Biocontrol of Fusarium graminearum Growth and Deoxynivalenol Production in Wheat Kernels with Bacterial Antagonists
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Media
2.2. Antagonistic Activities against the Fungal Growth in Dual-Culture and Tip-Culture Assays
2.3. Antagonistic Activities against Mycotoxin DON in Wheat Kernels Assay
2.4. Identification of the Bacterial Strains
2.5. Statistical Analysis
3. Results
3.1. Antagonism of Bacteria against Growth of F. graminearum in vitro
| Bacterial Strains | Inhibition Rate of Mycelia (%) by Dual-Culture Tip-Culture | Inhibition Rate of DON (%) by Wheat Kernels Assay | |
|---|---|---|---|
| N2PS25-2 | 54.55 ± 1.75 | ≈100 a | 36.00 ± 3.04 |
| WPP19-1 | 54.55 ± 0.00 | ≈100 a | 30.49 ± 5.26 |
| WPS2 | 52.53 ± 1.75 | 96.26 ± 0.57 | 27.80 ± 4.56 |
| WPP17 | 52.53 ± 1.75 | 95.93 ± 0.51 | 30.49 ± 10.56 |
| WPP10 | 51.52 ± 3.03 | 100 | 63.90 ± 2.00 |
| WPP9 | 51.52 ± 3.03 | 100 | 88.40 ± 4.41 |
| XPP6-1 | 51.52 ± 1.75 | ≈100 a | 70.99 ± 3.40 |
| WPS4-3 | 50.51 ± 1.75 | ≈100 a | 56.00 ± 3.88 |
| XPP6-2 | 49.49 ± 1.75 | 98.75 ± 0.85 | 26.80 ± 4.81 |
| WPP14 | 48.48 ± 0.00 | 94.64 ± 0.74 | 20.12 ± 3.84 |
| WPP19-2 | 47.47 ± 1.75 | ≈100 a | 37.71 ± 2.57 |
| XPP6-3 | 47.47 ± 1.75 | 96.93 ± 0.57 | 25.70 ± 10.70 |
| N1PB1-2 | 47.47 ± 1.75 | ≈100 a | 38.02 ± 3.60 |
| WPS4-2 | 46.46 ± 1.75 | 95.40 ± 0.46 | 40.09 ± 2.64 |
| WPP1-2 | 46.46 ± 1.75 | 97.84 ± 0.23 | 63.59 ± 3.08 |
| HPP8 | 46.46 ± 1.75 | 92.70 ± 0.10 | 0 |
| YPS8 | 45.45 ± 0.00 | ≈100 a | 16.69 ± 4.28 |
| WPP1-1 | 45.45 ± 0.00 | 97.44 ± 0.40 | 0 |
| WPP19-3 | 44.44 ± 1.75 | 98.26 ± 0.79 | 32.00 ± 4.54 |
| WPS4-1 | 41.41 ± 0.00 | 100 | 90.30 ± 1.01 |
| SPS3 | 35.35 ± 1.75 | – | – |
| N2PS9 | 33.33 ± 3.03 | – | – |
| WPP16 | 32.32 ± 1.75 | – | – |
| N2PS7 | 31.31 ± 1.75 | – | – |
| N1PB1-1 | 28.28 ± 1.75 | – | – |
| N2PS25-1 | 25.25 ± 1.75 | – | – |
| WPP5-1 | 24.24 ± 0.00 | – | – |
| WPP5-2 | 23.23 ± 1.75 | – | – |
| SPP1 | 19.19 ± 1.75 | – | – |
| WPP8-1 | 17.17 ± 1.75 | – | – |
| WPG2 | 16.16 ± 1.75 | – | – |
| WPP8-2 | 15.15 ± 0.00 | – | – |
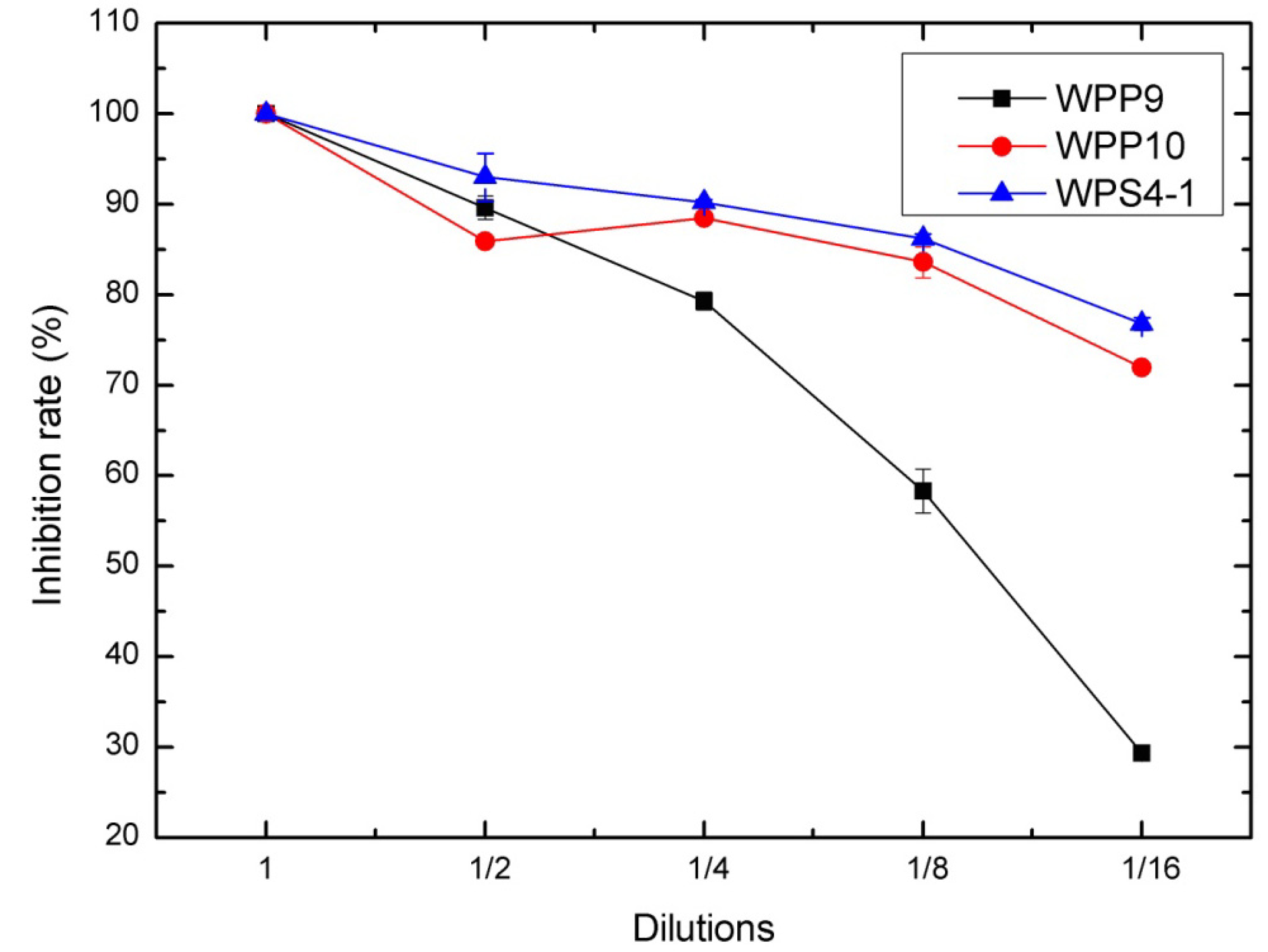
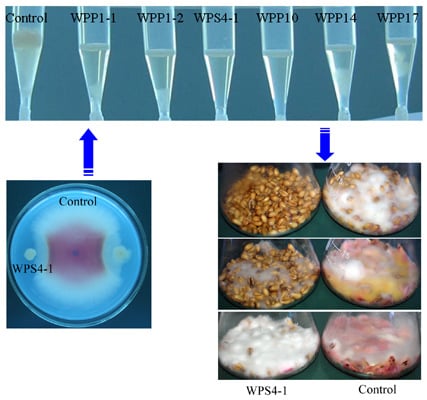
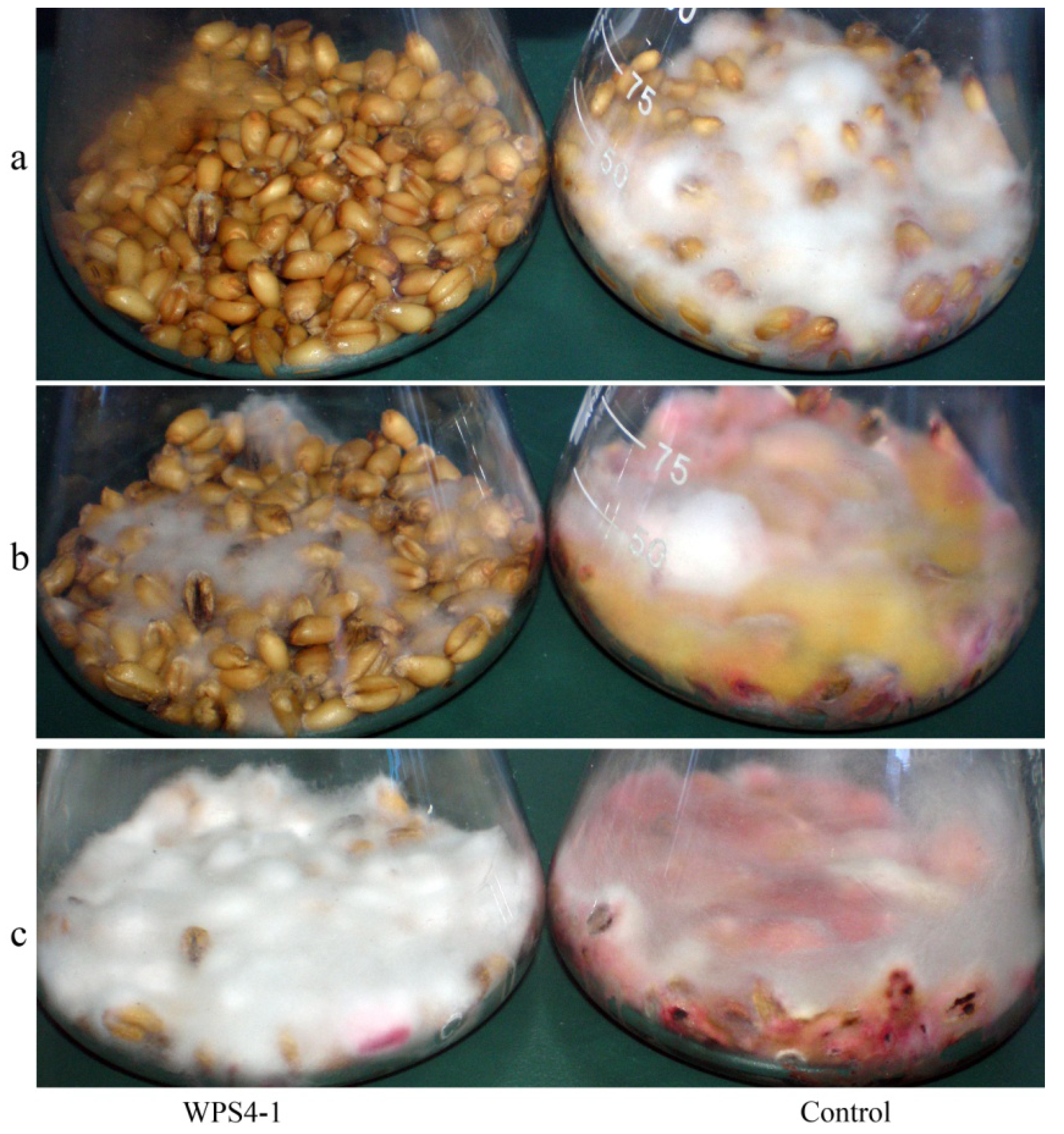
3.2. Antagonism of Bacteria against DON Production of F. graminearum in Wheat Kernels
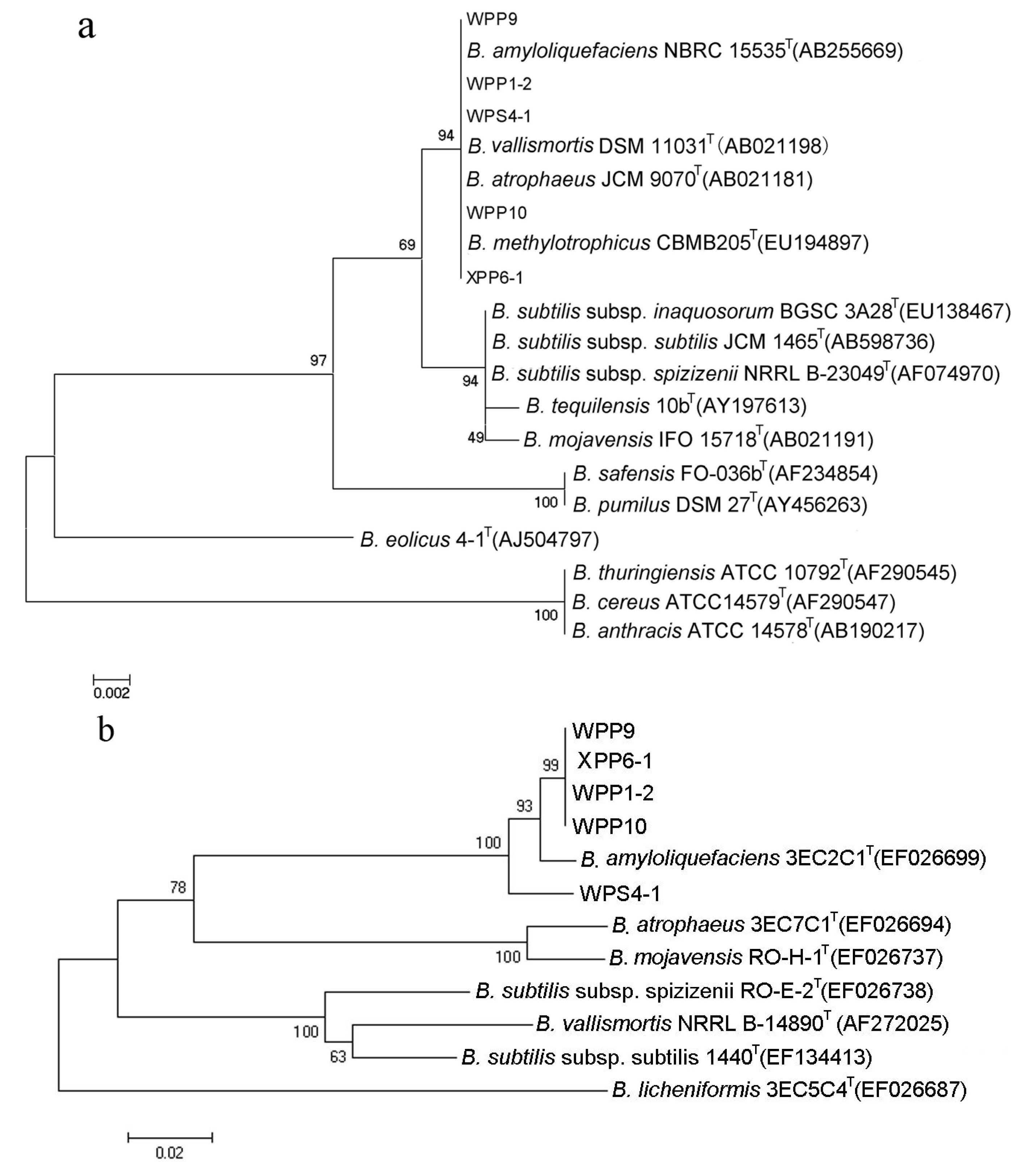
3.3. Identification of Bacterial Strains with High Antifungal and Anti-Mycotoxin Activities
4. Discussion
5. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Bai, G.-H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2001, 153, 91–98. [Google Scholar]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Hohn, T.M.; McCormick, S.P. Trichothecene biosynthesis in Fusarium species: Chemistry, genetics, and significance. Microbiol. Rev. 1993, 57, 595–604. [Google Scholar]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Voss, K.A. A new perspective on deoxynivalenol and growth suppression. Toxicol. Sci. 2010, 113, 281–283. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Cri. Rev. Food Sci. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Sliniger, P.J.; Bothast, R.J. Selection and evaluation of microorganisms for biocontrol of Fusarium head blight of wheat incited by Gibberella zeae. Plant Dis. 2001, 85, 1253–1258. [Google Scholar] [CrossRef]
- He, J.; Boland, G.J.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Ramirez, M.L.; Torres, A.M.; Chulze, S.N. Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat. Crop Prot. 2007, 26, 1702–1710. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals, Including Annexes on Ochratoxin A, Zearalenone, Fumonisins and Tricothecenes (CAC/RCP 51-2003). In Prevention and Reduction of Food and Feed Contamination, 1st ed.; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; pp. 1–13. [Google Scholar]
- Druverfors, U.; Jonsson, N.; Boysen, M.E.; Schnürer, J. Efficacy of the biocontrol yeast Pichia anolmala during long-term storage of moist feed grain under different oxygen and carbon dioxide regimens. FEMS Yeast Res. 2002, 2, 389–394. [Google Scholar]
- Mokiou, S.; Magan, N. Physiological manipulation and formulation of the biocontrol yeast Pichia anomala for control of Penicillium verrucosum and ochratoxin A contamination of moist grain. Biocontrol Sci. Technol. 2008, 18, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Laitila, A.; Alakomi, H.-L.; Raaska, L.; Mattila-Sandholm, T.; Haikara, A. Antifungal activities of two Lactobacillus plantarum strains against Fusarium moulds in vitro and in malting of barley. J. Appl. Microbiol. 2002, 93, 566–576. [Google Scholar] [CrossRef]
- Cheng, B.; Wan, C.; Yang, S.; Xu, H.; Wei, H.; Liu, J.; Tian, W.; Zeng, M. Detoxification of deoxynivalenol by Bacillus strains. J. Food Safety 2010, 30, 599–614. [Google Scholar]
- Zhou, T.; Gong, J.; Yu, H.; Li, X.Z. Bacterial Isolate and Methods for Detoxification of Trichothecene Mycotoxins. U.S. Patent 20100239537, 23 September 2010. [Google Scholar]
- Chen, Y.; Wang, W.; Zhang, A.; Gu, C.; Zhou, M.; Gao, T. Activity of the fungicide JS399-19 against Fusarium head blight of wheat and the risk of resistance. Agr. Sci. China 2011, 10, 1906–1913. [Google Scholar]
- Yan, P.; Gao, X.; Wu, H.; Li, Q.; Ning, L.; Guan, S. Isolation and screening of biocontrol bacterial strains against Aspergillus parasiticuns from groundnut geocarposphere. J. Earth Sci. 2010, 21, 309–311. [Google Scholar] [CrossRef]
- Yabe, K.; Nakamura, H.; Ando, Y.; Terakado, N.; Nakajima, H.; Hamasaki, T. Isolation and characterization of Aspergillus parasiticus mutants with impaired aflatoxin production by a novel tip culture method. Appl. Environ. Microbiol. 1988, 54, 2096–2100. [Google Scholar]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Struhl, K. Current Protocols in Molecular Biology; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/ (accessed on 16 January 2014).
- Jochum, C.C.; Osborne, L.E.; Yuen, G.Y. Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol. Control 2006, 39, 336–344. [Google Scholar] [CrossRef]
- Schisler, D.A.; Khan, N.I.; Boehm, M.J; Lipps, P.E; Slininger, P.J.; Zhang, S. Selection and evaluation of the potential of choline-metabolizing microbial strains to reduce Fusarium head blight. Biol. Control 2006, 39, 497–506. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact. 2009, 8, 1–12. [Google Scholar] [CrossRef]
- Cazzaniga, D.; Basílico, J.C.; González, R.J.; Torres, R.L.; De Greff, D.M. Mycotoxins inactivation by extrusion cooking of corn flour. Lett. Appl. Microbiol. 2001, 33, 144–147. [Google Scholar] [CrossRef]
- Fouler, S.G.; Triverdi, A.B.; Kitabatake, N. Detoxification of citrinin and ochratoxin A by hydrogen peroxide. J. AOAC Int. 1994, 77, 631–637. [Google Scholar]
- Wolf, C.E.; Bullerman, L.B. Heat and pH alter the concentration of deoxynivalenol in an aqueous environment. J. Food Protect. 1998, 1998, 365–367. [Google Scholar]
- Bata, Á.; Lásztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar] [CrossRef]
- Bakutis, B.; Baliukoniené, V.; Paškevičilus, A. Use of biological method for detoxification of mycotoxins. Botanica Lithuanica 2005, 7, 123–129. [Google Scholar]
- El-Nezami, H.S.; Chrevatidis, A.; Auriola, S.; Salminen, S.; Mykkänen, H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 2002, 19, 680–686. [Google Scholar] [CrossRef]
- Etcheverry, M.G.; Scandolara, A.; Nesci, A.; Vilas Boas Ribeiro, M.S.; Pereira, P.; Battilani, P. Biological interactions to select biocontrol agents against toxigenic strains of Aspergillus flavus and Fusarium verticillioides from maize. Mycopathologia 2009, 167, 287–295. [Google Scholar] [CrossRef]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agr. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, G.; Luo, Y.; Ran, W.; Shen, Q. Production of lipopeptides by Bacillus amyloliquefaciens XZ-173 in solid state fermentation using soybean flour and rice straw as the substrate. Bioresource Technol. 2012, 112, 254–260. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, C.; Yan, P.; Li, J.; Wu, H.; Li, Q.; Guan, S. Biocontrol of Fusarium graminearum Growth and Deoxynivalenol Production in Wheat Kernels with Bacterial Antagonists. Int. J. Environ. Res. Public Health 2014, 11, 1094-1105. https://doi.org/10.3390/ijerph110101094
Shi C, Yan P, Li J, Wu H, Li Q, Guan S. Biocontrol of Fusarium graminearum Growth and Deoxynivalenol Production in Wheat Kernels with Bacterial Antagonists. International Journal of Environmental Research and Public Health. 2014; 11(1):1094-1105. https://doi.org/10.3390/ijerph110101094
Chicago/Turabian StyleShi, Cuijuan, Peisheng Yan, Jiafei Li, Hanqi Wu, Qianwei Li, and Shanshan Guan. 2014. "Biocontrol of Fusarium graminearum Growth and Deoxynivalenol Production in Wheat Kernels with Bacterial Antagonists" International Journal of Environmental Research and Public Health 11, no. 1: 1094-1105. https://doi.org/10.3390/ijerph110101094