Intrahippocampal Infusion of Crotamine Isolated from Crotalus durissus terrificus Alters Plasma and Brain Biochemical Parameters †
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Drugs
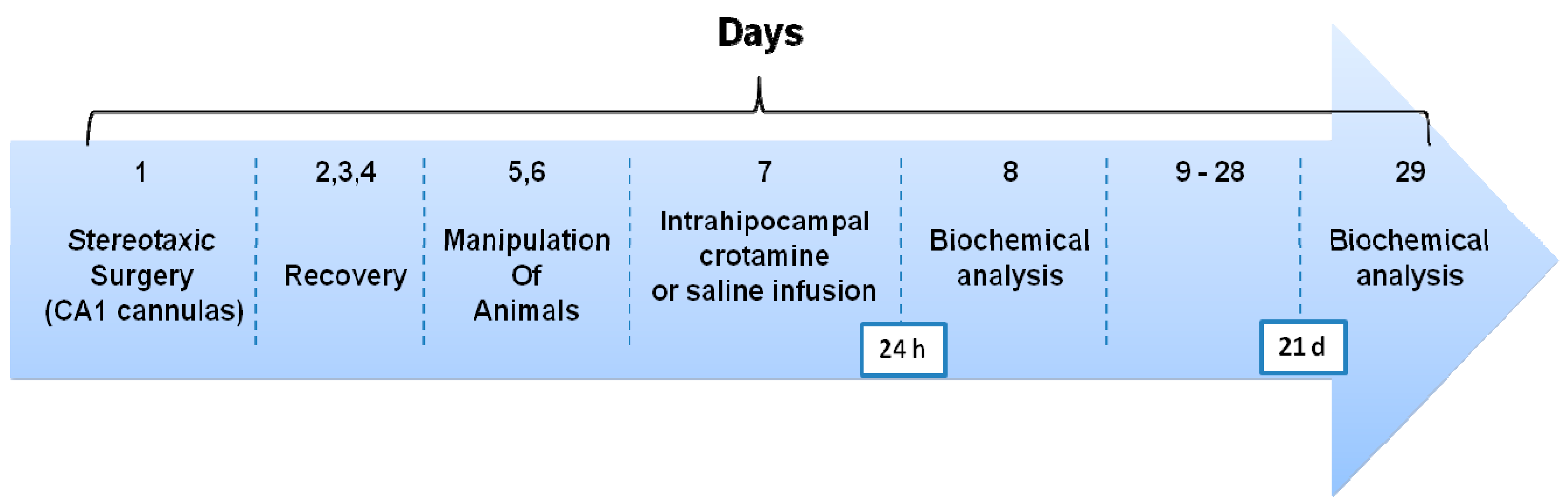
2.3. Surgery and Drug Infusion Procedures
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
| Blood Hematological and Cardiac Parameters | Saline-Group | Crotamine-Group | |
|---|---|---|---|
| 24 h | 21 Days | ||
| Creatinine (mg/dL) | 0.58 ± 6 × 10−3 | 0.57 ± 5 × 10−3 | 0.83 ± 0.01 #,* |
| Urea (mg/dL) | 45.50 ± 0.89 | 52.75 ± 1.26 * | 34.29 ± 0.30 #,* |
| GOT (U/L) | 120.00 ± 0.56 | 142.00 ± 2.22 * | 266.10 ± 3.32 #,* |
| GPT (U/L) | 73.25 ± 0.98 | 82.75 ± 2.75 * | 94.43 ± 1.11 #,* |
| CK (U/L) | 806.30 ± 4.69 | 947.00 ± 15.71 * | 15 × 102 ± 12.62 #,* |
| CK-MB (U/L) | 22 × 102 ± 19.11 | 23 × 102 ± 49.86 | 24 × 102 ± 0.06 |
| Red Blood Cells (106/µL) | 7.21 ± 0.01 | 6.66 ± 0.04 * | 7.84 ± 0.31 |
| Hemoglobin (g/dL) | 13.58 ± 0.11 | 13.10 ± 0.16 | 13.40 ± 0.25 |
| Hematocrit (%) | 40.88 ± 0.18 | 38.60 ± 0.57 * | 37.76 ± 0.50 * |
| Leukocytes (103/µL) | 58 × 102 ± 57.90 | 45 × 102 ± 56.41* | 93 × 102 ± 253.10 #,* |
| Platelets (103/µL) | 44 × 104 ± 20 × 102 | 53 × 104 ± 32 × 102 * | 89 × 104 ± 16 × 102 #,* |
| Plasma and Brain Oxidative Parameters | Saline-Group | Crotamine-Group | |
|---|---|---|---|
| 24 h | 21 Days | ||
| TBARS Plasma (nmol MDA/L) | 27.43 ± 0.27 | 27.43 ± 0.92 | 50.51 ± 1.37 #,* |
| TBARS Brain (nmol MDA/L) | 96.13 ± 1.32 | 97.73 ± 3.62 | 143.30 ± 4.34 #,* |
| Carbonyl Plasma (nmol carbonyl/mg protein) | 0.01 ± 2 × 10−4 | 0.01 ± 4 × 10−4 | 0.02 ± 6 × 10−4 #,* |
| Carbonyl Brain (nmol carbonyl/mg protein) | 0.01 ± 3 × 10−4 | 0.01 ± 5 × 10−4 | 0.02 ± 7 × 10−4 #,* |
| Micronucleus (% frequency) | 0.75 ± 0.13 | 1.00 ± 0.21 | 1.87 ± 0.19 #,* |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tribuiani, N.; da Silva, A.M.; Ferraz, M.C.; Silva, M.G.; Bentes, A.P.G.; Graziano, T.S.; dos Santos, M.G.; Cogo, J.C.; Varanda, E.A.; Groppo, F.C.; et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef]
- Jorge, M.T.; Ribeiro, L.A. Epidemiologia e quadro clínico do acidente por cascavel sul-americana (Crotalus durissus). Rev. Inst. Med. Trop. São Paulo 1992, 34, 347–354. [Google Scholar]
- Kerkis, I.; de Sá Silva, F.; Pereira, A.; Kerkis, A.; Rádis-Baptista, G. Biological versatility of crotamine—A cationic peptide from the venom of a South American rattlesnake. Expert Opin. Investig. Drugs 2010, 19, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Oliveira e Silva, S.; Rostelato-Ferreira, S.; Rocha-e-Silva, T.A.A.; Randazzo-Moura, P.; Dal-Belo, C.A.; Sanchez, E.F.; Borja-Oliveira, C.R.; Rodrigues-Simioni, L. Beneficial effect of crotamine in the treatment of myasthenic rats. Muscle Nerve 2013, 47, 591–593. [Google Scholar]
- Vargas, L.S.; Lara, M.V.; Gonçalves, R; Mandredini, V.; Ponce-Soto, L.A.; Marangoni, S; Dal-Belo, C.A.; Mello-Carpes, P.B. The intrahippocampal infusion of crotamine from Crotalus durissus terrificus venom enhances memory persistence in rats. Toxicon 2014, 85, 52–58. [Google Scholar] [CrossRef]
- Mancin, A.C.; Soares, A.M.; Andrião-Escarso, S.H.; Faça, V.M.; Greene, L.J.; Zuccolotto, S.; Pelá, I.R.; Giglio, J.R. The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon 1998, 36, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.L.; Lennon, V.A. End-plate voltage-gated sodium channels are lost in clinical and experimental myasthenia gravis. Ann. Neurol. 1998, 43, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.T.; Carvalho-de-Souza, J.L.; Schiavon, E.; Cassola, A.C.; Wanke, E.; Troncone, L.R.P. Crotamine inhibits preferentially fast-twitching muscles but is inactive on sodium channels. Toxicon 2007, 50, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Camillo, M.A.P.; Arruda Paes, P.C.; Troncone, L.R.P.; Rogero, J.R. Gyroxin fails to modify in vitro release of labelled dopamine and acetylcholine from rat and mouse striatal tissue. Toxicon 2001, 39, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Platt, B.; Riedel, G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011, 221, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Rossato, J.I.; Radiske, A.; Kohler, C.A.; Gonzalez, C.; Bevilaqua, L.R.; Medina, J.H.; Cammarota, M. Consolidation of object recognition memory requires simultaneous activation of dopamine D1/D5 receptors in the amygdala and medial prefrontal cortex but not in the hippocampus. Neurobiol. Learn. Mem. 2013, 106, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Berrih-Aknin, S.; Le Panse, R. Myasthenia gravis: A comprehensive review of immune dysregulation and etiological mechanisms. J. Autoimmun. 2014, 52, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Dal-Belo, C.A.; de Bairros Lucho, A.P.; Vinadé, L.; Rocha, L.; Seibert França, H.; Marangoni, S.; Rodrigues-Simioni, L. In vitro antiophidian mechanisms of Hypericum brasiliense choisy standardized extract: Quercetin dependent neuroprotection. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Habermann, E.; Cheng-Raude, D. Central neurotoxicity of apamin, crotamin, phospholipase A and alpha-amanitin. Toxicon 1975, 13, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.E.A.M.; Cavalheiro, E.A. Behavioural, electroencephalographic and neuropathological effects of the intrahippocampal injection of the venom of the South American rattlesnake (Crotalus durissus terrificus). Toxicon 1989, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1986. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Pinho, F.M.O.; Yu, L.; Burdmann, E.A. Snakebite-induced kidney injury in Latin America. Semin. Nephrol. 2008, 28, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ofidismo: Análise Epidemiológica; Ministério da Saúde: Brasília, Brazil, 1991. [Google Scholar]
- Amaral, C.F.S.; Rezende, N.A.; da Silva, O.A.; Ribeiro, M.M.F.; Magalhães, R.A.; Reis, R.J.D.; Carneiro, J.G.; Castro, J.R.S. Insuficiência renal aguda secundária a acidentes ofídicos botrópico e crotálico: Análise de 63 casos. Rev. Inst. Med. Trop. São Paulo 1896, 28, 220–227. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Calderón, L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon 2003, 42, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Radis-Baptista, G. New view on crotamine, a small basic polypeptide myotoxin from South American rattlesnake venom. Toxicon 2005, 46, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Orts, D.J.B.; da Silva, A.R.P.; Oguiura, N.; Boni-Mitake, M.; de Oliveira, E.B.; Zaharenko, A.J.; de Freitas, J.C.; Tytgat, J. Crotamine pharmacology revisited: Novel insights based on the inhibition of KV channels. Mol. Pharmacol. 2012, 82, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dal-Belo, C.A.; Leite, G.B.; Toyama, M.H.; Marangoni, S.; Corrado, A.P.; Fontana, M.D.; Souhan, A.; Rowan, E.G.; Hyslop, S.; Rodrigues-Simioni, L. Pharmacological and structural characterization of a novel phospholipase A2 from Micrurus dumerilii carinicauda venom. Toxicon 2005, 46, 736–750. [Google Scholar] [CrossRef] [PubMed]
- De Sousa-e-Silva, M.C.; Tomy, S.C.; Tavares, F.L.; Navajas, L.; Larsson, M.H.; Lucas, S.R.; Kogika, M.M.; Sano-Martins, I.S. Hematological, hemostatical and clinical chemistry disturbances induced by Crotalus durissus terrificus sanke venom in dogs. Hum. Exp. Toxicol. 2003, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sebastin Santhosh, M.; Thushara, R.M.; Hemshekhar, M.; Sunitha, K.; Devaraja, S.; Kemparaju, K.; Girish, K.S. Allevation of viper venom induced platelet apoptosis by crocin (Crocus sativus): implication for thrombocytopenia in viper bites. J. Thromb. Thrombolsys. 2013, 36, 424–432. [Google Scholar] [CrossRef]
- Waghmare, A.B.; Salvi, N.C.; Deopurkar, R.L.; Shenoy, P.A.; Sonpetkar, J.M. Evaluation of health status of horses immunized with snake venom and montanide adjuvants, IMS 3012 (nanoparticle), ISA 206 and ISA 35 (emulsion based) during polyvalent snake antivenom production: Hematological and biochemical assessment. Toxicon 2014, 82, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.D.; Lin, P.Y.; Chen, L.M.; Fang, W.H.; Lin, L.P.; Loh, C.H. Serum glutamic-oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res. Dev. Disabil. 2010, 31, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadoon, M.K.; Fahim, A. Possible recovery from an acute envenomation in male rats with LD50 of Echis coloratus crude venom: I-A seven days hematological follow-up study. Saudi J. Biol. Sci. 2012, 19, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in brain. Front. Aging Neurosci. 2010, 2. [Google Scholar] [CrossRef]
- Da Silva, J.G.; da Silva Soley, B.; Gris, V.; do Rocio Andrade Pires, A.; Caderia, S.M.S.C.; Eler, G.J.; Hermoso, A.P.M.; Bracht, A.; Dalsenter, P.R.; Acco, A. Effects of the Crotalus durissus terrificus snake venom on hepatic metabolism and oxidative stress. J. Biochem. Mol. Toxicol. 2011, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A. Lipid peroxidation of membrane phospholipids generate hidroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Kerkis, A.; Hayashi, M.A.F.; Pereira, A.S.P.; Silva, F.S.; Oliveira, E.B.; da Silva, A.R.B.P.; Yamane, T.; Rádis-Baptista, G.; Kerkis, I. Crotamine toxicity and efficacy in mouse models of melanoma. Expert. Opin. Investig. Drugs 2011, 20, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Boni-Mitake, M.; Costa, H.; Spencer, P.J.; Vassilieff, V.S.; Rogero, J.R. Effects of 60Co gamma radiation on crotamine. Braz. J. Med. Biol. Res. 2001, 34, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Boni-Mitake, M.; Costa, H.; Vassilieff, V.S.; Rogero, J.R. Distribution of 125I-labeled crotamine in mice tissues. Toxicon 2006, 48, 550–555. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.; Vargas, L.S.; Lara, M.V.S.; Güllich, A.; Mandredini, V.; Ponce-Soto, L.; Marangoni, S.; Belo, C.A.D.; Mello-Carpes, P.B. Intrahippocampal Infusion of Crotamine Isolated from Crotalus durissus terrificus Alters Plasma and Brain Biochemical Parameters. Int. J. Environ. Res. Public Health 2014, 11, 11438-11449. https://doi.org/10.3390/ijerph111111438
Gonçalves R, Vargas LS, Lara MVS, Güllich A, Mandredini V, Ponce-Soto L, Marangoni S, Belo CAD, Mello-Carpes PB. Intrahippocampal Infusion of Crotamine Isolated from Crotalus durissus terrificus Alters Plasma and Brain Biochemical Parameters. International Journal of Environmental Research and Public Health. 2014; 11(11):11438-11449. https://doi.org/10.3390/ijerph111111438
Chicago/Turabian StyleGonçalves, Rithiele, Liane S. Vargas, Marcus V. S. Lara, Angélica Güllich, Vanusa Mandredini, Luis Ponce-Soto, Sergio Marangoni, Cháriston A. Dal Belo, and Pâmela B. Mello-Carpes. 2014. "Intrahippocampal Infusion of Crotamine Isolated from Crotalus durissus terrificus Alters Plasma and Brain Biochemical Parameters" International Journal of Environmental Research and Public Health 11, no. 11: 11438-11449. https://doi.org/10.3390/ijerph111111438




