Water-Related Parasitic Diseases in China
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Amoebiasis
3.1.1. Parasite and Pathogenicity

| Syndrome | Symptoms |
|---|---|
| Asymptomatic colonization (most common) |
|
| Acute amoebic colitis |
|
| Fulminant colitis (occurs most often in children) |
|
| Amoeboma (approximately 1% of patients) |
|
3.1.2. Epidemiology
3.1.3. Recent Advances in Research
3.2. Giardiasis
3.2.1. Parasite and Pathogenicity

| Stage | Symptoms |
|---|---|
| Prodromal |
|
| Acute (often lasts 3–4 days, subsiding spontaneously) |
|
| Chronic |
|
3.2.2. Epidemiology

3.2.3. Recent Advances in Research
3.3. Cryptosporidiosis
3.3.1. Parasite and Pathogenicity
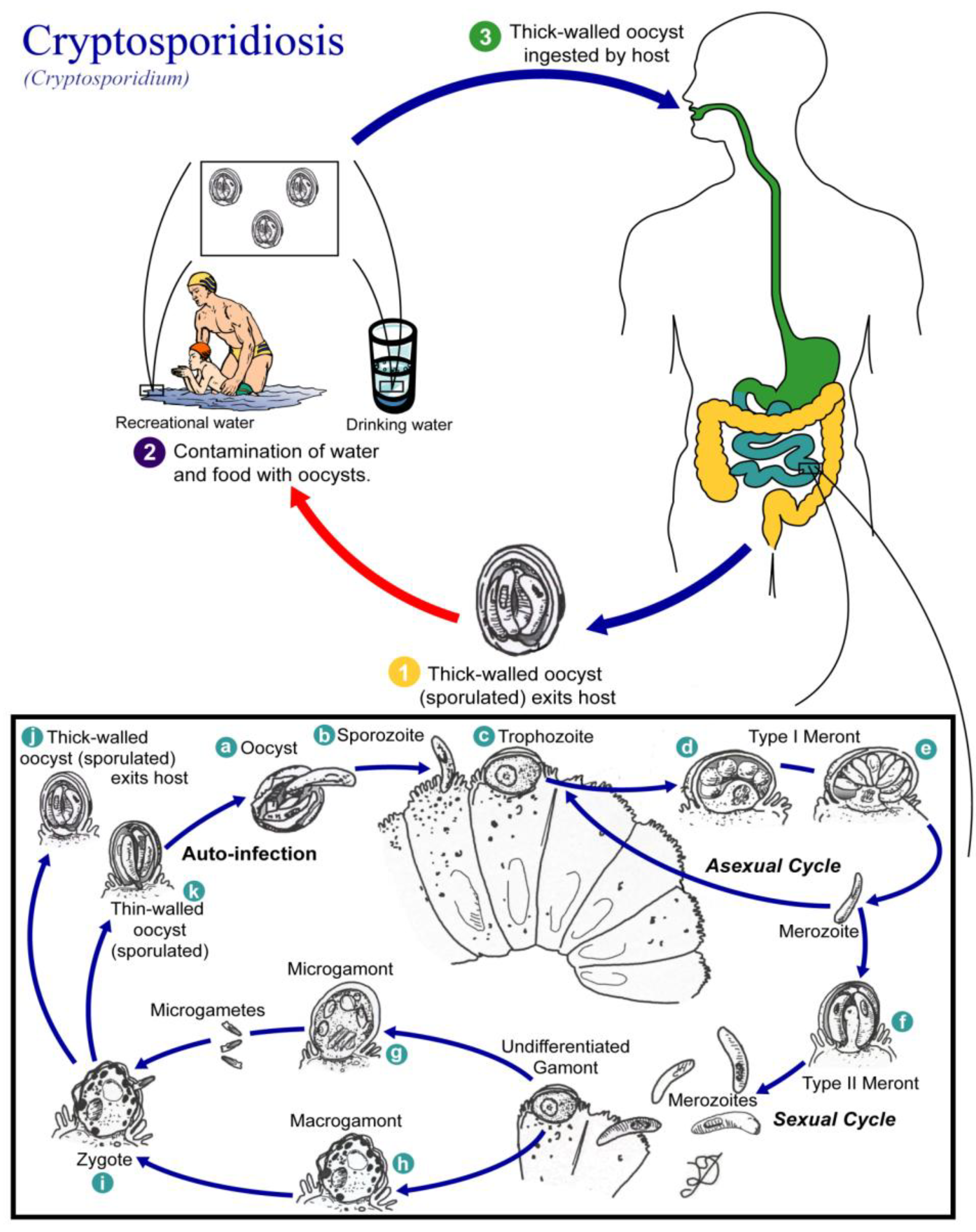
| Patient Health | Effects |
|---|---|
| Immunocompetent |
|
| Immunocompromised |
|
3.3.2. Epidemiology
| Report time | Location (City, Province) | No. exam | No. infected | Infection rate | Sex | Age | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| male | female | Children | Adults | ||||||
| 1992 | Yuxi, Yunnan | 1,640 | 84 | 5.12 | - | - | 7.99(52/651) (<5 years);
5.00(7/140) (6–10 years); 3.95(9/228) (11–20 years) | 2.41(11/457) (21–60years); 4.81(5/104) (>60 years) | Fan et al, [68] |
| 1993 | Kaifeng, Henan | 483 | 12 | 2.48 | - | - | 2.48(<4 years) | Su et al. [69] | |
| 1998 | Harbin, Heilongjiang | 931 | 13 | 1.40 | - | - | 1.40(<4 years) | Zhao et al. [70] | |
| 1999 | Wenzhou, Zhejiang | 1,060 | 60 | 5.66 | 5.18 | 6.38 | 6.13(59/962) (<15 years) | 1.02(1/98) (>15 years) | Xing et al. [71] |
| 2000 | Hangzhou, Zhejiang | 548 | 57 | 10.40 | 10.31 | 10.60 | 10.40(<10 years) | Lu et al. [72] | |
| 2001 | Chengdu, Sichuan | 406 | 12 | 2.96 | - | - | 2.96(<10 years) | Zhang et al. [73] | |
| 2001 | Gansu Province | 1,840 | 41 | 2.23 | 2.01 | 2.50 | 10.95(23/210) (<6 years);
1.82(7/385) (7–17 years) | 0.88(11/1245) (>18 years) | Chen et al. [74] |
| 2002 | Urumqi, Xinjiang | 190 | 4 | 2.11 | 0.86 | 4.05 | 1.56(1/64) (1–4 years.);
0.00(0/34) (5–11 years); | 0.00(0/12) (>60 years) | Luo et al. [75] |
| 2002 | Yunnan Province | 378 | 20 | 5.29 | 5.11 | 5.45 | 7.09(9/127) (<15 years) | 4.44(10/225) (15–60 years); 3.85(1/26) (>60 years) | Zhang et al. [76] |
| 2003 | Huainan, Anhui | 827 | 46 | 5.56 | 5.23 | 6.06 | 6.12(45/735) (<5 years) | 1.09(1/92) (>15 years) | Cai et al. [77] |
| 2004 | Longhai, Fujian | 248 | 7 | 2.82 | 1.48 | 5.95 | 3.75(3/80) (<4 year) | Xu et al. [78] | |
| 2006 | Qiqihar, Heilongjiang | 330 | 11 | 3.33 | 2.69 | 4.17 | 5.61(6/107) (<15 years) | 1.98(4/202) (15–60 years); 4.76(1/21) (>60 years) | Niu et al. [79] |
| 2006 | Heze, Shandong | 237 | 6 | 2.53 | 2.00 | 3.40 | 3.85(3/78) (<1 year);
2.17(2/92) (<5 years); 0.00(0/11) (5–18 years); | 1.79 (1/56) (18–72 years) | Zhou [80] |
| 2006 | Shenyang, Liaoning | 283 | 9 | 3.18 | 3.95 | 2.30 | 6.98(6/86) (<18 years) | 1.52 (3/198) (>18 years) | Li et al. [81] |
| Report time | Location (City, Province) | No. exam | No. Infected | Infection rate | Sex | Age | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| male | female | Children | Adult | ||||||
| 1991 | Nanjing, Jangsu | 2,018 | 16 | 0.79 | - | - | 0.79(<5 years) | Shen et al. [82] | |
| 1991 | Xuzhou and Huaiyin, Jiangsu | 2,613 | 67 | 2.56 | - | - | 3.29(59/1794) | 0.98(8/819) | Xu et al. [83] |
| 1992 | Qingdao, Shandong | 969 | 26 | 2.68 | 2.86 | 2.47 | 2.68(preschool and primary school) | Gong et al. [84] | |
| 1992 | Hunan Province | 3,739 | 69 | 1.85 | 1.86 | 1.83 | 2.52(57/2262) (<10 years); 1.99(10/503) (10–20 years) | 0.21(2/974) (>20 years.) | Lu et al. [85] |
| 1992 | Yuxi, Yunnan | 2,853 | 42 | 1.47 | 1.32 | 1.60 | 2.66(17/640) (<10 years); 1.47(10/680) (10–20 years) | 0.92(12/1308) (21–60 years); 1.33(3/225) (>60 years) | Fan et al, [68] |
| 1993 | Jiangsu Province | 5,089 | 89 | 1.75 | 1.91 | 1.54 | 3.18(57/1793) (<4 years); 0.97(32/3296) (4–15 years) | Chen et al. [86] | |
| 1995 | Xinjiang Province | 1,124 | 53 | 4.72 | 32 | 21 | 10.59(34/321) (<5 years); 2.37(19/803) (6–10 years) | Wang et al. [87] | |
| 2001 | Weifang, Shandong | 1,943 | 55 | 2.83 | 2.73 | 2.97 | 2.83(5–13 years) | Cui et al. [88] | |
| 2004 | Anhui Province | 1,204 | 42 | 3.49 | 3.59 | 3.37 | 3.45(preschool children) | Lu et al. [62] | |
| 2006 | Qiannan, Guizhou | 1,739 | 40 | 2.30 | 2.23 | 2.39 | 8.76(12/137) (<2 years); 3.76(7/186) (3–6 years); 1.99(4/201) (7–12 years); 2.77(8/289) (13–17 years) | 0.97(9/926) (>18 years) | Wang et al. [89] |
| 2007 | Shiyan, Hubei | 941 | 62 | 6.59 | 6.88 | 6.26 | 6.59 (7–16 years.) | Zhu et al. [90] | |
| 2007 | Shiyan, Hubei | 1,118 | 51 | 4.56 | 4.60 | 4.52 | 4.56 (3–6 years.) | Zhu et al. [91] | |
| 2009 | Shiyan, Hubei | 2,549 | 119 | 4.67 | 4.82 | 4.51 | 5.99(110/1836) | 1.26(9/713) | Zhu et al. [92] |
| 2009 | Nanjing, Jiangsu | 1,758 | 17 | 0.97 | 0.94 | 1.00 | 0.97 | Du et al. [61] | |
3.3.3. Recent Advances in Research
3.4. Cyclosporiasis
3.4.1. Parasite and Pathogenicity
| Stage | Symptoms |
|---|---|
| Prodromal |
|
| Acute (often lasts a few days to 1 or 2 weeks) |
|
| Chronic |
|
3.4.2. Epidemiology
3.4.3. Recent Advances in Research
3.5. Blastocystosis
3.5.1. Parasite and Pathogenicity
3.5.2. Epidemiology
| School | No. students | Source of drinking water | No. cases | Infection rate (%) |
|---|---|---|---|---|
| Chongyi middle school | 1,524 | running water | 106 | 6.96 |
| Technical school | 1,074 | spring water | 5 | 0.47 |
| Hengshui middle school | 608 | well water | 2 | 0.33 |
| Chengguan primary school | 2,326 | running water | 264 | 11.35 |
| Chengguan kindergarten | 411 | running water | 117 | 28.47 |
| Woman united kindergarten | 324 | running water | 62 | 19.14 |
| Total | 6,267 | 556 | 8.87 |
3.5.3. Recent Advances in Research
3.6. Schistosomiasis
3.6.1. Parasite and Pathogenicity
| Stages | Symptoms |
|---|---|
| Acute (exposure to high numbers of cercaria) |
|
| Chronic (untreated acute infection) |
|
| Infection outside intestines, liver and spleen |
|
3.6.2. Epidemiology
3.6.3. Recent Advances in Research
3.7. Fascioliasis
3.7.1. Parasites and Pathogenicity
| Stages | Symptoms |
|---|---|
| Acute (last a few months) |
|
| Chronic |
|
| Infection outside biliary system |
|
3.7.2. Epidemiology
3.7.3. Recent Advances in Research
3.8. Fasciolopsiasis
3.8.1. Parasite and Pathogenicity

| Worm Burden | Symptoms |
|---|---|
| Mild infection |
|
| Heavy infection |
|
| Prolonged infection |
|
3.8.2. Epidemiology
| Province | First National Survey | Second National Survey | ||||
|---|---|---|---|---|---|---|
| No. Examined | No. Infected | Infection Rate (%) | No. Examined | No. Infected | Infection Rate (%) | |
| Hubei | 53,382 | 1,002 | 1.877 | 15,524 | 1 | 0.006 |
| Shanghai | 62,134 | 482 | 0.776 | 11,372 | 2 | 0.018 |
| Hunan | 63,794 | 210 | 0.329 | 15,233 | 6 | 0.039 |
| Jiangsu | 62,699 | 181 | 0.284 | 14,700 | 3 | 0.020 |
| Jiangxi | 52,069 | 100 | 0.192 | 20,154 | 30 | 0.149 |
| Gansu | 28,700 | 54 | 0.188 | 9,255 | - | - |
| Guangxi | 51,883 | 58 | 0.112 | 13,990 | 1 | 0.007 |
| Anhui | 54,392 | 60 | 0.110 | 14,873 | 6 | 0.040 |
| Guangdong | 61,517 | 60 | 0.098 | 17,014 | - | - |
| Hainan | 7,958 | 6 | 0.075 | 7,924 | 0 | 0 |
| Zhejiang | 55,284 | 40 | 0.069 | 15,863 | 17 | 0.107 |
| Sichuan * | 97,222 | 57 | 0.059 | 81,359 | 0 | 0 |
| Fujian | 53,416 | 29 | 0.053 | 20,195 | + | + |
| Guizhou | 52,938 | 5 | 0.094 | 15,958 | - | - |
| Liaoning | 51,405 | 4 | 0.008 | 22,767 | - | - |
| Shandong | 87,825 | 4 | 0.005 | 15,152 | 0 | 0 |
| Henan | 85,554 | 1 | 0.001 | 25,894 | 2 | 0.008 |
3.8.3. Recent Advances in Research
3.9. Clonorchiasis
3.9.1. Parasites and Pathogenicity
| Stages | Symptoms |
|---|---|
| Acute |
|
| Chronic |
|
3.9.2. Epidemiology
3.9.3. Recent Advances in Research
3.10. Paragonimiasis
3.10.1. Parasites and Pathogenicity
| Stages | Symptoms |
|---|---|
| Acute |
|
| Chronic |
|
| Infection outside respiratory system |
|
3.10.2. Epidemiology
3.10.3. Recent Advances in Research
3.11. Control Strategies for Water-Related Diseases
3.11.1. Legal Framework
3.11.2. Water Supply and Sanitation
3.11.3. Innovative Approaches for the Control of Water-Related Parasitic Infections
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Zhou, X.N. Prioritizing Research for “One health-One world”. Inf Dis Poverty 2012, 1, 1. [Google Scholar] [CrossRef]
- Huntington, D. Health systems perspectives—Infectious diseases of poverty. Inf. Dis. Poverty 2012, 1, 12. [Google Scholar] [CrossRef]
- Wang, L.D. Report on the National Survey of Current Status of Major Human Parasitic Diseases in China; People’s Medical Publishing House: Beijing, China, 2008. [Google Scholar]
- Xu, L.Q.; Yu, S.H.; Xu, S.M. Distribution and Pathogenic Impact of Human Parasites in China; People’s Medical Publishing House: Beijing, China, 1999. [Google Scholar]
- WHO. Amoebiasis. Wkly. Epidemiol. Rec. 1997, 72, 97–99.
- Cox, F.E. History of human parasitology. Clin. Microbiol. Rev. 2002, 15, 595–612. [Google Scholar] [CrossRef]
- Garcia, L.S. Laboratory identification of the Microsporidia. J. Clin. Microbiol. 2002, 40, 1892–1901. [Google Scholar] [CrossRef]
- Diamond, L.S.; Clark, C.G. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 1993, 40, 340–344. [Google Scholar] [CrossRef]
- Reed, S.L. Amebiasis: An update. Clin. Infect. Dis. 1992, 14, 385–393. [Google Scholar] [CrossRef]
- Tu, X.G.; Yao, L.N.; Huang, X.M.; Chen, H.L.; Yu, K.G.; Jiang, M.G.; Zhu, W.M.; Chen, Y.M.; Liu, B.D.; Lei, C.Q. Second sampled survey on the distribution of human parasites in Zhejiang province. Chin. J. Parasitol. Parasit. Dis. 2001, 19, 145–148. [Google Scholar]
- Xu, B.L.; Zhao, X.D.; Su, Y.P.; Li, H.; He, L.J.; Lin, X.M.; Yan, Q.Y.; Huang, Q.; Yan, X.X.; Liu, H. Survey on present epidemic status of main human parasites infection in Henan province. Chin. J. Parasit. Dis. Control. 2005, 18, 454–457. [Google Scholar]
- Wei, Q.K.; Xu, F.Q.; Huang, B.C.; Cheng, P.; Shi, F.M.; Zhang, Z.H.; Fu, T.X.; Zou, E.S.; Tan, W.B.; Xu, X.L. Analysis of the infectious status of children with intestinal protozoa during 1981–2000 in Jining city. Chin. Trop. Med. 2003, 3, 758–760. [Google Scholar]
- Chen, H.; Li, C.H.; Xie, M.L.; Wei, X.Y.; Yao, L.J. The relationship between diarrhea and infection of intestinal protozoa in Fuzhou district. Strait J. Prev. Med. 2002, 8, 14–16. [Google Scholar]
- Liu, S.W.; Chen, F.L. Epidemiological analysis of amebic dysentery in Qiaotou township of Dongguan city in 2004. Chin. Trop. Med. 2005, 5, 1461–1462. [Google Scholar]
- Mao, L.X.; Zheng, J.J.; Wang, X.M.; Yao, L.N.; Cheng, H.L.; Zhu, Q.G. Epidemiological investigation on an outbreak of amoebic dysentery in Jiangshan city of Zhejiang province. Chin. J. Parasitol. Parasit. Dis. 2009, 27, 182–183. [Google Scholar]
- Tian, L.G.; Steinmann, P.; Chen, J.X.; Chen, S.H.; Zhou, X.N. HIV/AIDS, parasites and co-infections: Publication patterns in China. Parasit. Vectors 2009, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, Y.; Yang, B.; Qi, T.; Lu, H.; Cheng, X.; Tachibana, H. Seroprevalence of Entamoeba histolytica infection in HIV-infected patients in China. Am. J. Trop. Med. Hyg. 2007, 77, 825–828. [Google Scholar]
- Yang, G.Y.; Huang, D.C.; Jiang, B.; Niu, L.L.; Wang, Q.; Yu, M.X.; Zhao, B.; Deng, J.B.; Chen, W.G. Identification and culture of Entamoeba histolytica isolated from Manouria impressa. Chin. J. Prev. Vet. Med. 2007, 29, 248–251. [Google Scholar]
- Niu, L.L.; Wang, Q.; Yang, G.Y.; Huang, D.C.; Jiang, B.; Zhao, B.; Yu, X.M.; Deng, J.B.; Chen, W.G. Patho-histological observation of Manouria impressa with Entamoeba histolytica. Vet. Sci. Chin. 2006, 36, 478–481. [Google Scholar]
- Sun, X.D.; Manue, S.; Zhang, X.Y.; Liu, H.; Deng, Y.; Yang, Y.M.; Wang, L.B.; Li, C.F.; Guo, X.F.; Zhang, C.L. Epidemiological survey of amoebiasis by using TECHLAB E. histolytica II ELISA kits. Chin. Trop. Med. 2006, 6, 1931–1932. [Google Scholar]
- Zengzhu, G.; Bracha, R.; Nuchamowitz, Y.; Cheng, I.W.; Mirelman, D. Analysis by enzyme-linked immunosorbent assay and PCR of human liver abscess aspirates from patients in China for Entamoeba histolytica. J. Clin. Microbiol. 1999, 37, 3034–3036. [Google Scholar]
- Solaymani-Mohammadi, S.; Coyle, C.M.; Factor, S.M.; Petri, W.A., Jr. Amebic colitis in an antigenically and serologically negative patient: Usefulness of a small-subunit ribosomal RNA gene-based polymerase chain reaction in diagnosis. Diagn. Microbiol. Infect. Dis. 2008, 62, 333–335. [Google Scholar] [CrossRef]
- Thompson, R.C. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int. J. Parasitol. 2000, 30, 1259–1267. [Google Scholar] [CrossRef]
- Hunter, P.R.; Thompson, R.C. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 2005, 35, 1181–1190. [Google Scholar] [CrossRef]
- Traub, R.J.; Monis, P.T.; Robertson, I.; Irwin, P.; Mencke, N.; Thompson, R.C. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology 2004, 128, 253–262. [Google Scholar] [CrossRef]
- Luo, R.D. Clinical analysis of 7 cholecystitis cases due to giardiasis. Guangzhou Med. J. 1996, 27, 41–42. [Google Scholar]
- Tu, J.; Zhang, Y.L.; Wang, L.; Wu, T.J. A case of Giardia lamblia infection with cholecystitis and persistent fever. J. Appl. Clin. Pediatr. 2002, 17, 232. [Google Scholar]
- Yang, F.; Xu, J.; Zhang, J. Clinical analysis of 26 pediatric patients with cholecystitis due to Giardia lamblia. J. Appl. Clin. Pediatr. 1990, 5, 23. [Google Scholar]
- Lopez, J.J.; Wright, J.A.; Hammer, R.A.; Ertan, A. Chronic pancreatitis is associated with a high prevalence of giardiasis. Can. J. Gastroenterol. 1992, 6, 73–76. [Google Scholar]
- Cao, J.H.; Chen, Z.Z.; Rong, D.N.; Li, F. Case report: Discovery of Giardia lamblia from pleural effusion. Chin. J. Lab. Med. 2006, 21, 491. [Google Scholar]
- Wu, F.Y.; Liu, M.F. Case report: Discovery of Giardia lamblia from ascites. Sichuan Med. 1996, 17, 198. [Google Scholar]
- Gao, S.W.; Wang, X.G.; Wang, X.Q.; Chen, C.J. Case report: Giardia lamblia infection in joints and peripheral soft tissue. Chin. J. Radiol. 1996, 30, 252. [Google Scholar]
- Meng, X.Y.; Wang, D.N.; He, S.Y. Fatal case due to Giardia lamblia infection. New Med. 1981, 12, 461–462. [Google Scholar]
- Giboda, M.; Viengsay, M.; Bouaphan, S.; Ditrich, O. Epidemiology of intestinal parasitosis in Laos (with anti-amoebic antibody levels). Bull. Soc. Pathol. Exot. 1991, 84, 184–193. [Google Scholar]
- Steinmann, P.; Du, Z.W.; Wang, L.B.; Li, H.J.; Chen, S.R.; Yang, Z.; Fan, W.; Jia, T.W.; Li, L.H.; Vounatsou, P.; et al. Extensive multiparasitism in a village of Yunnan province, People’s Republic of China, revealed by a suite of diagnostic methods. Am. J. Trop. Med. Hyg. 2008, 78, 760–769. [Google Scholar]
- Jin, Q.Q.; Yu, K.M.; Tang, L.F.; Tian, C.L.; Lu, Z.C. Investigation of infectious status of Blastocystis hominis in 1,354 outpatients. Chin. Trop. Med. 2005, 5, 1469–1471. [Google Scholar]
- Chang, H.; Cao, M.F.; Hu, D.S.; Wu, S.B.; Zhang, Y.Q. Case report: Tonsillitis due to Giardia lamblia. J. Clin. Otorhinolaryngol. Head Neck Surg. 2009, 23, 716. [Google Scholar]
- Quan, T.T. Discovery of Giardia lamblia in bile. Med. J. Natl. Def. Forces Southwest China 2009, 19, 587. [Google Scholar]
- Hou, L.N.; Liang, K.Z.; Gao, C.F. Giardia lamblia infection in gallbladder cancer: A case report. Acad. J. Second Mil. Med. Uni. 2007, 28, 1305. [Google Scholar]
- Tian, Z.C.; Zhang, X.C. The discovery of virus from Giardia lambia in China. Chin. J. Vet. Sci. 2003, 23, 42. [Google Scholar]
- Tian, Z.C.; Zhang, X.C.; Li, J.H.; Yin, J.G.; Yang, J. Clone and sequencing of cDNA of complete genome of Giardia lamblia-virus. Chin. J. Vet. Sci. 2003, 23, 573–575. [Google Scholar]
- Bai, X.H.; Zeng, L.; Zhu, B.; Wang, H.L. Existence of Cryptosporidium and Giardia in the effluent from a WWTP and its receiving water in Shanghai. Chin. J. Health Lab. Technol. 2006, 16, 4–5. [Google Scholar]
- Wang, X.Y.; Li, Y.H.; Yu, S.Y.; Li, S.G.; Wang, B. Investigation on contamination of Cryptosporidium parvum and Giardia lamblia in surface water in Shengzhen. Chin. J. Public Health Manag. 2006, 22, 259–261. [Google Scholar]
- Cai, J.; Ye, J.; Du, H.L.; Hu, X.W.; Liu, J.; Huang, W.; Cui, X. Investigation of Cryptosporidium and Giardia contaminating drinking-water in Chengdu. Chin. J. Health Lab. Technol. 2007, 17, 2165–2167. [Google Scholar]
- Andrews, R.H.; Adams, M.; Boreham, P.F.; Mayrhofer, G.; Meloni, B.P. Giardia intestinalis: Electrophoretic evidence for a species complex. Int. J. Parasitol. 1989, 19, 183–190. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, Y.; Wen, J.; Wang, F. The intraspecific difference of the triose phosphate isomerase (tim) gene from Giardia lamblia. Chin. Med. J. 2002, 115, 763–766. [Google Scholar]
- Wen, S.F.; Lu, S.Q.; Feng, X.M.; Wang, F.Y. Molecular phylogeny of Giardia lamblia based on ITS1-5.8SrRNA-ITS2 sequence. Curr. Zool. 2006, 52, 954–958. [Google Scholar]
- Wang, A.L.; Wang, C.C. Viruses of parasitic protozoa. Parasitol. Today 1991, 7, 76–80. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol. Biochem. Parasitol. 1986, 21, 269–276. [Google Scholar] [CrossRef]
- Chen, L.F.; Li, J.H.; Zhang, X.C.; Liu, Q.; Zhao, Y.P.; Cao, L.L.; Chen, C. The analysis of full length cDNA sequence of Giardia canis virus Changchun strain. Acta. Vet. Zootech. Sin. 2006, 37, 408–411. [Google Scholar]
- Tian, Z.C.; Zhang, X.C.; Li, J.H.; Yi, J.G.; Yang, J. The expression of GLV1518-2322 gene from Giardiavirus in Giardia lamblia isolated from human in China and the preparation of its antibodies. Acta. Parasitol. Med. Entomol. Sin. 2004, 11, 5–7. [Google Scholar]
- Marshall, M.M.; Naumovitz, D.; Ortega, Y.; Sterling, C.R. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 1997, 10, 67–85. [Google Scholar]
- Meisel, J.L.; Perera, D.R.; Meligro, C.; Rubin, C.E. Overwhelming watery diarrhea associated with a Cryptosporidium in an immunosuppressed patient. Gastroenterology 1976, 70, 1156–1160. [Google Scholar]
- Nime, F.A.; Burek, J.D.; Page, D.L.; Holscher, M.A.; Yardley, J.H. Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterology 1976, 70, 592–598. [Google Scholar]
- Dang, H.L.; He, G.S.; Zhang, L.X.; Cao, J.; Jin, H.Y.; Yu, J.Y.; Zhu, S.H.; Huang, Y.; Xu, M.Q. Investigation on the prevalence of Cryptosporidium in reptiles and amphibians in Shanghai. Chin. J. Zoonoses 2008, 24, 179–181. [Google Scholar]
- Lu, Q.B.; Chou, S.X.; Ru, B.R.; Liu, W.; Wang, S.M.; Miao, T.; Wang, Y.; Duan, Z.X.; Ning, C.S.; Zhang, L.X. Epidemiological investigation of cryptosporidiosis in dairy calves in some prefectures of Henan province. Chin. Vet. Sci. 2008, 38, 261–267. [Google Scholar]
- Xu, T.S. Epidemiological investigation of Cryptosporidium from poultry in Shandong province. Chin. J. Vet. Med. 2009, 45, 3–5. [Google Scholar]
- Deng, M.; Rutherford, M.S.; Abrahamsen, M.S. Host intestinal epithelial response to Cryptosporidium parvum. Adv. Drug Deliv. Rev. 2004, 56, 869–884. [Google Scholar] [CrossRef]
- Han, F.; Tan, W.X.; Zhou, X.L. Two case reports of cryptosporidiosis in Nanjing. Jiangsu Med. J. 1987, 13, 692. [Google Scholar]
- Lu, S.H.; Lin, A.F.; Chen, R.; Wen, L.Y.; Chen, C.H.; Cheng, Y.Z.; Chen, X.J.; Zhu, G. The diagnosis and analysis of Cryptosporidium infection in children of Zhejiang Province. Chin. J. Zoonoses 2005, 16, 42–44. [Google Scholar]
- Du, X.L.; Ge, J.J.; Qin, Y.F.; Meng, R.; Liu, Y.; Chu, K.; Hou, M.; Yang, P.S.; Wu, H.W. The epidemiological study on cryptosporidiosis in outpatients of Nanjing Children’s Hospital. J. Trop. Med. 2009, 9, 382–385. [Google Scholar]
- Lu, J.; Li, C.P. The survey of Cryptosporidium infection among young children in kindergartens in Anhui Province. Chin. J. Parasitol. Parasit. Dis. 2004, 22, 331–333. [Google Scholar]
- Yue, X.H.; Wang, H.; Gou, J.Z.; Chen, X.C.; Yang, G.L.; Yang, Q.T.; Li, X.H.; Zhou, B.P.; Li, H.Q.; Cai, W.P. Investigation of Cryptosporidium parvum infection in AIDS patients in Guangdong and Yunnan provinces. Chin. J. Exp. Clin. Virol. 2008, 22, 339–341. [Google Scholar]
- Xin, L.; Cui, W.; Liang, R.W.; Ji, R.; Sun, X.N.; Li, R.F. Investigation on the infection of Cryptosporidium among malignant tumor patients. J. Pathogen Biol. 2007, 2, 307–308. [Google Scholar]
- Shen, L.J.; Li, W. Investigation on Cryptosporidium infection among injection drug users in Dali. Chin. J. Public Health 2005, 21, 1295–1296. [Google Scholar]
- Huang, M.Z.; Guan, L.; Xie, M.Z.; Li, D.Q.; Zhou, J.; Li, Z.Y.; Liu, H.; Dai, W.P. Study on condition of Cryptosporidium infection among male drug users in detoxification institute in Changsha city. Chin. J. Public Health 2003, 19, 301–303. [Google Scholar]
- Zhu, H.S.; Du, X.L.; Yu, R.B.; Xu, J.M.; Zhu, L.F.; Wu, H.W. Serum immunology investigation of Cryptosporidium infection among injection drug abusers. Chin. J. Schistosom. Contr. 2008, 20, 364–366. [Google Scholar]
- Fan, B.; He, X.Y.; Huang, Z.M.; Wang, W.L.; Bo, W.F.; Su, Q. Epidemiological investigation of cryptosporidiosis in Yuxi district. Chin. J. Pest Contr. 1992, 8, 228–230. [Google Scholar]
- Su, Y.P.; He, L.J.; Song, J.D.; Zhang, K.R.; Lu, L.F.; Zhang, Q.F. Cryptosporidium infection among children in Henan province. Chin. J. Parasit. Dis. Contr. 1993, 5, 313. [Google Scholar]
- Zhao, X.N.; Zhang, D.M.; Zhang, L.X.; Liu, X.M. Cryptosporidium infections among children in Helongjiang province. Chin. J. Zoonoses 1998, 14, 73–74. [Google Scholar]
- Xing, W.L.; Yang, L.; Liang, S.H.; Liu, Q.Z.; Zheng, X.Y. Cryptosporidiosis in patients with diarrhea in Wenzhou district. J. Wenzhou Med. Coll. 1999, 29, 117–118. [Google Scholar]
- Lu, S.H.; Lin, A.F.; Chen, R.; Wen, L.Y.; Chen, C.H.; Cheng, Y.Z.; Chen, X.J.; Zhu, G. The diagnosis and analysis of Cryptosporidium infection in children of Zhejiang province. Chin. J. Zoonoses 2000, 16, 42–44. [Google Scholar]
- Zhang, Y.J.; Luo, P.; Gao, R. Cryptosporidium infection among children with diarrea in a child hospital in Chengdu city. J. Prac. Parasit. Dis. 2001, 9, 72–73. [Google Scholar]
- Chen, H.; Mao, X.R.; Ling, X.M.; Song, J.J. Investigation on Cryptosporidium infection in population in three districts of Gansu province. Chin. J. Parasitol. Parasit. Dis. 2001, 19, 112. [Google Scholar]
- Luo, W.P.; Li, B.S.; Luo, L.; Tong, S.X.; Liu, Y.; Suo, F.Y.; Dong, Q.; Wang, L. Primary investigation on Cryptosporidium in patients with diarrea in Urumqi. End. Dis. Bull. 2002, 17, 93. [Google Scholar]
- Zhang, B.X.; Yu, H.; Zhang, L.L.; Tao, H.; Li, Y.Z.; Li, Y.; Cao, Z.K.; Bai, Z.M.; He, Y.Q. Prevalence survey on Cyclospora cayetanensis and Cryptosporidium ssp. in diarrhea cases in Yunnan province. Chin. J. Parasitol. Parasit. Dis. 2002, 20, 106–108. [Google Scholar]
- Cai, R.; Li, C.P.; Wang, J.; Xu, L.F.; He, J. An epidemiological survey of cryptosporidiosis with diarrhea in Huainan area. J. Trop. Med. Parasitol. 2003, 1, 26–28. [Google Scholar]
- Xu, H.Z.; Lin, G.H.; Meng, J.F.; Huang, Z.M. Cryptosporidium infection in Longhai city. Chin. J. Zoonoses 2005, 21, 282. [Google Scholar]
- Niu, Y.; Li, R.H.; Yu, X.H. Primary investigation of Cryptosporidium infection in diarrhea patients in Qiqihar district. J. Qiqihar Med. Coll. 2006, 27, 184–185. [Google Scholar]
- Zhou, Y.X. Investigation on Cryptosporidium infection among diarrea patients in Heze city during 2002-2003. End. Dis. Bull. 2006, 21, 38. [Google Scholar]
- Li, Y.; Bin, Y.Y.; Cong, F.; An, C.L. Cryptosporidium infection in humans in Shenyang district. Chin. J. Zoonoses 2006, 22, 473–474. [Google Scholar]
- Shen, J.P.; Ge, J.J. Cryptosporidiosis among 2018 children in kindergartens. J. Appl. Clin. Pediatr. 1991, 6, 132. [Google Scholar]
- Xu, Y.G.; Yao, F.B. Human cryptosporidiosis in Xuzhou and Huaiyin districts, Jiangsu province. J. Parasit. Dis. Contr. 1991, 4, 42–43. [Google Scholar]
- Gong, Y.X.; Cao, S.Q.; Shi, X.Z.; Zhou, S.C.; Liu, Y.X. An investigation of Cryptosporidium infection among children. Acta Acad. Med. Qingdao 1992, 28, 144–146. [Google Scholar]
- Lu, L.A.; LI, c.c.; Fan, Z.Z.; Chen, Y.L. The discovery of zoonotic cryptosporidiosis and epidemiological investigation in Hunan province. Chin. J. Zoonoses 1992, 8, 43–44. [Google Scholar]
- Chen, Y.G.; Guo, H.S.; Dai, X.M.; Yao, F.B.; Shi, W.S.; Lu, M. Cryptosporidium infection in infants and children of Jiangsu province. J. Xuzhou Med. Coll. 1993, 13, 1–4. [Google Scholar]
- Wang, Q.J.; Zhang, J. Cryptosporidium infections among 1124 children in Talimu district, Xinjiang province. Xijiang Med. J. 1995, 25, 187–188. [Google Scholar]
- Cui, W.; Liang, R.W.; Wang, Z.Z. The investigation of children Cryptosporidium parvum infection and epidemiology in Weifang south mountains area. J. Weifang Med. Coll. 2001, 23, 11–12. [Google Scholar]
- Wang, H.Y.; Rong, J.Q.; Wu, G.P. Investigation on cryptosporidiosis in some southern areas of Guizhou province. J. Trop. Med. 2006, 6, 717–718. [Google Scholar]
- Zhu, M.S.; Song, M.H. Cryptosporidium infection among school students in Shiyan district. Chin. J. Sch. Health 2007, 28, 549. [Google Scholar]
- Zhu, M.S.; Song, M.H. Cryptosporidium infection among young children in kindergartens in Shiyan district. Chin. J. Sch. Health 2007, 28, 1040. [Google Scholar]
- Zhu, M.S.; Zhu, J.; Wang, S.J.; Song, M.H. A survey of Cryptosporidium infection among humans being in Shiyan, China. J. Path. Biol. 2009, 4, 685–686. [Google Scholar]
- Wang, L.W.; Yan, G. Advanced on epidemiology and treatment of cryptosporidiosis. J. Pathogen. Biol. 2008, 3, 953–956. [Google Scholar]
- Tian, L.G.; Zhou, X.N. The neglected intestinal parasite co-infection with AIDS. Chin. J. Parasitol. Parasit. Dis. 2008, 26, 376–381. [Google Scholar]
- Wang, Q.; An, C.X.; Guan, F.C.; Zhu, J.J.; Ning, C.S.; Zhang, L.X. Advances in study of genotype marker of Cryptosporidium. Chin. J. Zoonoses 2010, 26, 279–282. [Google Scholar]
- Zhou, C.X.; He, G.S.; Zhang, L.X. Research on the identification technique of the species and genotype of Cryptosporidium in water. Chin. J. Animal Infect. Dis. 2009, 17, 81–86. [Google Scholar]
- Song, D.; Huang, Y.; He, G.S.; Yang, L.R. The progress of research for detective method on Cryptosporidium. J. Inner Mongolia Agric. Uni. 2008, 29, 216–220. [Google Scholar]
- Ortega, Y.R.; Sterling, C.R.; Gilman, R.H.; Cama, V.A.; Diaz, F. Cyclospora species—A new protozoan pathogen of humans. N Engl. J. Med. 1993, 328, 1308–1312. [Google Scholar] [CrossRef]
- Ortega, Y.R.; Gilman, R.H.; Sterling, C.R. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J. Parasitol. 1994, 80, 625–629. [Google Scholar] [CrossRef]
- Shlim, D.R.; Cohen, M.T.; Eaton, M.; Ramachandran, R.; Long, E.G.; Ungar, B.L.P. An alga-like organism associated with an outbreak of prolonged diarrhea among foreigners in Nepal. Am. J. Trop. Med. Hyg. 1991, 45, 383–389. [Google Scholar]
- Huang, P.; Weber, J.T.; Sosin, D.M.; Griffin, P.M.; Long, E.G.; Murphy, J.J.; Kocka, F.; Peters, C.; Kallick, C. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann. Intern. Med. 1995, 123, 409–414. [Google Scholar] [CrossRef]
- Hoge, C.W.; Shlim, D.R.; Rajah, R.; Triplett, J.; Shear, M.; Rabold, J.G.; Echeverria, P. Epidemiology of diarrhoeal illness associated with coccidian-like organism among travellers and foreign residents in Nepal. Lancet 1993, 341, 1175–1179. [Google Scholar] [CrossRef]
- Su, Q.P.; Lin, Q.J.; Chen, J.L. The first case of cyclosporiasis in China. Chin. J. Zoonoses 1995, 11, 6–7. [Google Scholar]
- Zhang, B.X.; Yu, H.; Zhang, L.L.; Tao, H.; Li, Y.Z.; Li, Y.; Cao, Z.K.; Bai, Z.M.; He, Y.Q. Prevalence survey on Cyclospora cayetanensis and Cryptosporidium ssp. in diarrhea cases in Yunnan province. Chin. J. Parasitol. Parasit. Dis. 2002, 20, 106–108. [Google Scholar]
- Chen, X.Y.; Xu, L.F. The infection of Cyclospora on diarrhea children patients in one city. Chin. J. Sch. Health 2006, 27, 817–818. [Google Scholar]
- Xing, W.L.; Wu, K.W.; Lin, X.Y.; Huang, H.C.; Liu, Q.Z.; Zheng, X.Y.; Jin, Y.G. Survey on Cyclospora infection among diarrheal patients in Whenzhou. Chin. J. Parasit. Dis. Control. 2002, 15, 320. [Google Scholar]
- Wang, K.X.; Li, C.P.; Wang, J.; Tian, H. A survey on Cyclospora infection in different population in Anhui province. Chin. J. Epidemiol. 2003, 24, 93. [Google Scholar]
- Ge, J.J. Cyclosporiasis in children misdiagnosed as general enteritis. Clin. Misdiagn. Misther. 1998, 11, 162. [Google Scholar]
- Zhou, Y.; Wang, Q.; Lv, B.; Qi, M.; Guan, F.C.; Zhang, L.X. Advances in epidemiology, taxology and genetics of Cyclospora. Chin. J. Zoonoses 2009, 25, 283–287. [Google Scholar]
- Ge, J.J.; Shen, J.P.; Jiang, X.R. The infection model of Cyclospora cayetanensis in rats. Jiangsu Health Care 2000, 2, 54–55. [Google Scholar]
- Xu, L.F.; Li, C.P. Study on the expression of immune function in cases infected by Cyclospora cayetanensis. Chin. J. Parasitol. Parasit. Dis. 2006, 24, 77–78. [Google Scholar]
- Xu, L.F.; Sun, S.C. Logical qualitative research on the change of T cell subsets counts in the patients with Cyclospora cayetanensis. Chin. J. Health Lab. Technol. 2005, 15, 910–911. [Google Scholar]
- Stenzel, D.J.; Boreham, P.F. Blastocystis hominis revisited. Clin. Microbiol. Rev. 1996, 9, 563–584. [Google Scholar]
- Tan, K.S.; Singh, M.; Yap, E.H. Recent advances in Blastocystis hominis research: Hot spots in terra incognita. Int. J. Parasitol. 2002, 32, 789–804. [Google Scholar] [CrossRef]
- Li, L.H.; Zhang, X.P.; Lv, S.; Zhang, L.; Yoshikawa, H.; Wu, Z.; Steinmann, P.; Utzinger, J.; Tong, X.M.; Chen, S.H.; et al. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol. Res. 2007, 102, 83–90. [Google Scholar] [CrossRef]
- Xie, Z.J.; Zhang, R.Q.; Huang, W.F.; Liao, Y.G.; Su, S.L. Infection status and clinical analysis of Blastocystis hominis in adult diarrheal patients in Ganzhou. J. South. Med. Univ. 2008, 28, 1035–1036. [Google Scholar]
- Liu, Y.; Qian, C.; Chen, X.R.; Zeng, D.Q.; He, A.J.; Yang, Y.H.; Lu, Z.C.; Liu, D.Y. Investigation of Blastocystis hominis from 180 patients. Appl. Prev. Med. 2008, 14, 285–286. [Google Scholar]
- Wu, G.H.; Xiong, Y.S.; Cao, G.L.; Li, G.M.; Liu, M.Z.; Zhu, J.L. An outbreak of Blastocystosis. hominis. Chin. J. Parasit. Dis. Control. 2000, 13, 25–27. [Google Scholar]
- Da, R.; Qiao, J.Y.; Lu, Z.H.; Li, X.Q.; Li, Y.Q.; Wang, W. Observation on the growth status of Blastocystis hominis in different mediums. J. Pathogen Biol. 2006, 1, 132–134. [Google Scholar]
- Li, L.H.; Zhou, X.N.; Du, Z.W.; Wang, X.Z.; Wang, L.B.; Jiang, J.Y.; Yoshikawa, H.; Steinmann, P.; Utzinger, J.; Wu, Z.; et al. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 2007, 56, 281–286. [Google Scholar] [CrossRef]
- He, L.J.; Su, Y.P.; Yan, Q.Y.; Zhu, X.P.; Liu, H. Study on morphology and pathogenicity of Blastocystis hominis. Chin. J. Parasit. Dis. Control. 1999, 12, 195–196. [Google Scholar]
- Jin, Q.Q.; Tang, G.D.; Yu, K.M. Measurement of impair and cytokines in intestinal mucous membrane in patients with Blastocystis hominis. Chin. J. Parasit. Dis. Control. 2005, 18, 352–354. [Google Scholar]
- Boorom, K.F.; Smith, H.; Nimri, L.; Viscogliosi, E.; Spanakos, G.; Parkar, U.; Li, L.H.; Zhou, X.N.; Ok, U.Z.; Leelayoova, S.; et al. Oh my aching gut: Irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit. Vectors 2008, 1, 40. [Google Scholar] [CrossRef]
- Utzinger, J.; Zhou, X.N.; Chen, M.G.; Bergquist, R. Conquering schistosomiasis in China: The long march. Acta Trop. 2005, 96, 69–96. [Google Scholar] [CrossRef]
- Zhou, X.N.; Bergquist, R.; Leonardo, L.; Yang, G.J.; Yang, K.; Sudomo, M.; Olveda, R. Schistosomiasis japonica: Control and research needs. Adv. Parasitol. 2010, 72, 145–178. [Google Scholar] [CrossRef]
- Collins, C.; Xu, J.; Tang, S. Schistosomiasis control and the health system in China. Inf. Dis. Poverty 2012, 1, 7. [Google Scholar] [CrossRef]
- Wang, L.D.; Guo, J.G.; Wu, X.H.; Chen, H.G.; Wang, T.P.; Zhu, S.P.; Zhang, Z.H.; Steinmann, P.; Yang, G.J.; Wang, S.P.; et al. China’s new strategy to block Schistosoma japonicum transmission: Experiences and impact beyond schistosomiasis. Trop. Med. Int. Health 2009, 14, 1475–1483. [Google Scholar] [CrossRef]
- Wu, X.H.; Wang, L.P.; Li, S.Z.; Wang, X.H.; Tao, H.Q.; Pan, H.D.; Li, Y.S.; Li, X.M.; Huang, S.Y.; Zhou, X.N. The process and strategy in elimination of Schistosomiasis in Guangdong, Shanghai, Fujian, Guangxi, Zhejiang Provinces. In The Course and Perspectives in Schistosomiasis Control in China; Wang, L.D., Ed.; People’s Health Press: Beijing, China, 2006; p. 401. [Google Scholar]
- Zhou, X.N.; Guo, J.G.; Wu, X.H.; Jiang, Q.W.; Zheng, J.; Dang, H.; Wang, X.H.; Xu, J.; Zhu, H.Q.; Wu, G.L.; et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg. Infect. Dis. 2007, 13, 1470–1476. [Google Scholar] [CrossRef]
- Hao, Y.; Zheng, H.; Zhu, R.; Guo, J.G.; Wu, X.H.; Wang, L.Y.; Chen, Z.; Zhou, X.N. Schistosomiasis situation in People’s Republic of China in 2008. Chin. J. Schistosomiasis. Control. 2009, 21, 451–456. [Google Scholar]
- Wang, L.; Utzinger, J.; Zhou, X.N. Schistosomiasis control: Experiences and lessons from China. Lancet 2008, 372, 1793–1795. [Google Scholar] [CrossRef]
- Wang, L.D.; Chen, H.G.; Guo, J.G.; Zeng, X.J.; Hong, X.L.; Xiong, J.J.; Wu, X.H.; Wang, X.H.; Wang, L.Y.; Xia, G.; et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl. J. Med. 2009, 360, 121–128. [Google Scholar] [CrossRef]
- Murray, C.J. Quantifying the burden of disease: The technical basis for disability-adjusted life years. Bull. World Health Organ. 1994, 72, 429–445. [Google Scholar]
- King, C.H.; Dickman, K.; Tisch, D.J. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005, 365, 1561–1569. [Google Scholar] [CrossRef]
- Jia, T.W.; Zhou, X.N.; Wang, X.H.; Utzinger, J.; Steinmann, P.; Wu, X.H. Assessment of the age-specific disability weight of chronic Schistosomiasis japonica. Bull. World Health Organ. 2007, 85, 458–465. [Google Scholar] [CrossRef]
- Mott, K.E. Schistosomiasis. In The Global Epidemiology of Infectious Diseases; Murray, C.J.L., Lopez, A.D., Mathers, C.D., Eds.; World Health Organization: Geneva, Switzerland, 2004; pp. 349–391. [Google Scholar]
- Zhou, X.N.; Yang, G.J.; Yang, K.; Wang, X.H.; Hong, Q.B.; Sun, L.P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on Schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar]
- Huang, Y.X.; Ren, Z.Y.; Hang, D.R.; Hong, Q.B.; Gao, Y.; Guo, J.H.; Sun, D.K.; Zuo, Y.P. Potential effect of climate changes on Schistosomiasis japonica transmission in east route of South-to-North Water Diversion Project. Chin. J. Schistosomiasis. Control. 2009, 21, 197–204. [Google Scholar]
- Yang, G.J.; Utzinger, J.; Sun, L.P.; Hong, Q.B.; Vounatsou, P.; Tanner, M.; Zhou, X.N. Effect of temperature on the development of Schistosoma japonicum within Oncomelania hupensis, and hibernation of O. hupensis. Parasitol. Res. 2007, 100, 695–700. [Google Scholar] [CrossRef]
- Yang, G.J.; Utzinger, J.; Lv, S.; Qian, Y.J.; Li, S.Z.; Wang, Q.; Bergquist, R.; Vounatsou, P.; Li, W.; Yang, K.; et al. Regional network for Asian schistosomiasis and other helminth zoonoses (RNAS+): Target diseases in face of climate change. Adv. Parasitol. 2010, 72, 101–135. [Google Scholar]
- Hu, W.; Yan, Q.; Shen, D.K.; Liu, F.; Zhu, Z.D.; Song, H.D.; Xu, X.R.; Wang, Z.J.; Rong, Y.P.; Zeng, L.C.; et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat. Genet. 2003, 35, 139–147. [Google Scholar] [CrossRef]
- Consortium TSjGSaFA. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 2009, 460, 345–351. [CrossRef]
- Liu, F.; Lu, J.; Hu, W.; Wang, S.Y.; Cui, S.J.; Chi, M.; Yan, Q.; Wang, X.R.; Song, H.D.; Xu, X.N.; et al. New perspectives on host-parasite interplay by comparative transcriptomic and proteomic analyses of Schistosoma japonicum. PLoS Pathog. 2006, 2, e29. [Google Scholar] [CrossRef]
- Wang, S.P.; Chen, X.C.; Gao, D.M. Research progress on Schistosome vaccine and prospect of its application in China. Chin. J. Parasitol. Parasit. Dis. 2009, 27, 402–411. [Google Scholar]
- Xiao, S.H.; Keiser, J.; Chen, M.G.; Tanner, M.; Utzinger, J. Research and development of antischistosomal drugs in People’s Republic of China: A 60-year review. Adv. Parasitol. 2010, 72, 231–295. [Google Scholar]
- Xu, J.; Feng, T.; Guo, J.G.; Zheng, H.; Wang, Q.; Wu, X.H.; Zhou, X.N. Comprehensive evaluation of several diagnosis agents of schistosomiasis japonica in China. Chin. J. Schistosomiasis. Control. 2005, 17, 116–119. [Google Scholar]
- Li, S.Z.; Wang, Y.X.; Yang, K.; Liu, Q.; Wang, Q.; Zhang, Y.; Wu, X.H.; Guo, J.G.; Bergquist, R.; Zhou, X.N. Landscape genetics: The correlation of spatial and genetic distances of Oncomelania hupensis, the intermediate host snail of Schistosoma japonicum in mainland China. Geospat. Health 2009, 3, 221–231. [Google Scholar]
- Zhao, Q.P.; Jiang, M.S.; Littlewood, D.T.; Nie, P. Distinct genetic diversity of Oncomelania hupensis, intermediate host of Schistosoma japonicum in mainland China as revealed by ITS sequences. PLoS Negl. Trop. Dis. 2010, 4, e611. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Fried, B. Development of Fasciola Hepatica in the Intermediate Host; CABI Publishing: Oxon, UK, 1999. [Google Scholar]
- Mas-coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, Lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar] [CrossRef]
- Fica, A.; Dabanch, J.; Farias, C.; Castro, M.; Jercic, M.I.; Weitzel, T. Acute fascioliasis—Clinical and epidemiological features of four patients in Chile. Clin. Microbiol. Infect. 2011, 18, 91–96. [Google Scholar]
- Marsden, P.D. Fascioliasis in man: An outbreak in Hampshire. Br. Med. J. 1960, 2, 619–625. [Google Scholar]
- Aksoy, D.Y.; Kerimoglu, U.; Oto, A.; Erguven, S.; Arslan, S.; Unal, S.; Batman, F.; Bayraktar, Y. Fasciola hepatica infection: Clinical and computerized tomographic findings of ten patients. Turk. J. Gastroenterol. 2006, 17, 40–45. [Google Scholar]
- Bjorland, J.; Bryan, R.T.; Strauss, W.; Hillyer, G.V.; McAuley, J.B. An outbreak of acute fascioliasis among Aymara Indians in the Bolivian Altiplano. Clin. Infect. Dis. 1995, 21, 1228–1233. [Google Scholar] [CrossRef]
- Xuan, T.; Hung, N.T.; Waikagul, J. Cutaneous fascioliasis: A case report in Vietnam. Am. J. Trop. Med. Hyg. 2005, 72, 508–509. [Google Scholar]
- Flores, M.; Merino-Angulo, J.; Aguirre Errasti, C. Pulmonary infiltrates as first sign of infection by Fasciola. hepatica. Eur. J. Respir. Dis. 1982, 63, 231–233. [Google Scholar]
- Chen, M.G. Fasciola. hepatica infection in China. Southeast Asian J. Trop. Med. Public Health 1991, 22, 356–360. [Google Scholar]
- Lin, R.Q.; Dong, S.J.; Nie, K.; Wang, C.R.; Song, H.Q.; Li, A.X.; Huang, W.Y.; Zhu, X.Q. Sequence analysis of the first internal transcribed spacer of rDNA supports the existence of the intermediate Fasciola between F. hepatica and F. gigantica in mainland China. Parasitol. Res. 2007, 101, 813–817. [Google Scholar]
- Lin, R.Q.; Dong, S.J.; Xu, C.X.; Huang, W.Y.; Song, H.Q.; Zhu, X.Q. DNA polymorphism in first internal transcribed spacer (ITS-1) rDNA of Fasciola. from Mainland China. Chin. J. Vet. Sci. Technol. 2004, 34, 8–12. [Google Scholar]
- Huang, W.Y.; Dong, S.J.; Yang, X.Y.; Lin, R.Q.; Qian, D.X.; Zhang, W.Y.; Song, H.Q.; Zhu, X.Q. DNA polymorphism of the mitochondrial NADH dehydrogenase subunit 1 gene (nad1) of Fasciola. from China. Chin. J. Prev. Vet. Med. 2005, 27, 273–276. [Google Scholar]
- Keiser, J.; Engels, D.; Buscher, G.; Utzinger, J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin. Investig. Drugs 2005, 14, 1513–1526. [Google Scholar] [CrossRef]
- Huang, W.Y.; He, B. Chinese Fasciola. detemination by ITS2 sequence and RFLP. Anim. Sci. Vet. Med. 2002, 19, 2–6. [Google Scholar]
- Dong, S.J.; Huang, W.Y.; Lin, R.Q.; Qian, D.X.; Wu, S.Q.; Song, H.D.; Zhu, X.Q. DNA polymophism of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) of Fasciola. from Mainland China. Chin. J. Vet. Sci. 2005, 25, 378–381. [Google Scholar]
- Weng, Y.L.; Zuang, Z.L.; Lu, C.X.; Yao, T.L. Drinking water is a new transmission route of Fasciolopsis buski for human and animals. Chin. J. Zool. 1980, 23, 48–50. [Google Scholar]
- Lin, Y.G.; Cai, J.Z.; Weng, Y.L.; Zhang, C.X.; Yan, R.L.; He, Y.C.; Zheng, Y.Z.; Chen, Q.Q.; Chen, M. Studies on the epidemiology and treatment of human fasciolopsiasis in Putian district, Fujian province. J. Xiamen Univ. 1978, 17, 118–129. [Google Scholar]
- Xiong, J.J.; Deng, Z.B. Epidemiology of intestinal parasitic diseases in 61,392 residents in Jiangling county. Chin. J. Rural Med. 1994, 22, 14–15. [Google Scholar]
- Xu, Y.C.; Xu, F. Severe anemia and oedema due to Fasciolopsis buski infection. J. Clin. Transfusion Lab. Med. 2009, 11, 274. [Google Scholar]
- Wang, K.X.; Wang, H.Y.; He, Q.; Ye, S. Case report: Intestinal obstruction due to Fasciolopsis buski infection. Chin. J. Surg. 2005, 43, 1168. [Google Scholar]
- Zhou, R.G. Case report: Fasciolopsis buski infection in biliary tract. Chin. J. General Surg. 2003, 12, 493. [Google Scholar]
- Chen, X.L.; Yuan, Y.; Chen, Q.; Zhou, Y.S.; Zhang, X.H.; Liu, H. Studies on detecting antibodies in human sera of Fasciolopsiasis buski by ELISA. J. Centr. Chin. Norm. Univ. 2004, 38, 85–87. [Google Scholar]
- Zhang, Y.; Yang, Z.N.; Jiang, N.; Hu, H.B. 13 cases of patients with fasciolopsiasis diagnosed by gastroscopy at early stage of infection. Zhejiang J. Clin. Med. 2002, 4, 356. [Google Scholar]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar]
- Qian, M.-B.; Chen, Y.-D.; Yan, F. Time to tackle clonorchiasis in China. Infect. Dis. Poverty 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Furst, T.; Keiser, J.; Utzinger, J. Global burden of human food-borne trematodiasis: A systematic review and meta-analysis. Lancet Infect. Dis. 2011, 12, 210–221. [Google Scholar]
- Qian, M.B.; Chen, Y.D.; Liang, S.; Yang, G.J.; Zhou, X.N. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect. Dis. Poverty 2012, 1, 4. [Google Scholar] [CrossRef]
- Yang, L.C.; Huang, B.Y.; Xue, G.F.; Li, X.L.; Mo, D.L. Relationship between infection of Clonorchis sinensis and hepatobiliary and pancreatic diseases. Chin. J. Hepatobiliary. Surg. 2004, 10, 165–166. [Google Scholar]
- Qu, H.M.; Qu, Z.M.; Liang, J.L.; Jia, B. Case report: 4096 patients with clonorchiasis. J. Pathogen. Biol. 2006, 1, 197. [Google Scholar]
- Hotez, P.J.; Ehrenberg, J.P. Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv. Parasitol. 2010, 72, 31–53. [Google Scholar] [CrossRef]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Huang, Y.; Sun, J.; Men, J.; Liu, H.; Luo, F.; Guo, L.; Lv, X.; Deng, C.; et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011, 12, R107. [Google Scholar] [CrossRef]
- Qian, M.B.; Chen, Y.D.; Fang, Y.Y.; Xu, L.Q.; Zhu, T.J.; Tan, T.; Zhou, C.H.; Wang, G.F.; Jia, T.W.; Yang, G.J.; Zhou, X.N. Disability weight of Clonorchis sinensis infection: Captured from community study and model simulation. PLoS Negl. Trop. Dis. 2011, 5, e1377. [Google Scholar] [CrossRef]
- Choi, M.H.; Park, S.K.; Li, Z.; Ji, Z.; Yu, G.; Feng, Z.; Xu, L.; Cho, S.Y.; Rim, H.J.; Lee, S.H.; Hong, S.T. Effect of control strategies on prevalence, incidence and re-infection of clonorchiasis in endemic areas of China. PLoS Negl. Trop. Dis. 2010, 4, e601. [Google Scholar] [CrossRef]
- Xiao, S.H.; Xue, J.; Wu, Z.X. Experimental study progress on tribendimidine, artemether and artesuante against Clonorchis sinensis and other trematodes. Chin. J. Parasitol. Parasit. Dis. 2009, 27, 65–69. [Google Scholar]
- Qian, M.B.; Yap, P.; Yang, Y.C.; Liang, H.; Jiang, Z.H.; Li, W.; Tan, Y.G.; Zhou, H.; Utzinger, J.; Zhou, X.N.; Keiser, J. Efficacy and safety of tribendimidine against Clonorchis sinensis. Clin. Infect. Dis. 2013, 56, e76–e82. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Steinmann, P.; Zhou, X.N.; Utzinger, J. Helminth infections of the central nervous system occurring in Southeast Asia and the Far East. Adv. Parasitol. 2010, 73, 351–408. [Google Scholar]
- Li, D.J.; Liao, Y.F.; Zhu, X.R.; Liu, S.X. Electroencephalogram analysis of 106 cases with cerebral paragonimiasis. J. Wenzhou Med. Coll. 1996, 26, 22–24. [Google Scholar]
- Zhang, E.X. Clinical analysis of 38 pediatric cases with cerebral paragonimiasis in the west mountainous area of Hubei province. Clin. Pediatr. J. 1990, 8, 177–179. [Google Scholar]
- Li, P.; Fu, P.; He, J.C. Analysis of the clinical type and therapeutic efficiency of 280 children cases with paragonimiasis. J. Pathogen Biol. 2008, 3, 2–3. [Google Scholar]
- Wang, B.K.; Wang, H.X. Cerebral hemorrhage due to Paragonimus infection in Sichuan Province. J. Stroke Neurol. Dis. 1990, 7, 137–138. [Google Scholar]
- Cui, J.; Wang, Z.Q.; Wu, F.; Jin, X.X. An outbreak of paragonimiosis in Zhengzhou city, China. Acta Trop. 1998, 70, 211–216. [Google Scholar] [CrossRef]
- Ruan, W.; Lin, C.P.; Yao, L.N.; Lai, J.; Xia, R.S.; Yao, S.R. An outbreak of paragonimiasis. Dis. Surveill. 2009, 24, 978–979. [Google Scholar]
- Wang, C.Q.; Yang, S.J.; Pan, H.M.; Wang, D.J. Study on Paragonimus infection by drinking water. Chin. Prev. Med. 2008, 9, 675–676. [Google Scholar]
- Huang, W.D.; Zhong, H.L. Successful infection of Paragonimus in dogs and cats by ingesting cercariae. Chin. J. Vet. Med. 2008, 44, 62–63. [Google Scholar]
- Cheng, Y.Z.; Wu, X.P.; Li, L.S.; Lin, C.X.; Jiang, D.W. A new species of Tricula as the first intermetiate host of Paragonimus skriabini (Mesogastropoda: Pomatiopsidae) from China. Mar. Sci. 2009, 33, 97–99. [Google Scholar]
- Cheng, Y.Z.; Li, L.S.; Lin, C.X.; Li, Y.S.; Fang, Y.Y.; Jiang, H.D.; Huang, C.Y.; Zhou, A.P.; Zhang, X. Two new species of Huananpotamonas the second intermediate host of Paragonimus skriabini (Decapoda: Potamidae). Chin. J. Zoonoses 2008, 24, 885–889. [Google Scholar]
- Li, Y.S.; Cheng, Y.Z.; Lin, C.X.; Zhang, Z.P.; Zhou, X.M.; Jiang, M. A new species of freshwater crab infected with metacercariae of Paragonimus: The genus Huananpotamon tangi sp. nov. Chin. J. Zoonoses. 2008, 24, 125–127. [Google Scholar]
- Cheng, Y.Z.; Lin, G.H.; Li, Y.S. Two new species of freshwater crabs (Decapoda: Potamidae) serving as intermediate hosts of Paragonimus in Fujian, China. Chin. J. Parasitol. Parasit. Dis. 2010, 26, 241–245. [Google Scholar]
- Dan, X.Y.; Lin, C.X.; Li, Y.S.; Hu, Y.; Sheng, X.S.; Lou, H.Q. A new species of Paragonimus sheni—With a key to the species adult worm and metaceycariae of the genus Paragonimus in China. Chin. J. Zoonoses 2009, 25, 1143–1148. [Google Scholar]
- Liu, C.Q.; Guan, F.; Chen, Y.; Niu, A.O. Preliminary analysis of genetic variation of 11 populations of Paragonimus in China by random amplified polymorphic DNA technique. Chin. J. Zoonoses 2008, 24, 1041–1044. [Google Scholar]
- Qian, B.Z.; Sugiyama, H.; Zhu, Z.H. Studied on genetic variation of Paragonimus westermani in Ninghai, Zhejiang province. Chin. J. Trop. Dis. Parasitol. 2007, 6, 69–72. [Google Scholar]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Tanner, M.; Utzinger, J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia 2005, 47, 127–134. [Google Scholar]
- McManus, D.P.; Gray, D.J.; Li, Y.; Feng, Z.; Williams, G.M.; Stewart, D.; Rey-Ladino, J.; Ross, A.G. Schistosomiasis in the People’s Republic of China: The era of the Three Gorges Dam. Clin. Microbiol. Rev. 2010, 23, 442–466. [Google Scholar]
- Li, Y.S.; Raso, G.; Zhao, Z.Y.; He, Y.K.; Ellis, M.K.; McManus, D.P. Large water management projects and schistosomiasis control, Dongting Lake region, China. Emerg. Infect. Dis. 2007, 13, 973–979. [Google Scholar]
- Sun, J.G.; Li, J.M.; An, J.A.; Hu, Y.H.; Zhang, G.; Sun, X.H.; Sun, F.Y.; Bai, X.T.; Niu, W.Y.; Li, L. Functions of biogas construction on public health in rural areas. Chin. J. Health Educ. 2006, 22, 822–825. [Google Scholar]
- Jiang, W.S.; Chen, H.G.; Zeng, X.J.; Hong, X.L.; Ge, J.; Hu, S.Z.; Li, J. Effectiveness analysis of harmless treatment of human faeces to control eggs contamination of soil-transmitted nematodes. J. Pathogen Biol. 2008, 3, 49–52. [Google Scholar]
- Waltner-Toews, D. An ecosystem approach to health and its applications to tropical and emerging diseases. Cad. Saude Publica 2001, 17, 7–36. [Google Scholar]
- Bergquist, R.; Whittaker, M. Strengthening control of neglected tropical diseases in the Asia Pacific region: Implications for health information priorities. Inf. Dis. Poverty 2012, 1, 4. [Google Scholar] [CrossRef]
- Zhou, X.N.; Bergquist, R.; Tanner, M. Elimination of tropical disease through surveillance and response. Inf. Dis. Poverty 2013, 2, 1. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lv, S.; Tian, L.-G.; Liu, Q.; Qian, M.-B.; Fu, Q.; Steinmann, P.; Chen, J.-X.; Yang, G.-J.; Yang, K.; Zhou, X.-N. Water-Related Parasitic Diseases in China. Int. J. Environ. Res. Public Health 2013, 10, 1977-2016. https://doi.org/10.3390/ijerph10051977
Lv S, Tian L-G, Liu Q, Qian M-B, Fu Q, Steinmann P, Chen J-X, Yang G-J, Yang K, Zhou X-N. Water-Related Parasitic Diseases in China. International Journal of Environmental Research and Public Health. 2013; 10(5):1977-2016. https://doi.org/10.3390/ijerph10051977
Chicago/Turabian StyleLv, Shan, Li-Guang Tian, Qin Liu, Men-Bao Qian, Qing Fu, Peter Steinmann, Jia-Xu Chen, Guo-Jing Yang, Kun Yang, and Xiao-Nong Zhou. 2013. "Water-Related Parasitic Diseases in China" International Journal of Environmental Research and Public Health 10, no. 5: 1977-2016. https://doi.org/10.3390/ijerph10051977




