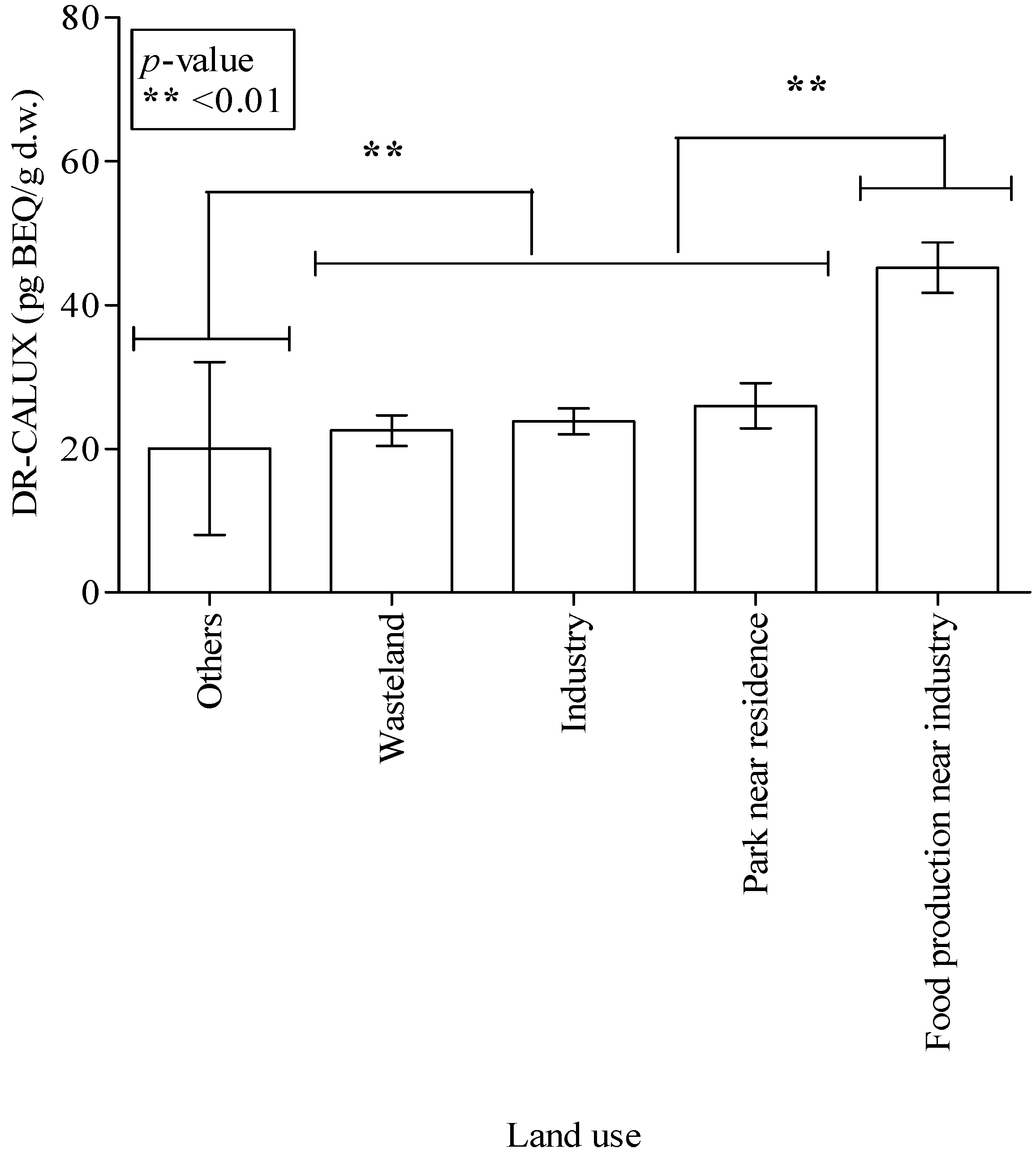
| Land use | <0.001 *** | |||||
| Wasteland | 42 | 39 | 6 | 57 | ||
| Industry | 44 | 23 | 88 | 43 | ||
| Park near residential area | 10 | 20 | 13 | 15 | ||
| Food production near industrial area b | 84 | 158 | 0 | 4 | ||
| Others c | 4 | 7 | 2 | 3 | ||
| Missing d | 0 | 0 | 23 | 0 | ||
| Dioxin concentration | Mean ± SD (pg-BEQ/g d.w.) | p e | ||||
| DR-CALUX® assay | 61.8 ± 62.3 | 22.2 ± 12.8 | 24.9 ± 26.3 | 7.80 ± 5.08 | <0.001 *** | |
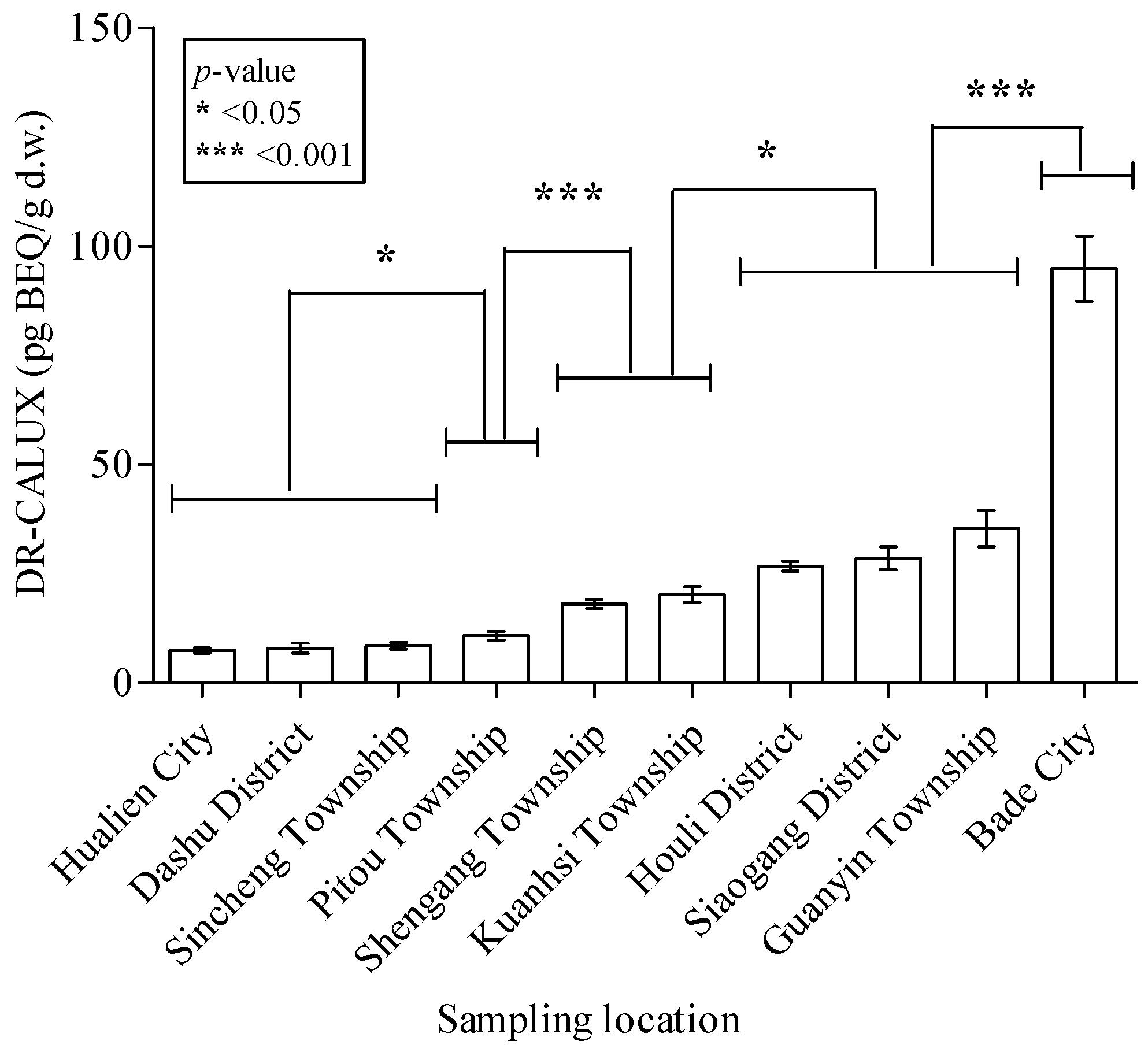
| DR-CALUX (pg-BEQ/g d.w.) | Odds Ratio | ||||
|---|---|---|---|---|---|
| Soil Sampling Location | Purpose of Land Use | Soil Brightness | Soil Moisture | Soil Depth | |
| <33.4 a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| >33.4 | 2.43 | 1.47 | 2.83 | 1.14 | 0.961 |
| p-value | <0.001 *** | <0.001 *** | 0.009 ** | 0.574 | 0.835 |
| Dependent Variable | Predictors | Beta | p-value | Adjusted R Square | p-value |
|---|---|---|---|---|---|
| Log10 DR-CALUX (pg-BEQ/g d.w.) | Soil sampling location | 0.097 | <0.001 *** | 0.947 | <0.001 *** |
| Purpose of land use | 0.065 | <0.001 *** | |||
| Soil brightness | 0.170 | <0.001 *** | |||
| Soil moisture | 0.051 | 0.020 * |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schecter, A.; Birnbaum, L.; Ryan, J.J.; Constable, J.D. Dioxins: An overview. Environ. Res. 2006, 101, 419–428. [Google Scholar] [CrossRef]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; de Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 world health organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Wang, L.C.; Lee, W.J.; Lee, W.S.; Chang-Chien, G.P. Emission estimation and congener-specific characterization of polybrominated diphenyl ethers from various stationary and mobile sources. Environ. Pollut. 2010, 158, 3108–3115. [Google Scholar] [CrossRef]
- Mi, H.H.; Wu, Z.S.; Lin, L.F.; Lai, Y.C.; Lee, Y.Y.; Wang, L.C.; Chang-Chien, G.P. Atmospheric dry deposition of polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs) in southern Taiwan. Aerosol Air Qual. Res. 2012, 12, 1016–1029. [Google Scholar]
- Chang, S.C.; Wang, Y.F.; You, S.J.; Kuo, Y.M.; Tsai, C.H.; Wang, L.C.; Hsu, P.Y. Toxicity evaluation of fly ash by Microtox®. Aerosol Air Qual. Res. 2013, 13, 1002–1008. [Google Scholar]
- Lohmann, R.; Ockenden, W.A.; Shears, J.; Jones, K.C. Atmospheric distribution of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/Fs), and non-ortho biphenyls (PCBs) along a north-south Atlantic transect. Environ. Sci. Technol. 2001, 35, 4046–4053. [Google Scholar] [CrossRef]
- Wang, L.C.; Lee, W.J.; Lee, W.S.; Chang-Chien, G.P.; Tsai, P.J. Characterizing the emissions of polychlorinated dibenzo-p-dioxins and dibenzofurans from crematories and their impacts to the surrounding environment. Environ. Sci. Technol. 2002, 37, 62–67. [Google Scholar]
- Du, Y.; Chen, T.; Lu, S.; Yan, J.; Li, X.; Cen, K.; Nakamura, M.; Handa, H. Comparative analysis of PCDD/Fs in soil around waste incineration plants in China using CALUX bioassay and HRGC/HRMS. J. Hazard. Mater. 2011, 192, 1729–1738. [Google Scholar] [CrossRef]
- Lee, W.J.; Shih, S.I.; Chang, C.Y.; Lai, Y.C.; Wang, L.C.; Chang-Chien, G.P. Thermal treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans from contaminated soils. J. Hazard. Mater. 2008, 160, 220–227. [Google Scholar] [CrossRef]
- Vassura, I.; Passarini, F.; Ferroni, L.; Bernardi, E.; Morselli, L. PCDD/Fs atmospheric deposition fluxes and soil contamination close to a municipal solid waste incinerator. Chemosphere 2011, 83, 1366–1373. [Google Scholar] [CrossRef]
- Chi, K.H.; Luo, S.; Kao, S.J.; Lee, T.Y. Sources and deposition fluxes of PCDD/Fs in a high-mountain lake in central Taiwan. Chemosphere 2013, 91, 150–156. [Google Scholar] [CrossRef]
- Lohmann, R.; Breivik, K.; Dachs, J.; Muir, D. Global fate of POPs: Current and future research directions. Environ. Pollut. 2007, 150, 150–165. [Google Scholar] [CrossRef]
- Lee, W.J.; Shih, S.I.; Li, H.W.; Lin, L.F.; Yu, K.M.; Lu, K.; Wang, L.C.; Chang-Chien, G.P.; Fang, K.; Lin, M. Assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans contribution from different media to surrounding duck farms. J. Hazard. Mater. 2009, 163, 1185–1193. [Google Scholar] [CrossRef]
- Muir, D.C.G.; Wagemann, R.; Hargrave, B.T.; Thomas, D.J.; Peakall, D.B.; Norstrom, R.J. Arctic marine ecosystem contamination. Sci. Total Environ. 1992, 122, 75–134. [Google Scholar] [CrossRef]
- Chao, H.R.; Wang, S.L.; Su, P.H.; Yu, H.Y.; Yu, S.T.; Päpke, O. Levels of polychlorinated dibenzo-p-dioxins and dibenzofurans in primipara breast milk from Taiwan: Estimation of dioxins and furans intake for breastfed infants. J. Hazard. Mater. 2005, 121, 1–10. [Google Scholar] [CrossRef]
- Chao, H.R.; Wang, Y.F.; Chen, H.T.; Ko, Y.C.; Chang, E.E.; Huang, Y.J.; Tsai, F.Y.; Tsai, C.H.; Wu, C.H.; Tsou, T.C. Differential effect of arecoline on the endogenous dioxin-responsive cytochrome p450 1A1 and on a stably transfected dioxin-responsive element-driven reporter in human hepatoma cells. J. Hazard. Mater. 2007, 149, 234–237. [Google Scholar] [CrossRef]
- Shih, T.S.; Shih, M.; Lee, W.J.; Huang, S.L.; Wang, L.C.; Chen, Y.C.; Tsai, P.J. Particle size distributions and health-related exposures of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) of sinter plant workers. Chemosphere 2009, 74, 1463–1470. [Google Scholar] [CrossRef]
- Piskorska-Pliszczynska, J.; Mikolajczyk, S.; Warenik-Bany, M.; Maszewski, S.; Strucinski, P. Soil as a source of dioxin contamination in eggs from free-range hens on a polish farm. Sci. Total Environ. 2014, 466–467, 447–454. [Google Scholar] [CrossRef]
- Reeuwijk, N.M.; Talidda, A.; Malisch, R.; Kotz, A.; Tritscher, A.; Fiedler, H.; Zeilmaker, M.J.; Kooijman, M.; Wienk, K.J.H.; Traag, W.A.; et al. Dioxins (polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-furans) in traditional clay products used during pregnancy. Chemosphere 2013, 90, 1678–1685. [Google Scholar] [CrossRef]
- Henriksson, S.; Hagberg, J.; Bäckström, M.; Persson, I.; Lindström, G. Assessment of PCDD/Fs levels in soil at a contaminated sawmill site in Sweden—A GIS and PCA approach to interpret the contamination pattern and distribution. Environ. Pollut. 2013, 180, 19–26. [Google Scholar] [CrossRef]
- Li, C.; Zheng, M.; Zhang, B.; Gao, L.; Liu, L.; Zhou, X.; Ma, X.; Xiao, K. Long-term persistence of polychlorinated dibenzo-p-dioxins and dibenzofurans in air, soil and sediment around an abandoned pentachlorophenol factory in China. Environ. Pollut. 2012, 162, 138–143. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Z.; Peng, X.; Ren, M.; Zhang, S.; Liu, X.; Wang, J. Pollution characteristics and potential health risk of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in soil/sediment from Baiyin city, North West, China. Environ. Geochem. Health 2013, 35, 593–604. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Zhu, X.; Yeung, L.W.Y.; Taniyasu, S.; Miyake, Y.; Falandysz, J.; Yamashita, N. Altitudinal distributions of PCDD/Fs, dioxin-like PCBs and PCNs in soil and yak samples from Wolong high mountain area, eastern Tibet-Qinghai Plateau, China. Sci. Total Environ. 2013, 444, 102–109. [Google Scholar] [CrossRef]
- Xu, P.; Tao, B.; Li, N.; Qi, L.; Ren, Y.; Zhou, Z.; Zhang, L.; Liu, A.; Huang, Y. Levels, profiles and source identification of PCDD/Fs in farmland soils of Guiyu, China. Chemosphere 2013, 91, 824–831. [Google Scholar] [CrossRef]
- Chao, H.R.; Wang, Y.F.; Lin, D.Y.; Cheng, Y.T.; Tsou, T.C. Fast cleanup system combined with a dioxin-responsive element-driven luciferase bioassay for analysis of polychlorinated dibenzo-p-dioxins/furans in sediments and soils. Bull. Environ. Contam. Toxicol. 2011, 86, 278–282. [Google Scholar] [CrossRef]
- Chao, H.R.; Wang, Y.F.; Wang, Y.N.; Lin, D.Y.; Gou, Y.Y.; Chen, C.Y.; Chen, K.C.; Wu, W.K.; Chiang, B.A.; Huang, Y.T.; et al. An improved ahr reporter gene assay for analyzing dioxins in soil, sediment and fish. Bull. Environ. Contam. Toxicol. 2012, 89, 739–743. [Google Scholar] [CrossRef]
- Dindal, A.; Thompson, E.; Aume, L.; Billets, S. Application of site-specific calibration data using the CALUX by XDS bioassay for dioxin-like chemicals in soil and sediment samples. Environ. Sci. Technol. 2007, 41, 8376–8382. [Google Scholar] [CrossRef]
- Haro-García, L.; Villa-Ibarra, M.; Jesús Chaín-Castro, T.; Lastra-Rodríguez, Á.; Juárez-Pérez, C.; Aguilar-Madrid, G.; Sánchez-Escalante, V.; Brito-Zurita, O. Are Mexican agricultural farmlands PCDD/F soil reservoirs? Bull. Environ. Contam. Toxicol. 2012, 88, 813–815. [Google Scholar] [CrossRef]
- Hong, S.; Khim, J.S.; Naile, J.E.; Park, J.; Kwon, B.O.; Wang, T.; Lu, Y.; Shim, W.J.; Jones, P.D.; Giesy, J.P. Ahr-mediated potency of sediments and soils in estuarine and coastal areas of the Yellow Sea region: A comparison between Korea and China. Environ. Pollut. 2012, 171, 216–225. [Google Scholar] [CrossRef]
- Tue, N.M.; Suzuki, G.; Takahashi, S.; Kannan, K.; Takigami, H.; Tanabe, S. Dioxin-related compounds in house dust from New York State: Occurrence, in vitro toxic evaluation and implications for indoor exposure. Environ. Pollut. 2013, 181, 75–80. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, B.; Kojima, H.; Takeuchi, S.; Takagi, Y.; Tateishi, N.; Iida, M.; Shiozaki, T.; Xu, P.; Qi, L.; et al. Simple and rapid determination of PCDD/Fs in flue gases from various waste incinerators in China using DR-EcoScreen cells. Chemosphere 2014, 102, 24–30. [Google Scholar] [CrossRef]
- Brown, D.J.; Orelien, J.; Gordon, J.D.; Chu, A.C.; Chu, M.D.; Nakamura, M.; Handa, H.; Kayama, F.; Denison, M.S.; Clark, G.C. Mathematical model developed for environmental samples: Prediction of GC/MS dioxin TEQ from XDS-CALUX bioassay data. Environ. Sci. Technol. 2007, 41, 4354–4360. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Amakura, Y.; Nakamura, M.; Brown, D.J.; Clark, G.C.; Sasaki, K.; Toyoda, M.; Maitani, T. Validation of the CALUX bioassay for the screening of PCDD/Fs and dioxin-like PCBs in retail fish. Analyst 2003, 128, 486–492. [Google Scholar] [CrossRef]
- Leng, J.H.; Kayama, F.; Wang, P.Y.; Nakamura, M.; Nakata, T.; Wang, Y. Levels of persistent organic pollutants in human milk in two Chinese coastal cities, Tianjin and Yantai: Influence of fish consumption. Chemosphere 2009, 75, 634–639. [Google Scholar] [CrossRef]
- Hoogenboom, L.; Traag, W.; Bovee, T.; Goeyens, L.; Carbonnelle, S.; Van Loco, J.; Beernaert, H.; Jacobs, G.; Schoeters, G.; Baeyens, W. The CALUX bioassay: Current status of its application to screening food and feed. Trends Anal. Chem. 2006, 25, 410–420. [Google Scholar] [CrossRef]
- Chao, H.R.; Tsou, T.C.; Chen, H.T.; Chang, E.E.; Tsai, F.Y.; Lin, D.Y.; Chen, F.A.; Wang, Y.F. The inhibition effect of 2,3,7,8-tetrachlorinated dibenzo-p-dioxin-induced aryl hydrocarbon receptor activation in human hepatoma cells with the treatment of cadmium chloride. J. Hazard. Mater. 2009, 170, 351–356. [Google Scholar] [CrossRef]
- Chao, H.R.; Tsou, T.C.; Li, L.A.; Tsai, F.Y.; Wang, Y.F.; Tsai, C.H.; Chang, E.E.; Miao, Z.F.; Wu, C.H.; Lee, W.J. Arsenic inhibits induction of cytochrome P450 1A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human hepatoma cells. J. Hazard. Mater. 2006, 137, 716–722. [Google Scholar] [CrossRef]
- Behnisch, P.A. Dioxins & Robotics. Int. Dairy Mag. 2011, 9, 29–30. [Google Scholar]
- Chen, Y.; Wu, C.; Peng, J.; Weng, Y. Soxtherm with CAPE coupled carbon-acid column as a method for fast analyses of PCDD/Fs and dioxin-like PCBs in environmental samples. Organohalogen Compd. 2007, 69, 473–476. [Google Scholar]
- Lin, D.Y.; Shy, C.G.; Chen, F.A.; Wang, Y.F.; Chen, K.C.; Hsieh, L.T.; Tsai, F.Y.; Tsou, T.C.; Chao, H.R. Use of a highly sensitive recombinant hepatoma cell method to determine dioxin concentrations in samples of fish and crab from a hotspot area. Environ. Sci.: Process. Impacts 2013, 15, 1264–1270. [Google Scholar] [CrossRef]
- Jou, J.J.; Chung, J.C.; Weng, Y.M.; Liaw, S.L.; Wang, M.K. Identification of dioxin and dioxin-like polychlorbiphenyls in plant tissues and contaminated soils. J. Hazard. Mater. 2007, 149, 174–179. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lin, L.F.; Shih, S.I.; Yu, K.M.; Hsieh, L.T.; Wang, L.C.; Chang-Chien, G.P. Atmospheric deposition of polychlorinated dibenzo-p-dioxins and dibenzofurans on the soils in the vicinity of municipal solid waste incinerators. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2009, 44, 1327–1334. [Google Scholar] [CrossRef]
- Jou, J.J.; Lin, K.L.; Chung, J.C.; Liaw, S.L. Soil dioxins levels at agriculture sites and natural preserve areas of Taiwan. J. Hazard. Mater. 2007, 147, 1–7. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Vafeiadi, M.; Agramunt, S.; Mathianaki, K.; Karakosta, P.; Spanaki, A.; Besselink, H.; Kiviranta, H.; Rantakokko, P.; KaterinaSarri; et al. Maternal diet, prenatal exposure to dioxins and other persistent organic pollutants and anogenital distance in children. Sci. Total Environ. 2013, 461–462, 222–229. [Google Scholar] [CrossRef]
- Porpora, M.G.; Medda, E.; Abballe, A.; Bolli, S.; De Angelis, I.; di Domenico, A.; Ferro, A.; Ingelido, A.M.; Maggi, A.; Panici, P.B.; et al. Endometriosis and organochlorinated environmental pollutants: A case-control study on Italian women of reproductive age. Environ. Health Perspect. 2009, 117, 1070–1075. [Google Scholar] [CrossRef]
- He, G.; Tsutsumi, T.; Zhao, B.; Baston, D.S.; Zhao, J.; Heath-Pagliuso, S.; Denison, M.S. Third-generation Ah receptor-responsive luciferase reporter plasmids: Amplification of dioxin-responsive elements dramatically increases CALUX bioassay sensitivity and responsiveness. Toxicol. Sci. 2011, 123, 511–522. [Google Scholar] [CrossRef]
- Kojima, H.; Takeuchi, S.; Tsutsumi, T.; Yamaguchi, K.; Anezaki, K.; Kubo, K.; Iida, M.; Takahashi, T.; Kobayashi, S.; Jin, K.; et al. Determination of dioxin concentrations in fish and seafood samples using a highly sensitive reporter cell line, DR-EcoScreen cells. Chemosphere 2011, 83, 753–759. [Google Scholar] [CrossRef]
- Kanematsu, M.; Shimizu, Y.; Sato, K.; Kim, S.; Suzuki, T.; Park, B.; Saino, R.; Nakamura, M. Origins and transport of aquatic dioxins in the Japanese watershed: Soil contamination, land use, and soil runoff events. Environ. Sci. Technol. 2009, 43, 4260–4266. [Google Scholar]
- Naile, J.E.; Khim, J.S.; Wang, T.; Wan, Y.; Luo, W.; Hu, W.; Jiao, W.; Park, J.; Ryu, J.; Hong, S.; et al. Sources and distribution of polychlorinated-dibenzo-p-dioxins and -dibenzofurans in soil and sediment from the Yellow Sea region of China and Korea. Environ. Pollut. 2011, 159, 907–917. [Google Scholar] [CrossRef]
- Booth, S.; Hui, J.; Alojado, Z.; Lam, V.; Cheung, W.; Zeller, D.; Steyn, D.; Pauly, D. Global deposition of airborne dioxin. Mar. Pollut. Bull. 2013, 75, 182–186. [Google Scholar] [CrossRef]
- Bulle, C.; Samson, R.; Deschênes, L. Enhanced migration of polychlorodibenzo-p-dioxins and furans in the presence of pentachlorophenol-treated oil in soil around utility poles: Screening model validation. Environ. Toxicol. Chem. 2010, 29, 582–590. [Google Scholar] [CrossRef]
- Bergknut, M.; Laudon, H.; Wiberg, K. Dioxins, PCBs, and HCB in soil and peat profiles from a pristine boreal catchment. Environ. Pollut. 2010, 158, 2518–2525. [Google Scholar] [CrossRef]
- Bittner, M.; Hilscherova, K.; Giesy, J.P. In vitro assessment of AhR-mediated activities of TCDD in mixture with humic substances. Chemosphere 2009, 76, 1505–1508. [Google Scholar] [CrossRef]
- Fukushima, M.; Tanabe, Y.; Yabuta, H.; Tanaka, F.; Ichikawa, H.; Tatsumi, K.; Watanabe, A. Water solubility enhancement effects of some polychlorinated organic pollutants by dissolved organic carbon from a soil with a higher organic carbon content. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006, 41, 1483–1494. [Google Scholar] [CrossRef]
- Kim, E.J.; Oh, J.E.; Chang, Y.S. Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil. Sci. Total Environ. 2003, 311, 177–189. [Google Scholar] [CrossRef]
- Nam, J.J.; Gustafsson, O.; Kurt-Karakus, P.; Breivik, K.; Steinnes, E.; Jones, K.C. Relationships between organic matter, black carbon and persistent organic pollutants in European background soils: Implications for sources and environmental fate. Environ. Pollut. 2008, 156, 809–817. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, D.-Y.; Lee, Y.-P.; Li, C.-P.; Chi, K.-H.; Liang, B.-W.P.; Liu, W.-Y.; Wang, C.-C.; Lin, S.; Chen, T.-C.; Yeh, K.-J.C.; et al. Combination of a Fast Cleanup Procedure and a DR-CALUX® Bioassay for Dioxin Surveillance in Taiwanese Soils. Int. J. Environ. Res. Public Health 2014, 11, 4886-4904. https://doi.org/10.3390/ijerph110504886
Lin D-Y, Lee Y-P, Li C-P, Chi K-H, Liang B-WP, Liu W-Y, Wang C-C, Lin S, Chen T-C, Yeh K-JC, et al. Combination of a Fast Cleanup Procedure and a DR-CALUX® Bioassay for Dioxin Surveillance in Taiwanese Soils. International Journal of Environmental Research and Public Health. 2014; 11(5):4886-4904. https://doi.org/10.3390/ijerph110504886
Chicago/Turabian StyleLin, Ding-Yan, Yi-Pin Lee, Chiu-Ping Li, Kai-Hsien Chi, Bo-Wei P. Liang, Wen-Yao Liu, Chih-Cheng Wang, Susana Lin, Ting-Chien Chen, Kuei-Jyum C. Yeh, and et al. 2014. "Combination of a Fast Cleanup Procedure and a DR-CALUX® Bioassay for Dioxin Surveillance in Taiwanese Soils" International Journal of Environmental Research and Public Health 11, no. 5: 4886-4904. https://doi.org/10.3390/ijerph110504886





