Histological Changes in Gills of Two Fish Species as Indicators of Water Quality in Jansen Lagoon (São Luís, Maranhão State, Brazil)
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
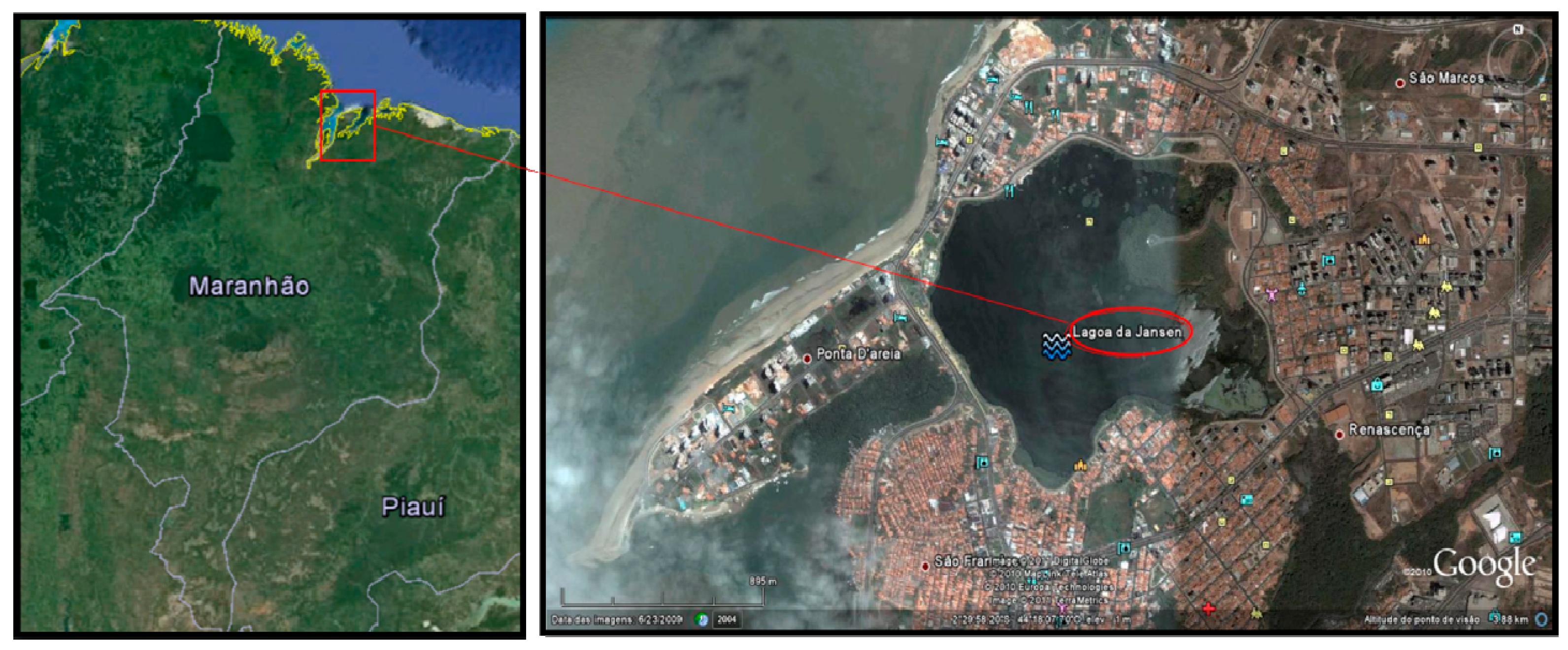
2.2. Fish Sampling
2.3. Histopathological Analysis
2.4. Microbiological Analysis of Water
3. Results and Discussion
3.1. Assessment of Microbiological Parameters of the Lagoon Water
| Collection/Period | Samples | Thermotolerant Coliforms |
|---|---|---|
| 1st/Dry | 2 | ≥1600/mL |
| 2nd/Dry | 2 | ≥1600/mL |
| 3rd/Rainy | 2 | ≥1600/mL |
| 4th/Rainy | 2 | ≥1600/mL |
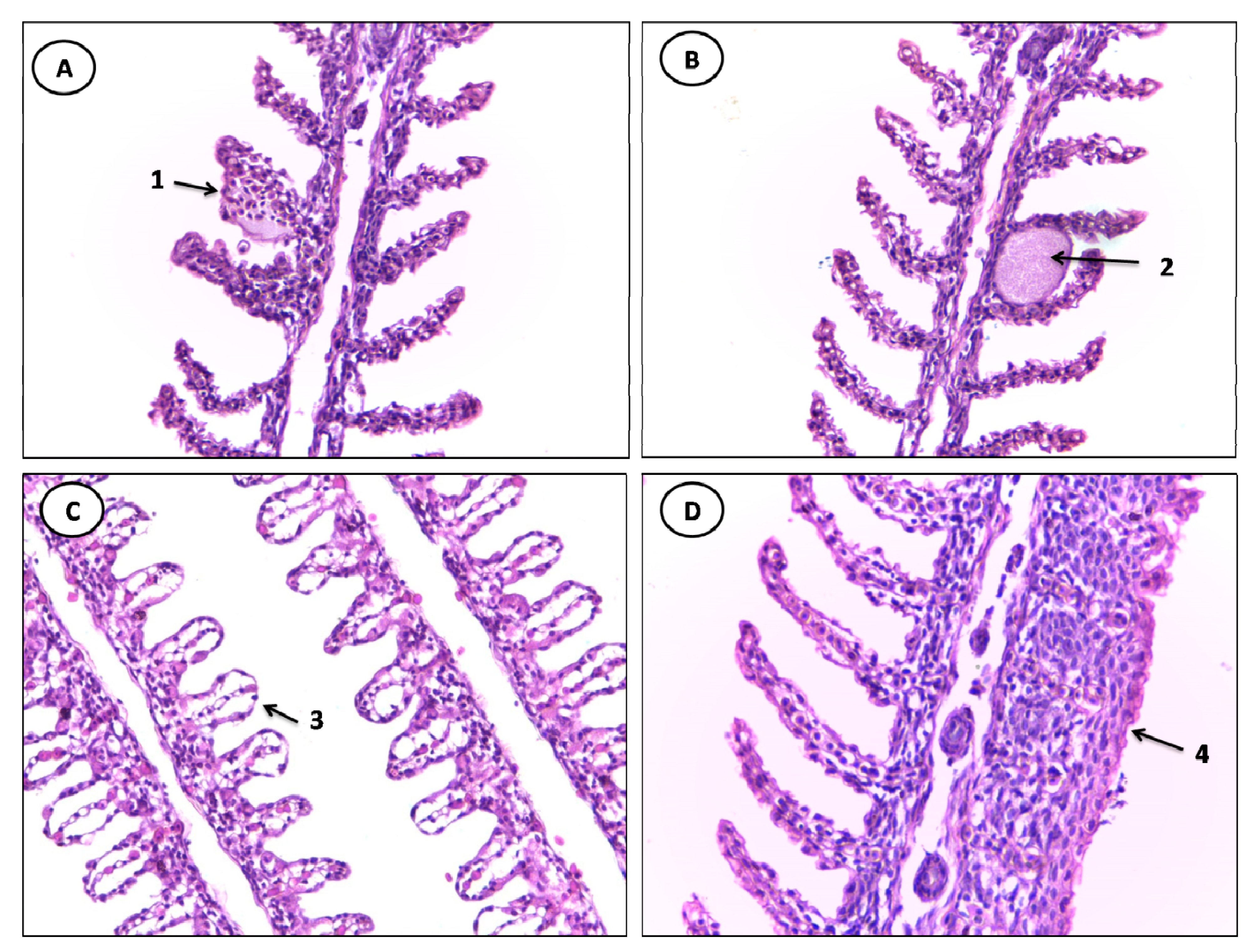
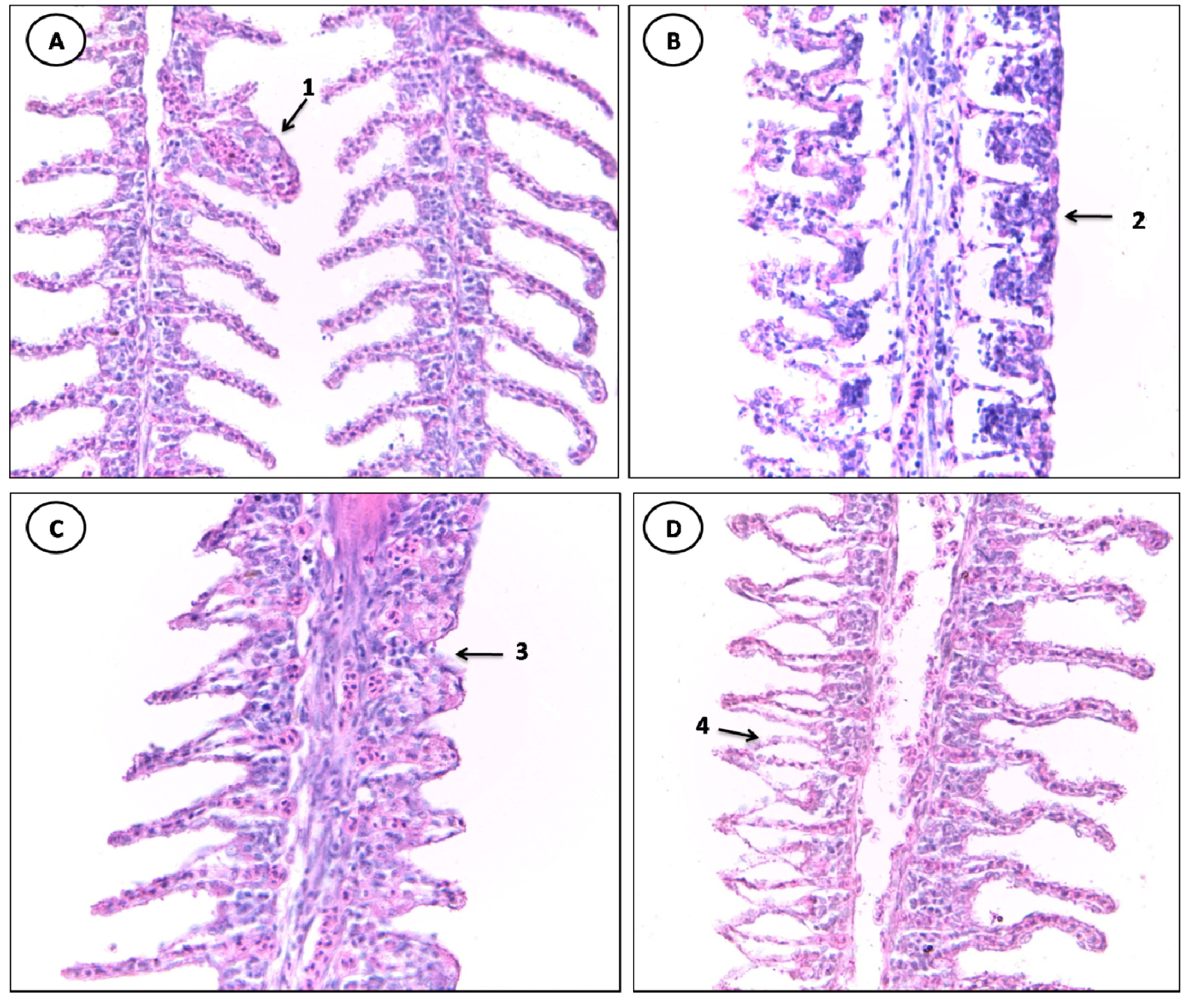
3.2. Histopathological Evaluation

| Stage I | Stage II |
|---|---|
| Lifting of the respiratory epithelium | Hemorrhage and rupture of the lamellar epithelium |
| Congestion of blood vessels | Aneurysm |
| Lamellar Disorganization | -- |
| Hyperplasia of the lamellar epithelium | -- |
| hypertrophy of the respiratory epithelium | -- |
| Incomplete and complete fusion of several lamellae Parasite | -- |
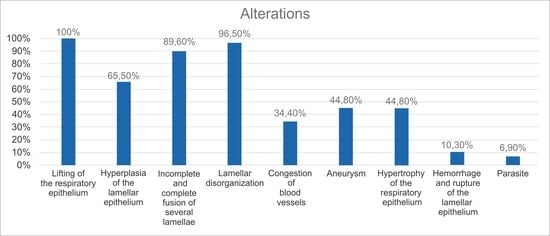
3.3. Gill Alterations
| Alterations | Species | Total | |
|---|---|---|---|
| Sardinella sp | Centropomus undecimalis | ||
| Lifting of the respiratory epithelium | 18/18 (100%) | 11/11 (100%) | 29/29 (100%) |
| Hyperplasia of the lamellar epithelium | 9/18 (50%) | 10/11 (90.9%) | 19/29 (65.5%) |
| Incomplete and complete fusion of several lamellae | 16/18 (88.8%) | 10/11 (90.9%) | 26/29 (89.6%) |
| Lamellar disorganization | 18/18 (100%) | 10/11 (90.9%) | 28/29 (96.5%) |
| Congestion of blood vessels | 8/18 (44.4%) | 2/11 (18.1%) | 10/29 (34.4%) |
| Aneurysm | 11/18 (61.1%) | 2/11 (18.1%) | 13/29 (44.8%) |
| Hypertrophy of the respiratory epithelium | 11/18 (61.1%) | 2/11 (18.1%) | 13/29 (44.8%) |
| Hemorrhage and rupture of the lamellar epithelium | 3/18 (16.6%) | 0/11 (0%) | 3/29 (10.3%) |
| Parasite | 2/18 (11.1%) | 0/11 (0%) | 2/29 (6.9%) |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lins, J.A.P.N.; Kirschnik, P.G; Queiroz, V.S.; Cirio, S.M. Uso de peixes como biomarcadores para monitoramento ambiental aquático. Rev. Acad. Ciên. Agrár. Ambient. 2010, 8, 469–484. [Google Scholar]
- Ramade, F. Dictionaire Encyclopédique Des Sciences De L’ Eau, 2nd ed.; Ediscience International: Paris, France, 1998. [Google Scholar]
- Depledge, M.H. Conceptual paradigms in marine ecotoxicology. In Proceedings of the 12 TH Baltic Marine Biologists Symposium, Helsingor, Denmark; Bjornstad, E., Hagerman, L., Jensen, K., Eds.; Fredensborg, Olsen & Olsen, 1992; pp. 47–52. [Google Scholar]
- Hinton, D.E. Histopathological biomarkers. In Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress; Huggett, R.J., Kimerli, R.A., Mehrle, P.M., Bergman, H.L., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 155–210. [Google Scholar]
- Poleksic, V.; Mitrovic-Tutundzic, V. Fish gills as a monitor of sublethal and chronic effects of pollution. In Sblethal and Chronic Effects of Pollutants on Freshwater Fish; Muller, R., Lloyd, R., Eds.; Oxford, Fishing News Books: Osney, England, 1994; pp. 339–352. [Google Scholar]
- Arellano, J.M.; Storch, V.; Sarasquete, C. Histological changes and copper accumulation in liver and gills of the Senegales Sole, Solea senegalensis. Ecotoxicol. Environ. Safety 1999, 44, 62–72. [Google Scholar]
- Cerqueira, C.C.C.; Fernandes, M.N. Gill tissue recovery copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol. Environ. Safety 2002, 52, 83–91. [Google Scholar]
- Pacheco, M.; Santos, M.A. Biotransformation, genotoxic and histopathological effects of environmental contaminants in European EEL (Anguilla anguilla L.). Ecotoxicol. Environ. Safety 2002, 53, 331–347. [Google Scholar]
- Koneman, E.W.; Allen, S.D.; Janda, W.M.; Schreckenberger, M.P.C.; Winn, W.C., Jr. Diagnostic Microbiology: Text and Color Atlas, 5th ed.; Medsi: São Paulo, Brazil, 2001. [Google Scholar]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill Book Company: New York, NY, USA, 1968; pp. 53–54. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21th ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Resolução No. 357, 17 de Março 2005. Available online: http://www.mma.gov.br/port/conama/res/res05/res35705.pdf (accessed on 10 December 2014).
- Laudo Geoambiental e Biológico para Reavaliação do Parque Ecológico da Lagoa da Jansen. Available online: http://www.sema.ma.gov.br/pdf/Laudo%20T%C3%A9cnico%20Lagoa%20da%20Jansen.pdf (accessed on 10 December 2014).
- Rojas, M.O.A.I; Costa-Neto, J.J.G.; Barbiere, R.; Siqueiraos, S. A valiação físico-química da água da Laguna da Jansen, São Luis, MA. Acta Tecnol. 2013, 8, 19–24. [Google Scholar]
- Vigilância E Controle Da Qualidade Da Água Para Consumo Humano. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigilancia_controle_qualidade_agua.pdf (accessed on 10 December 2014).
- Manual Prático De Análise De Água. Available online: http://www.funasa.gov.br/site/wp-content/files_mf/eng_analAgua.pdf (accessed on 10 December 2014).
- Cantanhêde, S.M.; Medeiros, A.M.; Ferreira, J.R.C.; Alves, L.M.C.; Ferreira, F.S.; Cutrim, M.V.J.; Santos, D.M.S. Uso de biomarcador histopatológico em brânquias de Centropomus undecimalis (Bloch, 1972) na avaliação da qualidade da água do Parque Ecológico Laguna da Jansen, São Luís—MA. Arq. Bra. Med. Vet. Zootec. 2014, 66, 593–601. [Google Scholar]
- Lupi, C.; Nhacarini, N.I.; Mazon, A.F.; Rigolinsá, O. Avaliação da poluição ambiental através das alterações morfológicas nas brânquias de Oreochromis niloticus (Tilapia) nos córregos Retiro consulta e Bebedouro, município de Bebedouro-sp. Rev. Hispeci & Lema. 2007, 3, 1–6. [Google Scholar]
- Nogueira, D.J.; de Castro, S.C.; de Sá, O.R. Utilização das brânquias de Astyanax altiparanae (Garutti & Britski, 2000) (Teleostei, Characidae) como biomarcador de poluição ambiental no reservatório UHE Furnas—MG. Rev. Bras. Zooc. 2009, 11, 227–232. [Google Scholar]
- Winkaler, E.U.; Silva, A.G.; Galindo, H.C.; Martinez, C.B.R. Biomarcadores histológicos e fisiológicos para o monitoramento da saúde de peixes de ribeirões de Londrina, Estado do Paraná. Acta Scient. Biol. Sci. 2001, 23, 507–514. [Google Scholar]
- Fracário, R.; Verani, N.F.; Espíndola, E.L.G.; Rocha, O.; Rigolin-Sá, O.; Andrade, C.A. Alterations on growth and gill morphology of danio rerio (pisces, ciprinidae) exposed to the toxic sediments. Brazil. Arc. Biol. Technol. 2003, 46, 685–695. [Google Scholar]
- Martinez, C.B.; Nagae, M.Y.; Zaia, C.T.B.; Zaia, D.M.A. Morphological and physiological acute effects of lead in the neotropical fish Prochilodus lineatus. Brazil. J. Biol. 2004, 64, 797–807. [Google Scholar]
- Meletti, P.C.; Rocha, O.; Martinez, C.B.R. Avaliação da degradação ambiental na bacia do rio Mogi-Guaçu por meio de testes de toxidade com sedimento e de análises histopatológicas em peixes. In Limnologia Fluvial: Um Estudo No Rio Mogi-Guaçú; Brigante, J., Espíndola, E.L.G., Eds.; São Paulo: São Carlos, Brasil, 2003; pp. 149–180. [Google Scholar]
- Pereira, D.P.; Santos, D.M.S.; Carvalho-Neta, A.V.; Cruz, C.F.; Carvalho Neta, R.N.F. Alterações morfológicas em brânquias de Oreochromis niloticus (Pisces, Cichlidae) como biomarcadores de poluição aquática na Laguna da Jansen, São Luís, MA (Brasil). Biosci. J. 2014, 30, 1213–1221. [Google Scholar]
- Gernhöfer, M.; Pawert, M.; Schramm, M.; Müller, E.; Triebskorn, R. Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. J. Aquat. Ecosyst. Stress Recov. 2001, 8, 241–260. [Google Scholar]
- Stentiford, G.D.; Longshaw, M.; Lyons, B.P.; Jones, G.; Green, M.; Feist, S.W. Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar. Environ. Res. 2003, 55, 137–159. [Google Scholar]
- Farombi, E.O.; Adelowo, O.A.; Ajimoko, Y.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health 2007, 4, 158–165. [Google Scholar]
- Flores-Lopes, F.; Thomaz, AT. Histopathologic alterations observed in fish gills as a tool in environmental monitoring. Braz. J. Biol. 2011, 71, 179–188. [Google Scholar]
- Paulo, D.V.; Fontes, F.M.; Flores-Lopes, F. Histopathological alterations observed in the liver of Poecilia vivipara (Cyprinodontiformes: Poeciliidae) as a tool for the environmental quality assessment of the Cachoeira River, BA. Braz. J. Biol. 2012, 72, 131–140. [Google Scholar]
- Peakall, D.B. The role of biomarkers in environmental assessment (1) Introduction. Ecotoxicology 1994, 3, 157–160. [Google Scholar]
- Adams, S.M.; Greeley, M.S.; Ryon, M.G. Evaluating effects of contaminants in fish health at multiple levels of biological organization: Extrapolating from lower to higher levels. Hum. Ecol. Risk Assess. 2000, 6, 15–27. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.M.S.; Melo, M.R.S.; Mendes, D.C.S.; Rocha, I.K.B.S.; Silva, J.P.L.; Cantanhêde, S.M.; Meletti, P.C. Histological Changes in Gills of Two Fish Species as Indicators of Water Quality in Jansen Lagoon (São Luís, Maranhão State, Brazil). Int. J. Environ. Res. Public Health 2014, 11, 12927-12937. https://doi.org/10.3390/ijerph111212927
Santos DMS, Melo MRS, Mendes DCS, Rocha IKBS, Silva JPL, Cantanhêde SM, Meletti PC. Histological Changes in Gills of Two Fish Species as Indicators of Water Quality in Jansen Lagoon (São Luís, Maranhão State, Brazil). International Journal of Environmental Research and Public Health. 2014; 11(12):12927-12937. https://doi.org/10.3390/ijerph111212927
Chicago/Turabian StyleSantos, Débora M. S., Mércia Regina S. Melo, Denise Carla S. Mendes, Iolanda Karoline B. S. Rocha, Jakeline Priscila L. Silva, Sildiane M. Cantanhêde, and Paulo C. Meletti. 2014. "Histological Changes in Gills of Two Fish Species as Indicators of Water Quality in Jansen Lagoon (São Luís, Maranhão State, Brazil)" International Journal of Environmental Research and Public Health 11, no. 12: 12927-12937. https://doi.org/10.3390/ijerph111212927