Racial Differences in Survival among Hemodialysis Patients after Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Definitions
2.3. Setting
2.4. Data Collection and Follow-up
2.5. Statistical Analysis
3. Results and Discussion
3.1. Results
| Characteristic | Black | White | UnivariableHR (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | 1, 3, 5 Year Survival | n (%) | 1, 3, 5 Year Survival | ||
| Overall | 127 (61) | 85, 62, 44 | 80 (39) | 78, 48, 30 | 1.6 (1.1–2.3) ‡ |
| Age (Years) | |||||
| Q1 (≤53) | 38 (30) | 92, 74, 67 | 21 (26) | 61, 25, 20 | 1.0 Referent |
| Q2 (>53–60) | 35 (28) | 88, 66, 30 | 14 (18) | 100, 85, 53 | 1.04 (0.60–1.8) |
| Q3 (>60–66) | 25 (20) | 83, 68, 54 | 23 (29) | 87, 51, 32 | 1.1 (0.64–2.0) |
| Q4 (>66) | 29 (23) | 72, 39, 20 | 22 (28) | 73, 44, 23 | 1.9 (1.2–3.2) |
| Mean ± SD | 59 ± 9.7 | 60 ± 9.2 | PTrend = 0.012 | ||
| Median (Range) | 60 (35–80) | 62 (39–77) | |||
| Sex | |||||
| Male | 65 (51) | 92, 72, 54 | 53 (66) | 81, 53, 35 | 1.0 Referent |
| Female | 62 (49) | 77, 52, 33 | 27 (34) * | 73, 38, 21 | 1.6 (1.1–2.3) |
| BMI (kg/m2) | |||||
| Obese (≥30) | 50 (39) | 83, 67, 41 | 23 (29) | 87, 75, 58 | 1.0 Referent |
| Overweight (25–29.9) | 48 (38) | 89, 63, 46 | 32 (40) | 81, 47, 30 | 1.3 (0.81–2.1) |
| Normal (18.5–24.9) | 27 (21) | 80, 53, 44 | 22 (28) | 73, 27, 5 | 1.9 (1.2–3.1) |
| Underweight (<18.5) | 2 (2) | 50, 50 † | 3 (4) | 33, 33, 33 | 2.1 (0.64–6.9) |
| Mean ± SD | 29 ± 5.8 | 27 ± 5.2 | PTrend = 0.0091 | ||
| Median (Range) | 28 (18–49) | 27 (17–41) | |||
| CAD Severity | |||||
| 1 Vessel | 8 (6) | 100, 73, 55 | 2 (3) | 100, 100, 100 | 1.0 Referent |
| 2 Vessel | 37 (29) | 86, 61, 44 | 27 (34) | 77, 61, 48 | 2.0 (0.61–6.5) |
| 3 Vessel | 82 (65) | 82, 61, 43 | 51 (64) | 78, 38, 16 | 2.6 (0.83–8.4) |
| PTrend = 0.035 | |||||
| Non-Elective | |||||
| No | 58 (46) | 89, 64, 49 | 33 (41) | 81, 63, 51 | 1.0 Referent |
| Yes | 69 (54) | 81, 61, 40 | 47 (59) | 76, 38, 16 | 1.6 (1.1–2.4) |
| Hypertension | |||||
| No | 11 (9) | 73, 45, 27 | 7 (9) | 83, 33, 17 | 1.0 Referent |
| Yes | 116 (91) | 86, 64, 46 | 73 (91) | 78, 49, 31 | 0.61 (0.34–1.1) |
| Diabetes | |||||
| No | 54 (43) | 88, 60, 45 | 27 (34) | 78, 44, 28 | 1.0 Referent |
| Yes | 73 (57) | 82, 63, 42 | 53 (66) | 79, 50, 31 | 1.0 (0.71–1.5) |
| Heart Failure | |||||
| No | 72 (57) | 93, 69, 49 | 46 (58) | 82, 51, 32 | 1.0 Referent |
| Yes | 55 (43) | 73, 53, 37 | 34 (42) | 73, 44, 27 | 1.5 (1.1–2.3) |
| Prior Stroke | |||||
| No | 105 (83) | 87, 64, 49 | 71 (89) | 81, 51, 33 | 1.0 Referent |
| Yes | 22 (17) | 71, 51, 19 | 9 (11) | 53, 20 † | 2.1 (1.3-3.4) |
| Previous MI | |||||
| No | 71 (56) | 86, 65, 49 | 42 (53) | 88, 47, 30 | 1.0 Referent |
| Yes | 56 (44) | 83, 58, 36 | 38 (47) | 68, 49, 30 | 1.2 (0.85–1.8) |
| Medication Medication | Black n (%) | White n (%) | p-value |
|---|---|---|---|
| Aspirin | 73 (57) | 53 (66) | 0.24 |
| Lipid Lowering Agents | 55 (43) | 34 (43) | 1.0 |
| Anticoagulants | 30 (24) | 17 (21) | 0.74 |
| Antiplatelet Agents | 36 (28) | 34 (43) | 0.049 |
| β-Blockers | 75 (59) | 50 (63) | 0.66 |
| Calcium Channel Blockers | 55 (43) | 33 (41) | 0.89 |
| Diuretics | 17 (13) | 16 (20) | 0.24 |
| ACE Inhibitors/ARBs | 51 (40) | 36 (45) | 0.56 |
| Digitalis | 9 (7) | 7 (9) | 0.79 |
| Nitrates | 18 (14) | 11 (14) | 1.0 |
| Inotropic Agents | 1 (1) | 1 (1) | 1.0 |
| Complication | Black n (%) | White n (%) | p-value |
|---|---|---|---|
| MI | 0 (0) | 1 (1) | 0.39 |
| Stroke | 2 (2) | 1 (1) | 1.0 |
| ARDS | 1 (1) | 1 (1) | 1.0 |
| Pneumonia | 3 (2) | 2 (3) | 1.0 |
| GI Event * | 4 (3) | 4 (5) | 0.71 |
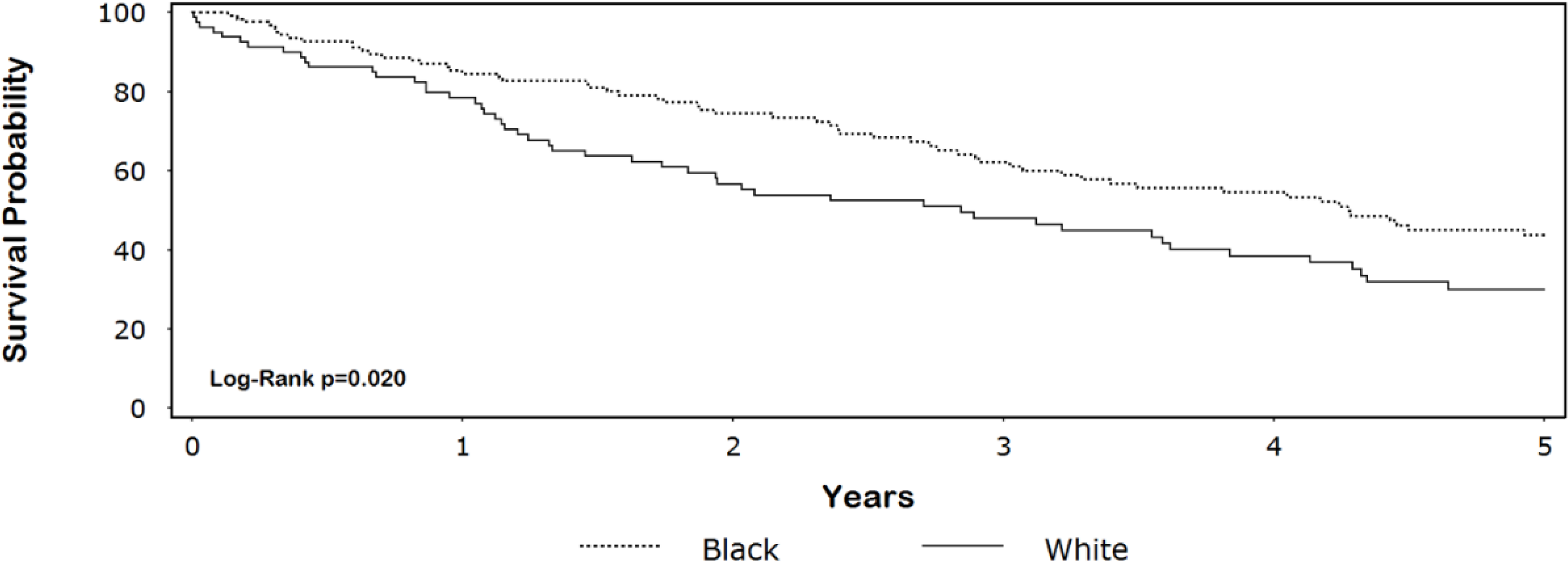
3.2. Discussion
3.3. Strengths and Limitations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Filsoufi, F.; Rahmanian, P.B.; Castillo, J.G.; Silvay, G.; Carpentier, A.; Adams, D.H. Predictors and early and late outcomes of dialysis-dependent patients in contemporary cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 522–529. [Google Scholar] [CrossRef]
- Dacey, L.J.; Liu, J.Y.; Braxton, J.H.; Weintraub, R.M.; DeSimone, J.; Charlesworth, D.C.; Lahey, S.J.; Ross, C.S.; Hernandez, F., Jr.; Leavitt, B.J.; et al. Long-term survival of dialysis patients after coronary bypass grafting. Ann. Thorac. Surg. 2002, 74, 458–462. [Google Scholar] [CrossRef]
- Boulton, B.J.; Kilgo, P.; Guyton, R.A.; Puskas, J.D.; Lattouf, O.M.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; Halkos, M.E.; Thourani, V.H. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann. Thorac. Surg. 2011, 92, 595–601. [Google Scholar] [CrossRef]
- Efird, J.T.; O’Neal, W.T.; Kennedy, W.L.; Kypson, A.P. Grand challenge: Understanding survival paradoxes in epidemiology. Front. Public Health 2013, 1, 1–2. [Google Scholar]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Derose, S.F.; Horwich, T.B.; Fonarow, G.C. Racial and survival paradoxes in chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 493–506. [Google Scholar] [CrossRef]
- Efird, J.T.; O’Neal, W.T.; Anderson, C.A.; O’Neal, J.B.; Kindell, L.C.; Ferguson, T.B.; Chitwood, W.R.; Kypson, A.P. The effect of race and chronic obstructive pulmonary disease on long-term survival after coronary artery bypass grafting. Front. Public Health 2013, 1, 1–7. [Google Scholar]
- Efird, J.T.; O’Neal, W.T.; Gouge, C.A.; Kindell, L.C.; Kennedy, W.L.; Bolin, P., Jr.; O’Neal, J.B.; Anderson, C.A.; Rodriguez, E.; Ferguson, T.B.; et al. Implications of hemodialysis in patients undergoing coronary artery bypass grafting. Int. J. Cardiovasc. Res. 2013, S1. [Google Scholar] [CrossRef]
- Efird, J.T.; O’Neal, W.T.; O’Neal, J.B.; Ferguson, T.B.; Chitwood, W.R.; Kypson, A.P. Effect of peripheral arterial disease and race on survival after coronary artery bypass grafting. Ann. Thorac. Surg. 2013, 96, 112–118. [Google Scholar] [CrossRef]
- Morris, P.J. Heart disease and stroke in North Carolina. N. C. Med. J. 2012, 73, 448–449. [Google Scholar]
- Morales, D.L.; McClellan, A.J.; Jacobs, J.P. Empowering a database with national long-term data about mortality: The use of national death registries. Cardiol. Young 2008, 18, 188–195. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Herzog, C.A.; Ma, J.Z.; Collins, A.J. Long-term survival of dialysis patients in the United States with prosthetic heart valves: Should ACC/AHA practice guidelines on valve selection be modified? Circulation 2002, 105, 1336–1341. [Google Scholar] [CrossRef]
- Bloembergen, W.E.; Port, F.K.; Mauger, E.A.; Wolfe, R.A. Causes of death in dialysis patients: Racial and gender differences. J. Am. Soc. Nephrol.: JASN 1994, 5, 1231–1242. [Google Scholar]
- Kucirka, L.M.; Grams, M.E.; Lessler, J.; Hall, E.C.; James, N.; Massie, A.B.; Montgomery, R.A.; Segev, D.L. Association of race and age with survival among patients undergoing dialysis. JAMA 2011, 306, 620–626. [Google Scholar] [CrossRef]
- Owen, W.F., Jr.; Chertow, G.M.; Lazarus, J.M.; Lowrie, E.G. Dose of hemodialysis and survival: Differences by race and sex. JAMA J. Am. Med. Assoc. 1998, 280, 1764–1768. [Google Scholar] [CrossRef]
- Agodoa, L.; Eggers, P. Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin. Dial. 2007, 20, 577–585. [Google Scholar] [CrossRef]
- Gray, R.J.; Nessim, S.; Khan, S.S.; Denton, T.; Matloff, J.M. Adverse 5-year outcome after coronary artery bypass surgery in blacks. Arch. Intern. Med. 1996, 156, 769–773. [Google Scholar] [CrossRef]
- Taylor, H.A., Jr.; Mickel, M.C.; Chaitman, B.R.; Sopko, G.; Cutter, G.R.; Rogers, W.J. Long-term survival of African Americans in the Coronary Artery Surgery Study (CASS). J. Am. Coll. Cardiol. 1997, 29, 358–364. [Google Scholar] [CrossRef]
- Streja, E.; Kovesdy, C.P.; Molnar, M.Z.; Norris, K.C.; Greenland, S.; Nissenson, A.R.; Kopple, J.D.; Kalantar-Zadeh, K. Role of nutritional status and inflammation in higher survival of African American and hispanic hemodialysis patients. Am. J. Kidney Dis. 2011, 57, 883–893. [Google Scholar]
- Farooq, V.; Serruys, P.W.; Garcia-Garcia, H.M.; Zhang, Y.L.; Bourantas, C.V.; Holmes, D.R.; Mack, M.; Feldman, T.; Morice, M.C.; Ståhle, E.; et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: The SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J. Am. Coll. Cardiol. 2013, 61, 282–294. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Srikanthan, P.; Costanzo, M.R.; Cintron, G.B.; Lopatin, M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am. Heart J. 2007, 153, 74–81. [Google Scholar]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Yan, G.; Norris, K.C.; Yu, A.J.; Ma, J.Z.; Greene, T.; Yu, W.; Cheung, A.K. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 953–961. [Google Scholar] [CrossRef]
- Efird, J.T.; Lea, S.; Toland, A.; Phillips, C.J. Informational odds ratio: A useful measure of epidemiologic association in environment exposure studies. Environ. Health Insights 2012, 6, 17–25. [Google Scholar]
- Koch, C.G.; Li, L.; Kaplan, G.A.; Wachterman, J.; Shishehbor, M.H.; Sabik, J.; Blackstone, E.H. Socioeconomic position, not race, is linked to death after cardiac surgery. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 267–276. [Google Scholar] [CrossRef]
- United States Census Bureau. State and County Quick Facts. Available online: http://quickfacts.census.gov/qfd/index.html (accessed on 9 May 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Efird, J.T.; O'Neal, W.T.; Bolin, P., Jr.; Davies, S.W.; O'Neal, J.B.; Anderson, C.A.; Ferguson, T.B.; Chitwood, W.R.; Kypson, A.P. Racial Differences in Survival among Hemodialysis Patients after Coronary Artery Bypass Grafting. Int. J. Environ. Res. Public Health 2013, 10, 4175-4185. https://doi.org/10.3390/ijerph10094175
Efird JT, O'Neal WT, Bolin P Jr., Davies SW, O'Neal JB, Anderson CA, Ferguson TB, Chitwood WR, Kypson AP. Racial Differences in Survival among Hemodialysis Patients after Coronary Artery Bypass Grafting. International Journal of Environmental Research and Public Health. 2013; 10(9):4175-4185. https://doi.org/10.3390/ijerph10094175
Chicago/Turabian StyleEfird, Jimmy T., Wesley T. O'Neal, Paul Bolin, Jr., Stephen W. Davies, Jason B. O'Neal, Curtis A. Anderson, T. Bruce Ferguson, W. Randolph Chitwood, and Alan P. Kypson. 2013. "Racial Differences in Survival among Hemodialysis Patients after Coronary Artery Bypass Grafting" International Journal of Environmental Research and Public Health 10, no. 9: 4175-4185. https://doi.org/10.3390/ijerph10094175






