Monitoring the Transmission of Schistosoma japonicum in Potential Risk Regions of China, 2008 – 2012
Abstract
:1. Introduction
2. Methods
2.1. Study Areas
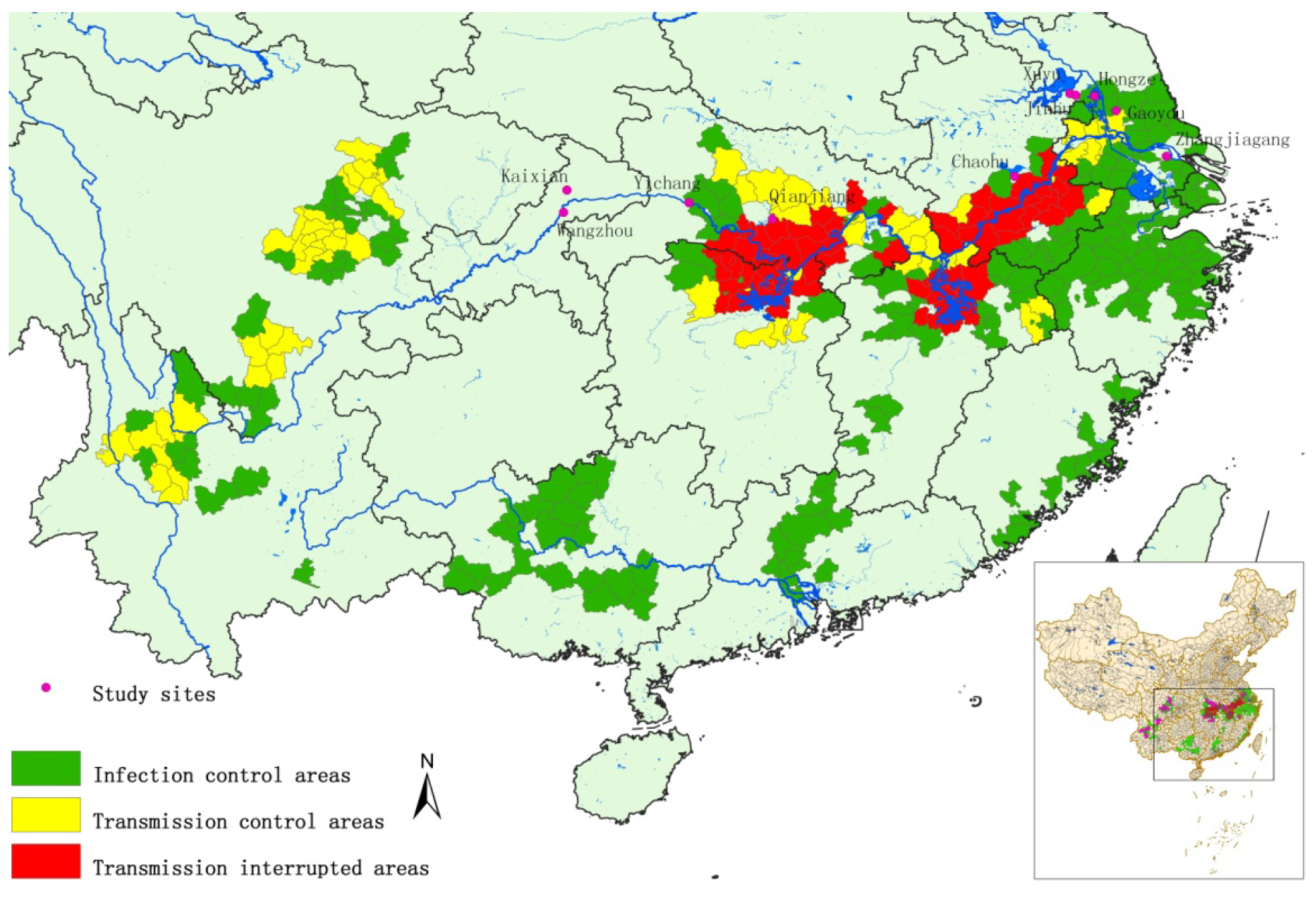
2.2. Monitoring S. japonicum Infection in Humans and Livestock
2.3. Snail Surveillance
2.4. Monitoring Snail Survival and Reproduction
2.5. Statistical Analysis
3. Results
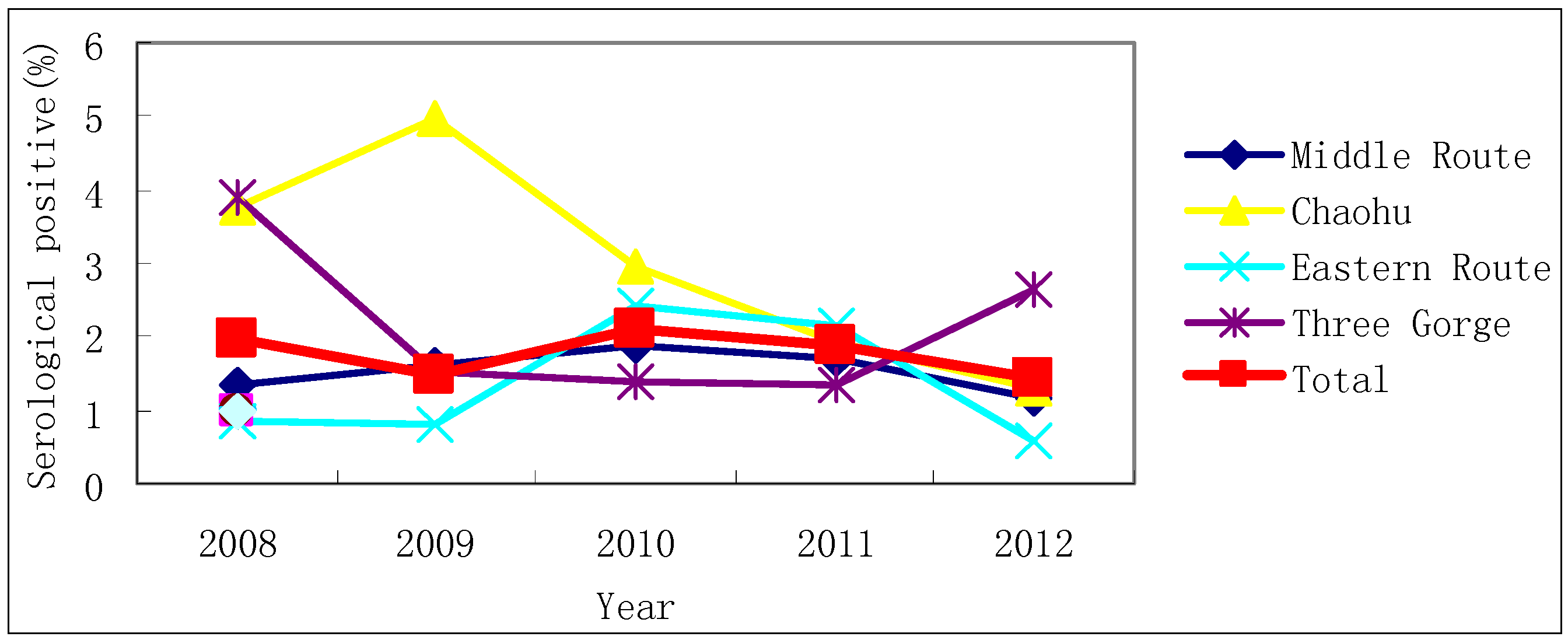
3.1. S. japonicum Infection in Humans and Livestock
| Province | County | Serological Test, 2008 | Serological Test, 2012 | ||||
|---|---|---|---|---|---|---|---|
| No. detected | No. positive | Positive rate (%) | No. detected | No. positive | Positive rate (%) | ||
| Anhui | Chaohu | 301 | 0 | 0 | 314 | 0 | 0 |
| Chongqing | Wanzhou | 501 | 5 | 1 | 501 | 2 | 0.4 |
| Kaixian | 500 | 18 | 3.6 | 300 | 4 | 1.33 | |
| Hubei | Qianjiang | 2,275 | 26 | 1.14 | 313 | 3 | 0.96 |
| Yichang | 101 | 0 | 0 | 303 | 1 | 0.33 | |
| Jiangsu | Xuyu | 1,035 | 6 | 0.58 | 638 | 5 | 0.78 |
| Hongze | 514 | 3 | 0.58 | 555 | 5 | 0.9 | |
| Jinhu | 1,539 | 0 | 0 | 1,021 | 5 | 0.49 | |
| Gaoyou | 671 | 0 | 0 | 494 | 0 | 0 | |
| Zhangjianggang | - | - | - | 500 | 0 | 0 | |
| Total | 7,437 | 58 | 0.78 | 4,939 | 25 | 0.51 | |
3.2. Snail Status
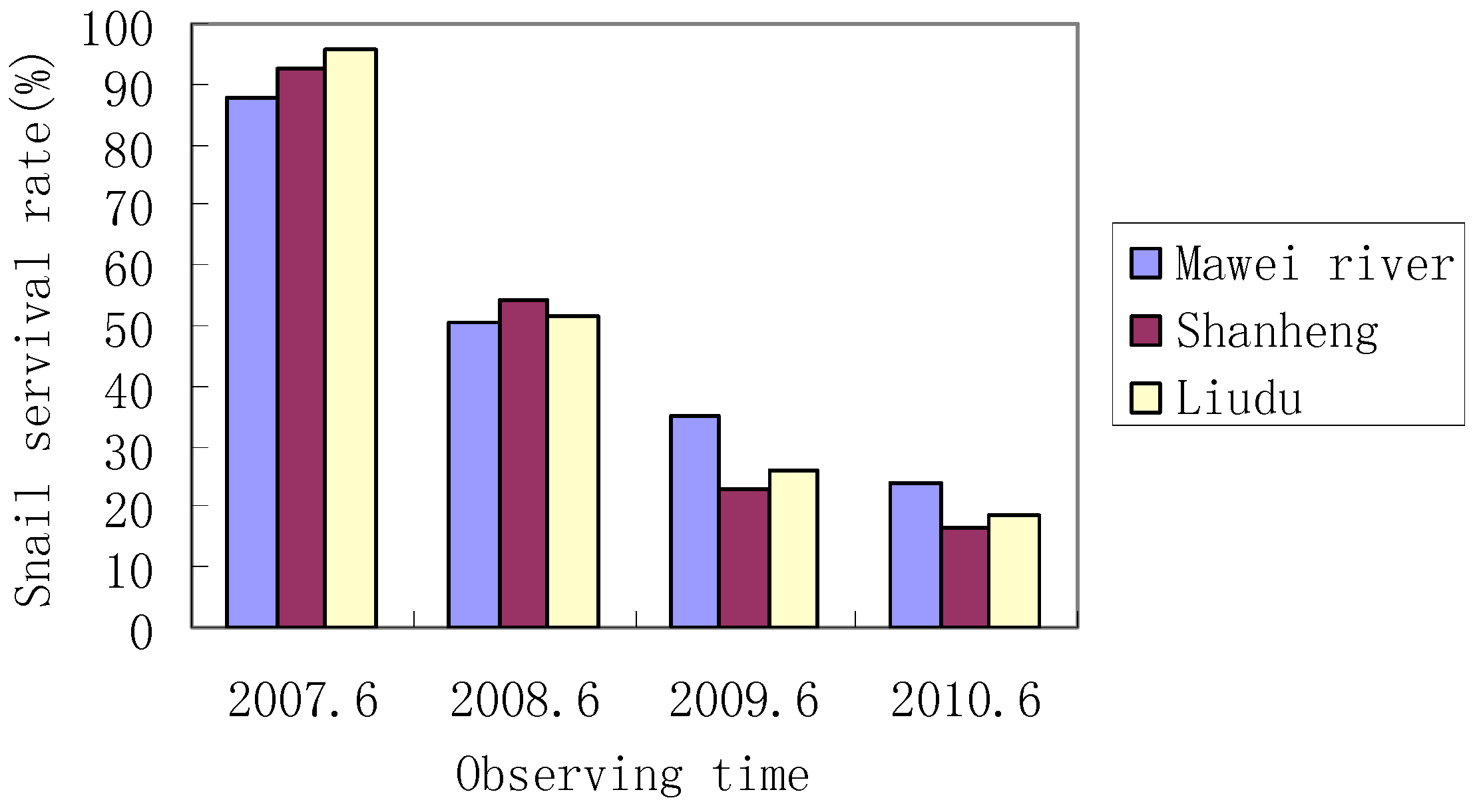
3.3. Snail Survival and Reproduction
| Snail population | April | June | August | October | December | Next February |
|---|---|---|---|---|---|---|
| Nanjing field-derived snails | 60 | 60 | 60 | 800 | 1,334 | 1,610 |
| Laboratory offspring snails | 60 | 60 | 60 | 911 | 1,326 | 1,620 |
| Site | 2007–2006 | 2008–2006 | 2009–2006 | 2010–2006 |
|---|---|---|---|---|
| Mawei River | 100 | 272 | 411 | 396 |
| Shanheng | 100 | 256 | 346 | 374 |
| Liudu | 100 | 289 | 387 | 412 |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X.N.; Bergquist, R.; Leonardo, L.; Yang, G.J.; Yang, K.; Sudomo, M.; Olveda, R. Schistosomiasis japonica control and research needs. Adv. Parasitol. 2010, 72, 145–178. [Google Scholar] [CrossRef]
- Wang, L.; Utzinger, J.; Zhou, X.N. Schistosomiasis control: Experiences and lessons from China. Lancet 2008, 372, 1793–1795. [Google Scholar] [CrossRef]
- Utzinger, J.; Zhou, X.N.; Chen, M.G.; Bergquist, R. Conquering schistosomiasis in China: The long march. Acta Trop. 2005, 96, 69–96. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.J.; Dong, H.F.; Jiang, M.S.; Zhu, H.G. Transmission control of Schistosomiasis japonica: Implementation and evaluation of different snail control interventions. Acta Trop. 2005, 96, 191–197. [Google Scholar] [CrossRef]
- Ohmae, H.; Iwanaga, Y.; Nara, T.; Matsuda, H.; Yasuraoka, K. Biological characteristics and control of intermediate snail host of Schistosoma japonicum. Parasitol Int. 2003, 52, 409–417. [Google Scholar] [CrossRef]
- Zhou, X.N.; Guo, J.G.; Wu, X.H.; Jiang, Q.W.; Zheng, J.; Dang, H.; Wang, X.H.; Xu, J.; Zhu, H.Q.; Wu, G.L.; et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg. Infect. Dis. 2007, 13, 1470–1476. [Google Scholar] [CrossRef]
- Wang, W.; Dai, J.R.; Liang, Y.S.; Huang, Y.X.; Coles, G.C. Impact of the South-to-North Water Diversion Project on the transmission of Schistosoma japonicum in China. Ann. Trop. Med. Parasitol. 2009, 103, 17–29. [Google Scholar]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Tanner, M.; Utzinger, J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia 2005, 47, 127–134. [Google Scholar]
- Zheng, J.; Gu, X.G.; Xu, Y.L.; Ge, J.H.; Yang, X.X.; He, C.H.; Tang, C.; Cai, K.P.; Jiang, Q.W.; Liang, Y.S.; et al. Relationship between the transmission of Schistosomiasis japonica and the construction of the Three Gorge Reservoir. Acta Trop. 2002, 82, 147–156. [Google Scholar] [CrossRef]
- Zhu, H.M.; Xiang, S.; Yang, K.; Wu, X.H.; Zhou, X.N. Three Gorges Dam and its impact on the potential transmission of Schistosomiasis in regions along the Yangtze River. Ecohealth 2008, 5, 137–148. [Google Scholar] [CrossRef]
- Seto, E.Y.; Wu, W.; Liu, H.Y.; Chen, H.G.; Hubbard, A.; Holt, A.; Davis, G.M. Impact of changing water levels and weather on Oncomelania hupensis populations, the snail host of Schistosoma japonicum, downstream of the Three Gorges Dam. Ecohealth 2008, 5, 149–158. [Google Scholar] [CrossRef]
- Sutherst, R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef]
- Zhou, X.N.; Yang, G.J.; Yang, K.; Wang, X.H.; Hong, Q.B.; Sun, L.P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on Schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar]
- Liang, Y.S.; Wang, W.; Li, H.J.; Shen, X.H.; Xu, Y.L.; Dai, J.R. The South-to-North Water Diversion Project: Effect of the water diversion pattern on transmission of Oncomelania hupensis, the intermediate host of Schistosoma japonicum in China. Parasit. Vect. 2012. [Google Scholar] [CrossRef]
- Cao, Z.G.; Wang, T.P.; Wu, W.D.; Zhang, S.Q.; Lv, D.B.; Fang, G.R.; Zhao, F.; Ling, X.S.; Sha, J.J.; Wang, F.F.; et al. Potential impact of water transfer project from Yangtze River to Huaihe River on snail spread and Schistosomiasis transmission. Chin. J. Parasitol. Parasit. Dis. 2007, 25, 385–389. [Google Scholar]
- Zhu, H.; Yuan, Y.; Xu, X.J. Risk assessment of the Water Transfer Project from the Yangtze River to Han River in middle scheme of the South-to-North Water Diversion Project on Schistosomiasis transmission and intervention measures. Chin. J. Schisto. Control 2010, 22, 415–419. [Google Scholar]
- Zhou, Y.B.; Yang, M.X.; Tao, P.; Jiang, Q.L.; Zhao, G.M.; Wei, J.G.; Jiang, Q.W. A longitudinal study of comparison of the Kato-Katz technique and Indirect Hemagglutination Assay (IHA) for the detection of Schistosomiasis japonica in China, 2001–2006. Acta Trop. 2008, 107, 251–254. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar]
- Yu, J.M.; de Vlas, S.J.; Jiang, Q.W.; Gryseels, B. Comparison of the Kato-Katz technique, hatching test and Indirect Hemagglutination Assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 2007, 56, 45–49. [Google Scholar] [CrossRef]
- Poda, J.N.; Sondo, B.; Parent, G. Impact of water resource installations on the distribution of Schistosomiasis and its intermediary hosts in Burkina Faso. Sante 2003, 13, 49–53. [Google Scholar]
- Ghebreyesus, T.A.; Witten, K.H.; Getachew, A.; Haile, M.; Yohannes, M.; Lindsay, S.W.; Byass, P. Schistosome transmission, water-resource development and altitude in northern Ethiopia. Ann. Trop. Med. Parasitol. 2002, 96, 489–495. [Google Scholar] [CrossRef]
- Sow, S.; de Vlas, S.J.; Engels, D.; Gryseels, B. Water-related disease patterns before and after the construction of the Diama dam in northern Senegal. Ann. Trop. Med. Parasitol. 2002, 96, 575–586. [Google Scholar] [CrossRef]
- Kloos, H. Water resources development and Schistosomiasis ecology in the Awash Valley, Ethiopia. Soc. Sci. Med. 1985, 20, 609–625. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human Schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Yang, G.J.; Vounatsou, P.; Tanner, M.; Zhou, X.N.; Utzinger, J. Remote sensing for predicting potential habitats of Oncomelania hupensis in Hongze, Baima and Gaoyou lakes in Jiangsu province, China. Geospat. Health 2006, 1, 85–92. [Google Scholar]
- Lin, D.D.; Liu, J.X.; Liu, Y.M.; Hu, F.; Zhang, Y.Y.; Xu, J.M.; Li, J.Y.; Ji, M.J.; Bergquist, R.; Wu, G.L.; et al. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: A case study in an endemic area of the People’s Republic of China. Parasitol. Int. 2008, 57, 281–286. [Google Scholar] [CrossRef]
- Zheng, Q.; Vanderslott, S.; Jiang, B.; Xu, L.L.; Liu, C.S.; Huo, L.L.; Duan, L.P.; Wu, N.B.; Li, S.Z.; Xia, Z.G.; et al. Research gaps for three main tropical diseases in the People’s Republic of China. Infect. Dis. Poverty 2013, 2. [Google Scholar] [CrossRef]
- Ross, A.G.; Olveda, R.M.; Acosta, L.; Harn, D.A.; Chy, D.; Li, Y.; Gray, D.J.; Gordon, C.A.; McManus, D.P.; Williams, G.M. Road to the elimination of Schistosomiasis from Asia: The journey is far from over. Microbes Infect. 2013, 15, 858–865. [Google Scholar] [CrossRef]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuenté, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for Schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef]
- Zhou, X.N.; Bergquist, R.; Tanner, M. Elimination of tropical disease through surveillance and response. Infect. Dis. Poverty 2013. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dang, H.; Xu, J.; Li, S.-Z.; Cao, Z.-G.; Huang, Y.-X.; Wu, C.-G.; Tu, Z.-W.; Zhou, X.-N. Monitoring the Transmission of Schistosoma japonicum in Potential Risk Regions of China, 2008 – 2012. Int. J. Environ. Res. Public Health 2014, 11, 2278-2287. https://doi.org/10.3390/ijerph110202278
Dang H, Xu J, Li S-Z, Cao Z-G, Huang Y-X, Wu C-G, Tu Z-W, Zhou X-N. Monitoring the Transmission of Schistosoma japonicum in Potential Risk Regions of China, 2008 – 2012. International Journal of Environmental Research and Public Health. 2014; 11(2):2278-2287. https://doi.org/10.3390/ijerph110202278
Chicago/Turabian StyleDang, Hui, Jing Xu, Shi-Zhu Li, Zhi-Guo Cao, Yi-Xin Huang, Cheng-Guo Wu, Zu-Wu Tu, and Xiao-Nong Zhou. 2014. "Monitoring the Transmission of Schistosoma japonicum in Potential Risk Regions of China, 2008 – 2012" International Journal of Environmental Research and Public Health 11, no. 2: 2278-2287. https://doi.org/10.3390/ijerph110202278




