Relationship between Obesity, Adipocytokines and Inflammatory Markers in Type 2 Diabetes: Relevance for Cardiovascular Risk Prevention
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
| Characteristic | Group A Obese T2D | Group B Overweight T2D | Group C Lean T2D | Group D Control Subjects |
|---|---|---|---|---|
| Number of subjectsMale/Female | 21 10/11 | 18 10/8 | 25 13/12 | 15 7/8 |
| Duration of T2D (y) 1 | 5.84 ± 0.1 | 7.3 ± 0.2 | 6.5 ± 0.1 | / |
| HbAlc (%) 1 | 6.3 ± 0.7 | 6.6 ± 0.4 | 6.5 ± 0.5 | 4.6 ± 0.4 |
| BMI (kg/ m2) 2 | 33.4 ± 2.8 | 28.3 ± 1.0 | 23.8 ± 1.5 | 22.7 ± 2.9 |
| Waist (cm) 3 Fat mass (kg) 4 | 110.4 ± 9.6 36.09 ± 7.0 | 100.7 ± 6.0 24.5 ± 6.0 | 85.5 ± 6.2 17.2 ± 4.5 | 82.4 ± 7.8 15.7 ± 6.8 |
2.2. Measurement of Anthropometric and Metabolic Characteristics
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Insulin and HOMA-IR Levels
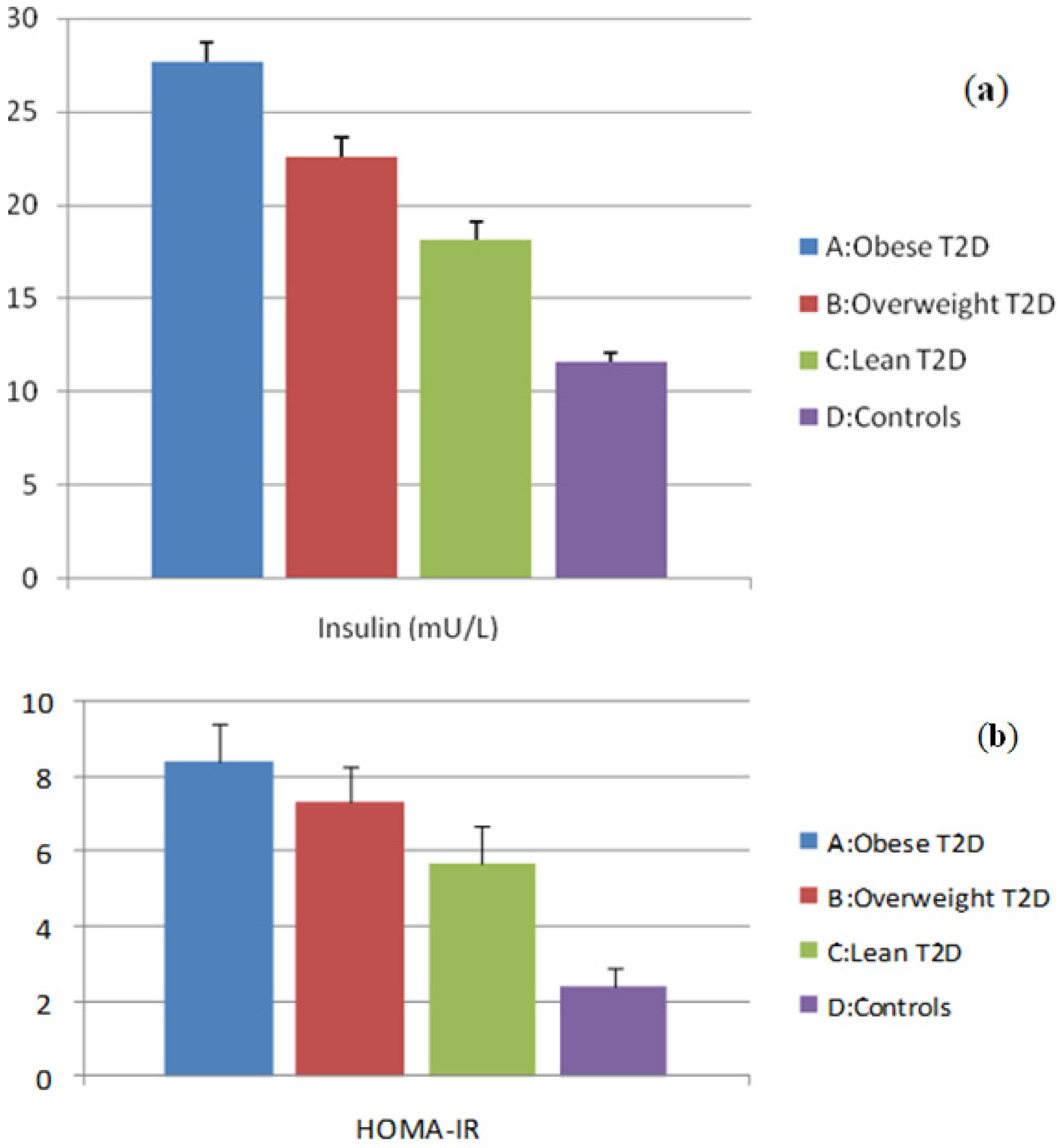
3.2. Evaluation of Adipocytokine Levels

3.3. Evaluation of Inflammatory Marker Levels

3.4. Correlations
| Adipocytokines and Inflammatory Markers | BMI | Waist | Fat Mass | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Adiponectin | −0.53 | ˂0.01 | −0.60 | ˂0.01 | −0.40 | ˂0.01 |
| Leptin | 0.58 | ˂0.01 | 0.35 | ˂0.01 | 0.65 | ˂0.01 |
| Resistin | 0.45 | ˂0.01 | 0.43 | ˂0.01 | 0.41 | ˂0.01 |
| TNF-α | 0.57 | ˂0.01 | 0.55 | ˂0.01 | 0.48 | ˂0.01 |
| IL-6 | 0.57 | ˂0.01 | 0.50 | ˂0.01 | 0.51 | ˂0.01 |
| hsCRP | 0.29 | ˂0.01 | 0.20 | ˂0.05 | 0.32 | ˂0.01 |
| Adipocytokinesand Inflammatory Markers | Insulin | HOMA-IR | ||
|---|---|---|---|---|
| r | p | r | p | |
| Adiponectin | −0.55 | <0.01 | −0.57 | <0.01 |
| Leptin | 0.24 | NS | 0.24 | NS |
| Resistin | 0.49 | <0.01 | 0.51 | <0.01 |
| TNF-α | 0.50 | <0.01 | 0.54 | <0.01 |
| IL-6 | 0.33 | <0.01 | 0.37 | <0.01 |
| hsCRP | 0.31 | <0.01 | 0.33 | <0.01 |
| Inflammatory Markers | Adiponectin | Leptin | Resistin | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| TNF-α | −0.34 | <0.01 | 0.08 | NS | 0.26 | <0.05 |
| IL-6 | −0.10 | NS | 0.35 | <0.01 | 0.34 | ˂0.01 |
| hsCRP | −0.09 | NS | 0.43 | <0.01 | 0.49 | NS |
3.5. Discussion
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Eeg-Olofsson, K.; Cederholm, J.; Nilsson, P.M.; Zethelius, B.; Nunez, L.; Gudbjörnsdóttir, S.; Eliasson, B. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: An observational study in 13,087 patients. Diabetologia 2009, 52, 65–73. [Google Scholar] [CrossRef]
- Kiadaliri, A.; Clarke, P.M.; Gerdtham, U.G.; Nilsson, P.; Eliasson, B.; Gudbjörnsdottir, S.; Steen, K.A. Predicting changes in cardiovascular risk factors in type 2 diabetes in the post-UKPDS era: Longitudinal analysis of the Swedish national diabetes register. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef]
- Gallagher, R.; Kirkness, A.; Zelestis, E.; Hollams, D.; Kneale, C.; Armari, E.; Bennett, T.; Daly, J.; Tofler, G. A randomised trial of a weight loss intervention for overweight and obese people diagnosed with coronary heart disease and/or type 2 diabetes. Ann. Behav. Med. 2012, 44, 119–128. [Google Scholar]
- Espeland, M.A.; Rejeski, W.J.; West, D.S.; Action for Health in Diabetes Research Group. Intensive weight loss intervention in older individuals: Results from the action for health in diabetes type 2 diabetes mellitus trial. J. Amer. Geriat. Soc. 2013, 61, 912–922. [Google Scholar] [CrossRef]
- Hwang, C.L.; Wu, Y.T.; Chou, C.H. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: A meta-analysis. J. Cardiopulm. Rehabil. Prev. 2011, 31, 378–385. [Google Scholar] [CrossRef]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Ouchi, N.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Walsh, K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003, 14, 561–566. [Google Scholar] [CrossRef]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int. J. Obesity 2010, 34, 165–171. [Google Scholar] [CrossRef]
- Brennan, A.M.; Li, T.Y.; Kelesidis, I.; Gavrila, A.; Hu, F.B.; Mantzoros, C.S. Circulating leptin levels are not associated with cardiovascular morbidity and mortality in women with diabetes: A prospective cohort study. Diabetologia 2007, 50, 1178–1185. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Hartge, M.M.; Unger, T.; Kontscher, U. The endothelium and vascular inflammation in diabetes. Diab. Vasc. Res. 2007, 4, 84–88. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, J.; Li, H.; Yu, Z.; Ye, X.; Hu, F.B.; Franco, O.H.; Pan, A.; Liu, Y.; Lin, X. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur. J. Endocrinol. 2008, 159, 585–593. [Google Scholar] [CrossRef]
- Wallace, A.M.; McMahon, A.D.; Packard, C.J.; Kelly, A.; Shepherd, J.; Gaw, A.; Sattar, N.; The WOSCOPS Executive Committee. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 2001, 104, 3052–3056. [Google Scholar] [CrossRef]
- Wolk, R.; Berger, P.; Lennon, R.J.; Brilakis, E.S.; Johnson, B.D.; Somers, V.K. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J. Amer. Coll. Cardiol. 2004, 44, 1819–1824. [Google Scholar] [CrossRef]
- Vozarova de Courten, B.; Degawa-Yamauchi, M.; Considine, R.V.; Tataranni, P.A. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 2004, 53, 1279–1284. [Google Scholar] [CrossRef]
- Azuma, K.; Katsukawa, F.; Oguchi, S.; et al. Correlation between serum resistin level and adiposity in obese individuals. Obesity 2003, 11, 997–1001. [Google Scholar] [CrossRef]
- Youn, B.S.; YU, K.Y.; Park, H.J.; Roberts, C.K.; Manson, J.A.E.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2004, 89, 150–156. [Google Scholar] [CrossRef]
- McTernan, P.G.; Fisher, F.M.; Valsamakis, G.; Chetty, R.; Harte, A.; MCternan, C.L.; Clark, P.M.S.; Smith, S.A.; Barnett, A.H.; Kumars, S. Resistin and type 2 diabetes: Regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J. Clin. Endocrinol. Metab. 2003, 88, 6098–6106. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Rood, J.; Janderova, L.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Smith, S.R. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848. [Google Scholar] [CrossRef]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef]
- Devaraj, S.; Singh, U.; Jialal, I. The evolving role of C-reactive protein in atherothrombosis. Clin. Chem. 2009, 55, 229–238. [Google Scholar] [CrossRef]
- Bastard, J.P.; Maachi, M.; van Nhieu, T.J.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.J.; Capeau, J.; Hainque, B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol Metab. 2002, 87, 2084–2089. [Google Scholar] [CrossRef]
- Pannacciulli, N.; Cantatore, F.P.; Minenna, A.; Bellacicco, M.; Giorgino, R.; de pergola, G. C–reactive protein is independetly associated with total body fat and insulin resistance in adult women. Int. J. Obesity 2001, 25, 1461–1420. [Google Scholar]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Sola, E.; Jover, A.; López-Ruiz, A.; Jarabo, M.; Vayá, A.; Morillas, C.; Gómez-Balaguer, M.; Hernández-Mijares, A. Parameters of inflammation in morbid obesity: Lack of effect of moderate weight loss. Obes. Surg. 2009, 19, 571–576. [Google Scholar] [CrossRef]
- Bastard, J.P.; Jardel, C.; Bruckert, E.; Blondy, P.; Capeau, J.; Laville, M.; Vidal, H.; Hainque, B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol Metab. 2000, 85, 3338–3342. [Google Scholar]
- Ziccardi, P.; Nappo, F.; Giugliano, G.; Esposito, K.; Marfella, R.; Cioffi, M.; D’Andrea, F.; Molinari, A.M.; Giugliano, D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002, 105, 804–809. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar]
- Skalicky, J.; Muzakova, V.; Kandar, R.; Meloun, M.; Rousar, T.; Palicka, V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin. Chem. Lab. Med. 2008, 46, 499–505. [Google Scholar]
- Olson, C.N.; Callas, W.P.; Hanley, A.J.G.; Festa, A.; Haffner, S.M.; Wagenknecht, L.E.; Tracy, R.P. Circulating levels od TNF are associated with impaired glucose tolerance, icreased insulin reistance, and ethinicity: The insulin resistance atherosclerosis study. J. Clin. Endocrinol. Metab. 2012, 97, 1032–1040. [Google Scholar] [CrossRef]
- Bahceci, M.; Gokalp, D.; Bahceci, S.; Tuzcu, A.; Atmaca, S.; Arikan, S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high-sensitivity C-reactive protein levels. Is adipocyte size associated with the inflammation in adults? J. Endocrinol. Invest. 2007, 30, 210–214. [Google Scholar]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.G.; de Faire, U.; Nilsson, J.; Eriksson, P.; Hamsten, A. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- National Committee for the Clinical Guidelines for Good Clinical Practice. Group for Diabetes. Nacionalni Vodič Dobre Kliničke Prakse Diabetes Mellitus; Agencija za Akreditaciju Zdravstvenih Ustanova Srbije: Beograd, Serbia, 2012. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S. Homeostasis model assessment: Insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 13, 412–419. [Google Scholar] [CrossRef]
- Goto, M.; Goto, A.; Morita, A; Deura, K.; Sasaki, S.; Aiba, N.; Shimbo, T.; Terauchi, Y.; Miyachi, M.; Noda, M.; Watanabe, S.; Saku Cohort Study Group. Low-molecular-weight adiponectin and high-molecular-weight adiponectinlevels in relation to diabetes. Obesity 2013, 1. [Google Scholar] [CrossRef]
- Hasegawa, G.; Ohta, M.; Ichida, Y.; Obayashi, H.; Shigeta, M.; Yamasaki, M.; Fukui, M.; Yoshikawa, T.; Nakamura, N. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetol. 2005, 42, 104–109. [Google Scholar] [CrossRef]
- Hansen, D.; Dendale, P.; Beelen, M.; Jonkers, R.A.; Mullens, A.; Corluy, L.; Meeusen, R.; van Loon, L.J. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur. J. Appl. Physiol. 2010, 109, 397–404. [Google Scholar] [CrossRef]
- Marita, A.R.; Sarkar, J.A.; Rane, S. Type 2 diabetes in non-obese Indian subjects is associated with reduced leptin levels: Study from Mumbai, western India. Mol. Cell. Biochem. 2005, 275, 143–151. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.L.; Zhang, J.; Yu, H.Y.; Wang, H.W.; Zhang, M.X.; Cianflone, K. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs. obese type 2 diabetes. Int. J. Obesity 2006, 30, 439–446. [Google Scholar] [CrossRef]
- Gao, H.; Fall, T.; van Dam, R.M.; Flyvbjerg, A.; Zethelius, B.; Ingelsson, E.; Hägg, S. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: A Mendelian randomization study. Diabetes 2013, 62, 1338–1344. [Google Scholar] [CrossRef]
- Abdelgadir, M.; Karlsson, A.F.; Berglund, L.; Berne, C. Low serum adiponectin concentrations are associated with insulin sensitivity independent of obesity in Sudanese subjects with type 2 diabetes mellitus. Diabetol. Metab. Synd. 2013, 5((1)), 15. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Khan, A.K.A.; Mahtab, H.; Ahsan, K.A.; Banu, A.; Khanam, P.A.; Ahrén, B. Leptin is reduced in lean subjects with type 2 diabetes in Bangladesh. Diabetes Care 2003, 26. [Google Scholar] [CrossRef]
- Mohammadzadeh, G.; Zarghami, N. Serum leptin level is reduced in non-obese subjects with type 2 diabetes. Int. J. Endocrinol. Metab. 2013, 11, 3–10. [Google Scholar]
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, 17–36. [Google Scholar] [CrossRef]
- Morton, G.J.; Gelling, R.W.; Niswender, K.D.; Morrison, C.D.; Rhodes, C.J.; Schwartz, M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell. Metab. 2005, 2, 411–420. [Google Scholar] [CrossRef]
- German, P.J.; Wisse, B.E.; Thaler, J.P.; Oh-I, S.; Sarruf, D.A.; Ogimoto, K.; Kaiyala, K.J.; Fischer, J.D.; Matsen, M.E.; Taborsky, G.J., Jr.; Schwartz, M.W.; Morton, G.J. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes 2010, 59, 1626–1634. [Google Scholar] [CrossRef]
- Chanchay, S.; Tungtrongchitr, R.; Harnroongroj, T.; Phonrat, B.; Rungseesakorn, O.; Paksanont, S.; Pooudong, S.; Saowakontha, S.; Varongchayakul, C. Plasma resistin, insulin concentration in non-diabetic and diabetic, overweight/obese Thai. Int. J. Vitam. Nutr. Res. 2006, 76, 125–131. [Google Scholar] [CrossRef]
- Lee, J.H.; Chan, J.L.; Yiannakouris, N.; Kontogianni, M.; Estrada, E.; Seip, R.; Orlova, C.; Mantzoros, C.S. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: Cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J. Clin. Endocrinol. Metab. 2003, 88, 4848–4856. [Google Scholar] [CrossRef]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.L.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef]
- Al Harithy, R.N.; Al Ghamdi, S. Serum resistin, adiposity and insulin resistance in Saudi women with type 2 diabetes mellitus. Ann. Saudi Med. 2005, 25, 283–287. [Google Scholar]
- Nilsson, J.; Jovinge, S.; Niemann, A.; Reneland, R.; Lithell, H. Relationship between plasma tumor necrosis factor α and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1199–1202. [Google Scholar] [CrossRef]
- Katsuki, A.; Sumida, Y.; Murashima, S.; Murata, K.; Takarada, Y.; Ito, K.; Fujii, M.; Tsuchihashi, K.; Goto, H.; Nakatani, K.; Yano, Y. Serum levels of tumor necrosis factor-α are increased in obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1998, 83, 859–862. [Google Scholar]
- Zinman, B.; Hanley, A.J.G.; Harris, S.B.; Kwan, J.; Fantus, I.G. Circulating tumor necrosis factor-αconcentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 1999, 84, 272–278. [Google Scholar]
- Shamsuzzaman, A.S.; Winnicki, M.; Wolk, R.; Svatikova, A.; Phillips, B.G.; Davison, D.E.; Berger, P.B.; Somers, V.K. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation 2004, 109, 2181–2185. [Google Scholar] [CrossRef]
- Van Dielen, F.M.; van’t Veer, C.; Schols, A.M.; Soeters, P.B.; Buurman, W.A.; Greve, J.W. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1759–1766. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Salvador, J.; Paramo, J.A.; Orbe, J.; de Irala, J.; Diez-Caballero, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Involvement of leptin in the association between percentage of body fat and cardiovascular risk factors. Clin. Biochem. 2002, 35, 315–320. [Google Scholar] [CrossRef]
- Gomez-Ambrosi, J.; Salvador, J.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Leptin therapy does not affect inflammatory markers. J. Clin. Endocrinol. Metab. 2005, 90, 3803. [Google Scholar]
- Shetty, G.K.; Economides, P.A.; Horton, E.S.; Mantzoros, C.S.; Veves, A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 2004, 27, 2450–2457. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rajkovic, N.; Zamaklar, M.; Lalic, K.; Jotic, A.; Lukic, L.; Milicic, T.; Singh, S.; Stosic, L.; Lalic, N.M. Relationship between Obesity, Adipocytokines and Inflammatory Markers in Type 2 Diabetes: Relevance for Cardiovascular Risk Prevention. Int. J. Environ. Res. Public Health 2014, 11, 4049-4065. https://doi.org/10.3390/ijerph110404049
Rajkovic N, Zamaklar M, Lalic K, Jotic A, Lukic L, Milicic T, Singh S, Stosic L, Lalic NM. Relationship between Obesity, Adipocytokines and Inflammatory Markers in Type 2 Diabetes: Relevance for Cardiovascular Risk Prevention. International Journal of Environmental Research and Public Health. 2014; 11(4):4049-4065. https://doi.org/10.3390/ijerph110404049
Chicago/Turabian StyleRajkovic, Natasa, Miroslava Zamaklar, Katarina Lalic, Aleksandra Jotic, Ljiljana Lukic, Tanja Milicic, Sandra Singh, Ljubica Stosic, and Nebojsa M. Lalic. 2014. "Relationship between Obesity, Adipocytokines and Inflammatory Markers in Type 2 Diabetes: Relevance for Cardiovascular Risk Prevention" International Journal of Environmental Research and Public Health 11, no. 4: 4049-4065. https://doi.org/10.3390/ijerph110404049




