Lead Ions Encapsulated in Liposomes and Their Effect on Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
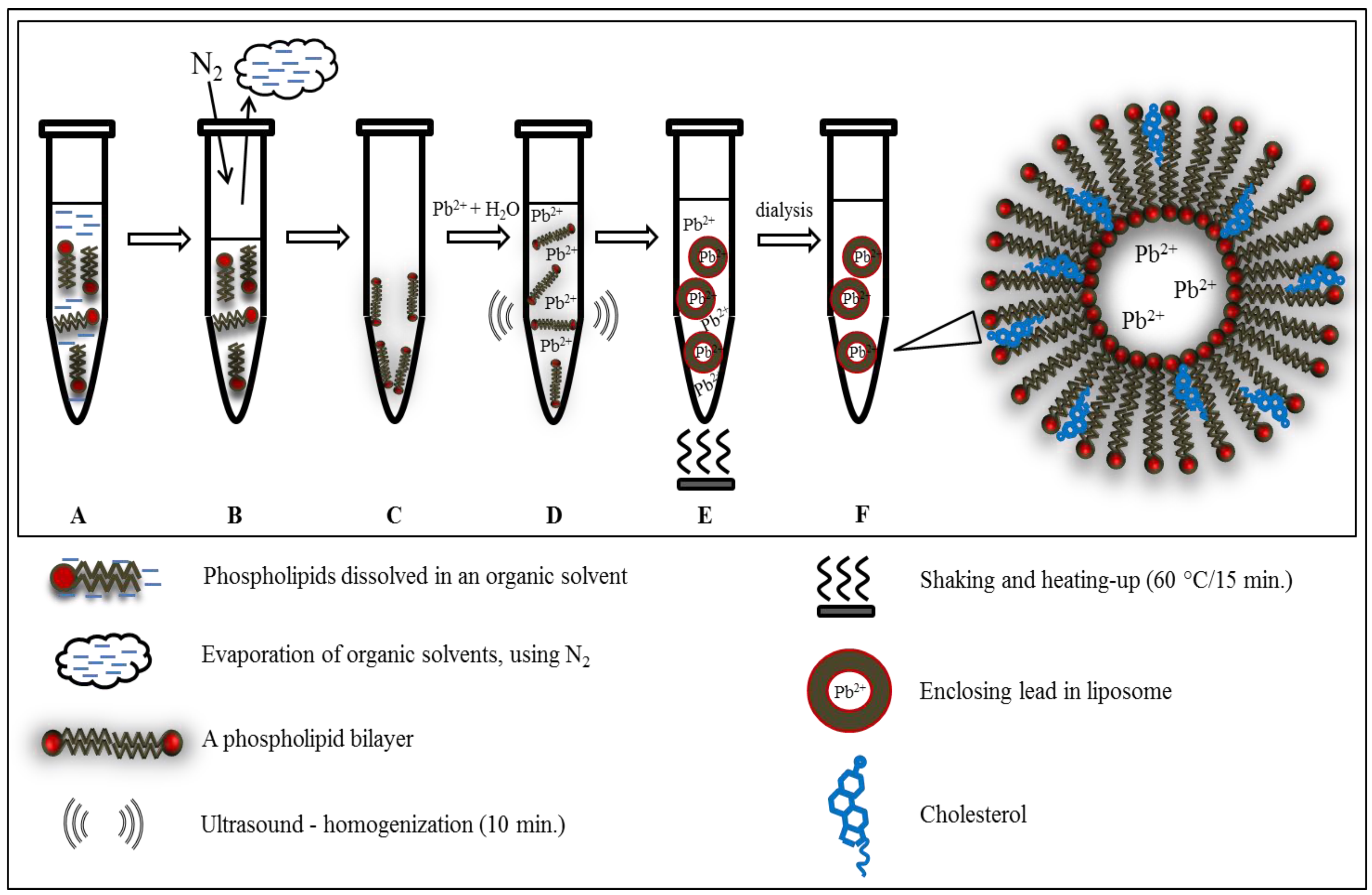
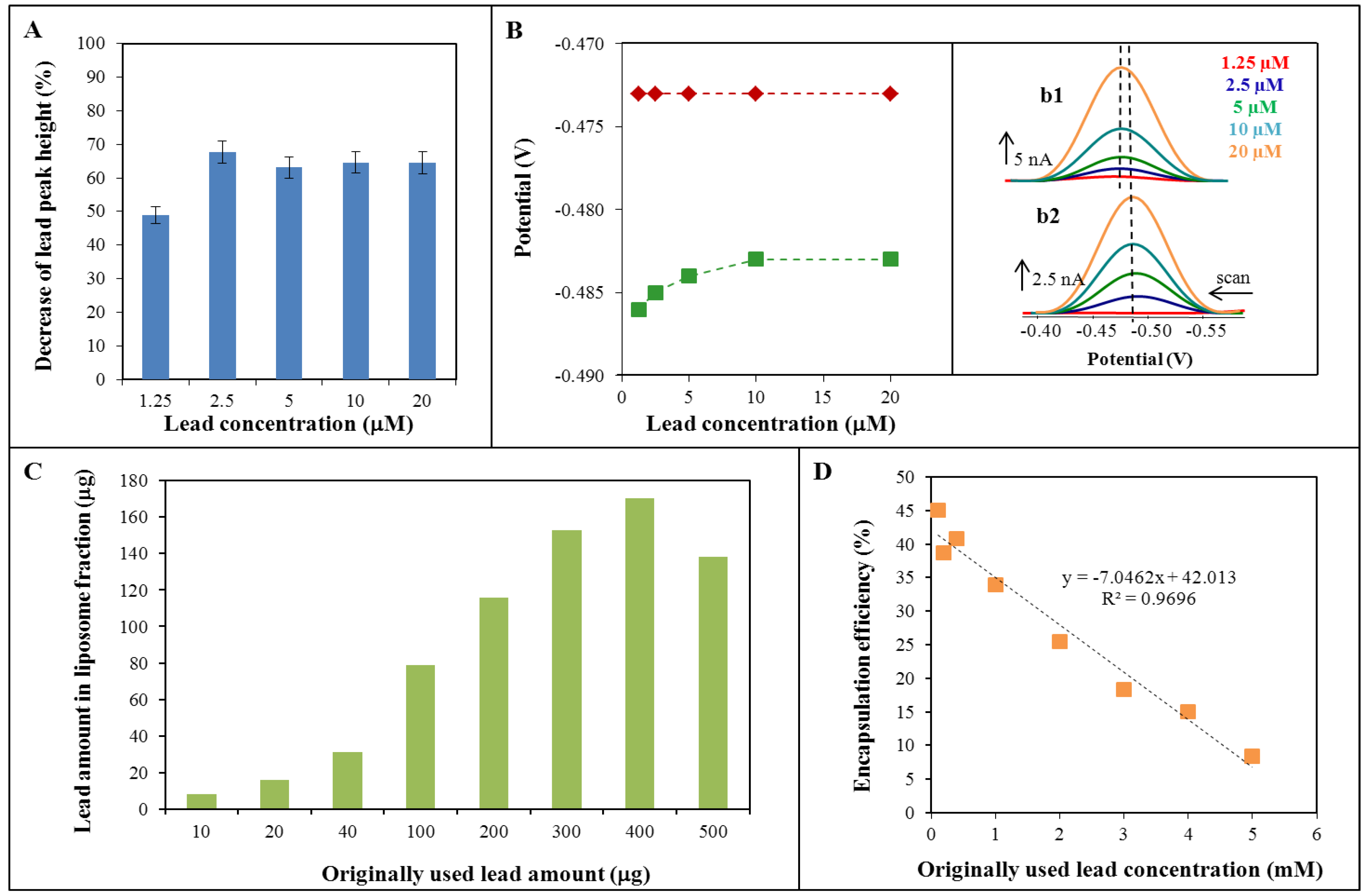
2.1. Cyclic Voltammetry of Lead—Liposome Complex

2.2. Lead Encapsulation in Liposome
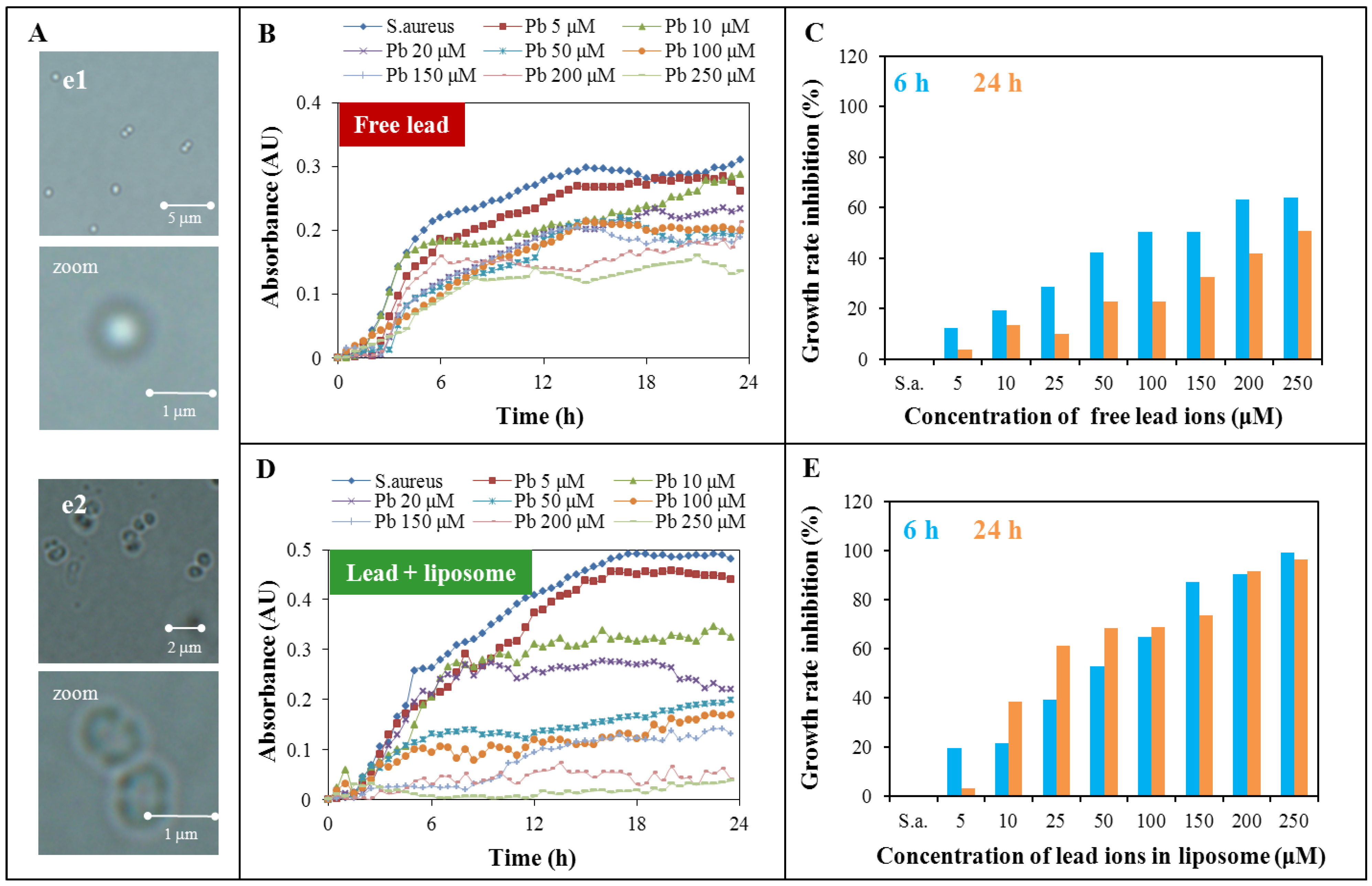
2.3. Toxicity Determination of Free Lead Ions and Lead Encapsulated in the Liposome
| Hours | Free lead | Encapsulated lead |
|---|---|---|
| 6 | 10.3 | 43.4 |
| 12 | 57.9 | 31.9 |
| 18 | 315.6 | 25.5 |
| 24 | 236.9 | 28.0 |
3. Experimental Section
3.1. Chemicals and Materials
3.2. Preparation of Liposome
3.3. Preparation of Liposome Filled with Lead
3.4. Electrochemical Determination
3.4.1. Differential Pulse Voltammetry (DPV)
3.4.2. Cyclic Voltammetry (CV)
3.5. Atomic Absorption Spectrometry (AAS)
3.6. Toxicity Determination of Free Lead Ions and Lead Encapsulated in the Liposome
3.6.1. Cultivation of Staphylococcus aureus
3.6.2. Growth Curves
3.6.3. Statistical Evaluation
3.6.4. Cell Microscopy
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Neal, A.P.; Guilarte, T.R. Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. 2013, 2, 99–114. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Arain, M.B.; Jamali, M.K.; Kazi, G.H.; Jalbani, N. Determination of cadmium and lead in biological samples by three ultrasonic-based samples treatment procedures followed by electrothermal atomic absorption spectrometry. J. AOAC Int. 2007, 90, 470–478. [Google Scholar]
- Najafi, N.M.; Massumi, A.; Shafaghizadeh, M. In situ digestion of serum samples in graphite furnace prior to determination by ETAAS. Sci. Iran. 2005, 12, 324–328. [Google Scholar]
- Afridi, H.I.; Kazi, T.G.; Kazi, A.G.; Shah, F.; Wadhwa, S.K.; Kolachi, N.F.; Shah, A.Q.; Baig, J.A.; Kazi, N. Levels of arsenic, cadmium, lead, manganese and zinc in biological samples of paralysed steel mill workers with related to controls. Biol. Trace Elem. Res. 2011, 144, 164–182. [Google Scholar] [CrossRef]
- Parsons, P.J. Monitoring human exposure to lead—An assessment of current laboratory performance for the determination of blood lead. Environ. Res. 1992, 57, 149–162. [Google Scholar] [CrossRef]
- Blazewicz, A.; Orlicz-Szczesna, G.; Prystupa, A.; Szczesny, P. Use of ion chromatography for the determination of selected metals in blood serum of patients with type 2 diabetes. J. Trace Elem. Med. Biol. 2010, 24, 14–26. [Google Scholar] [CrossRef]
- Vamvakaki, V.; Fournier, D.; Chaniotakis, N.A. Fluorescence detection of enzymatic activity within a liposome based nano-biosensor. Biosens. Bioelectron. 2005, 21, 384–388. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Pantos, A.; Tsiourvas, D.; Paleos, C.M.; Nounesis, G. Enhanced drug transport from unilamellar to multilamellar liposomes induced by molecular recognition of their lipid membranes. Langmuir 2005, 21, 6696–6702. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Dos Santos, N.; Waterhouse, D.; Masin, D.; Tardi, P.G.; Karlsson, G.; Edwards, K.; Bally, M.B. Substantial increases in idarubicin plasma concentration by liposome encapsulation mediates improved antitumor activity. J. Control. Release 2005, 105, 89–105. [Google Scholar] [CrossRef]
- Oja, C.; Tardi, P.; Schutze-Redelmeier, M.P.; Cullis, P.R. Doxorubicin entrapped within liposome-associated antigens results in a selective inhibition of the antibody response to the linked antigen. Biochim. Biophys. Acta-Biomembr. 2000, 1468, 31–40. [Google Scholar] [CrossRef]
- Petersen, A.L.; Hansen, A.E.; Gabizon, A.; Andresen, T.L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1417–1435. [Google Scholar] [CrossRef]
- Viswanathan, S.; Wu, L.C.; Huang, M.R.; Ho, J.A.A. Electrochemical immunosensor for cholera toxin using liposomes and poly(3,4-ethylenedioxythiophene)-coated carbon nanotubes. Anal. Chem. 2006, 78, 1115–1121. [Google Scholar] [CrossRef]
- Zhong, Z.Y.; Peng, N.; Qing, Y.; Shan, J.L.; Li, M.X.; Guan, W.; Dai, N.; Gu, X.Q.; Wang, D. An electrochemical immunosensor for simultaneous multiplexed detection of neuron-specific enolase and pro-gastrin-releasing peptide using liposomes as enhancer. Electrochim. Acta 2011, 56, 5624–5629. [Google Scholar]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum dots—Characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef]
- Hynek, D.; Prasek, J.; Pikula, J.; Adam, V.; Hajkova, P.; Krejcova, L.; Trnkova, L.; Sochor, J.; Pohanka, M.; Hubalek, J.; et al. Electrochemical analysis of lead toxicosis in vultures. Int. J. Electrochem. Sci. 2011, 6, 5980–6010. [Google Scholar]
- Krystofova, O.; Trnkova, L.; Adam, V.; Zehnalek, J.; Hubalek, J.; Babula, P.; Kizek, R. Electrochemical microsensors for the detection of cadmium(II) and lead(II) ions in plants. Sensors 2010, 10, 5308–5328. [Google Scholar] [CrossRef]
- Hynek, D.; Krejcova, L.; Krizkova, S.; Ruttkay-Nedecky, B.; Pikula, J.; Adam, V.; Hajkova, P.; Trnkova, L.; Sochor, J.; Pohanka, M.; et al. Metallomics study of lead-protein interactions in albumen by electrochemical and electrophoretic methods. Int. J. Electrochem. Sci. 2012, 7, 943–964. [Google Scholar]
- Krizkova, S.; Zitka, O.; Adam, V.; Beklova, M.; Horna, A.; Svobodova, Z.; Sures, B.; Trnkova, L.; Zeman, L.; Kizek, R. Possibilities of electrochemical techniques in metallothionein and lead detection in fish tissues. Czech J. Anim. Sci. 2007, 52, 143–148. [Google Scholar]
- Sochor, J.; Majzlik, P.; Salas, P.; Adam, V.; Trnkova, L.; Hubalek, J.; Kizek, R. A study of availability of heavy metal ions by using various exracction procedures and electrochemical detection. Lis. Cukrov. Repar. 2010, 126, 414–415. [Google Scholar]
- Percival, S.L.; Thomas, J.; Linton, S.; Okel, T.; Corum, L.; Slone, W. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. Int. Wound J. 2012, 9, 488–493. [Google Scholar] [CrossRef]
- Martinez-Abad, A.; Sanchez, G.; Lagaron, J.M.; Ocio, M.J. On the different growth conditions affecting silver antimicrobial efficacy on Listeria monocytogenes and Salmonella enterica. Int. J. Food Microbiol. 2012, 158, 147–154. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Soler, C.; Lagaron, J.M.; Ocio, M.J. Effects of chitosan films on the growth of Listeria monocytogenes, Staphylococcus aureus and Salmonella spp. in laboratory media and in fish soup. Int. J. Food Microbiol. 2010, 137, 287–294. [Google Scholar] [CrossRef]
- Borneman, D.L.; Ingham, S.C.; Ane, C. Mathematical approaches to estimating lag-phase duration and growth rate for predicting growth of Salmonella serovars, Escherichia coli O157:H7, and Staphylococcus aureus in raw beef, bratwurst, and poultry. J. Food Prot. 2009, 72, 1190–1200. [Google Scholar]
- Rufian-Henares, J.A.; Morales, F.J. Microtiter plate-based assay for screening antimicrobial activity of melanoidins against E. coli and S. aureus. Food Chem. 2008, 111, 1069–1074. [Google Scholar] [CrossRef]
- Mahdavi, B.; Yaacob, W.A.; Din, L.B.; Nazlina, I. Antimicrobial activity of consecutive extracts of Etlingera brevilabrum. Sains Malays. 2012, 41, 1233–1237. [Google Scholar]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar] [CrossRef]
- Belley, A.; Harris, R.; Beveridge, T.; Parr, T.; Moeck, G. Ultrastructural effects of oritavancin on methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Antimicrob. Agents Chemother. 2009, 53, 800–804. [Google Scholar] [CrossRef]
- Webster, D.; Rennie, R.P.; Brosnikoff, C.L.; Chui, L.; Brown, C. Methicillin-resistant Staphylococcus aureus with reduced susceptibility to vancomycin in Canada. Diagn. Microbiol. Infect. Dis. 2007, 57, 177–181. [Google Scholar] [CrossRef]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, C.H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.F.; Zhang, L.F. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar]
- Kilian, G.; Tshanga, S.S.; Oidu, B.; Milne, P.J. Antimicrobial activity of liposome encapsulated cyclo(l-tyrosyl-l-prolyl). Pharmazie 2011, 66, 421–423. [Google Scholar]
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial efficacy of liposome-encapsulated silver ions and tea tree oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Lett. Appl. Microbiol. 2013, 57, 33–39. [Google Scholar] [CrossRef]
- Omri, A.; Ravaoarinoro, M. Preparation, properties and the effects of amikacin, netilmicin and tobramycin in free and liposomal formulations on Gram-negative and Gram-positive bacteria. Int. J. Antimicrob. Agents 1996, 7, 9–14. [Google Scholar] [CrossRef]
- Kunjachan, S.; Blauz, A.; Mockel, D.; Theek, B.; Kiessling, F.; Etrych, T.; Ulbrich, K.; van Bloois, L.; Storm, G.; Bartosz, G.; et al. Overcoming cellular multidrug resistance using classical nanomedicine formulations. Eur. J. Pharm. Sci. 2012, 45, 421–428. [Google Scholar] [CrossRef]
- Gonzalez, N.; Sevillano, D.; Alou, L.; Cafini, F.; Gimenez, M.-J.; Gomez-Lus, M.-L.; Prieto, J.; Aguilar, L. Influence of the MBC/MIC ratio on the antibacterial activity of vancomycin versus linezolid against methicillin-resistant Staphylococcus aureus isolates in a pharmacodynamic model simulating serum and soft tissue interstitial fluid concentrations reported in diabetic patients. J. Antimicrob. Chemother. 2013, 68, 2291–2295. [Google Scholar]
- Chudobova, D.; Dobes, J.; Nejdl, L.; Maskova, D.; Rodrigo, M.A.M.; Nedecky, B.R.; Krystofova, O.; Kynicky, J.; Konecna, M.; Pohanka, M.; et al. Oxidative stress in Staphylococcus aureus treated with silver(I) ions revealed by spectrometric and voltammetric assays. Int. J. Electrochem. Sci. 2013, 8, 4422–4440. [Google Scholar]
- Gangwar, A. Antimicrobial effectiveness of different preparations of calcium hydroxide. Ind. J. Dental Res. 2011, 22, 66–70. [Google Scholar] [CrossRef]
- Chudobova, D.; Nejdl, L.; Gumulec, J.; Krystofova, O.; Rodrigo, M.A.M.; Kynicky, J.; Ruttkay-Nedecky, B.; Kopel, P.; Babula, P.; Adam, V.; et al. Complexes of silver(I) ions and silver phosphate nanoparticles with hyaluronic acid and/or chitosan as promising antimicrobial agents for vascular grafts. Int. J. Mol. Sci. 2013, 14, 13592–13614. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kensova, R.; Blazkova, I.; Konecna, M.; Kopel, P.; Chudobova, D.; Zitka, O.; Vaculovicova, M.; Hynek, D.; Adam, V.; Beklova, M.; et al. Lead Ions Encapsulated in Liposomes and Their Effect on Staphylococcus aureus. Int. J. Environ. Res. Public Health 2013, 10, 6687-6700. https://doi.org/10.3390/ijerph10126687
Kensova R, Blazkova I, Konecna M, Kopel P, Chudobova D, Zitka O, Vaculovicova M, Hynek D, Adam V, Beklova M, et al. Lead Ions Encapsulated in Liposomes and Their Effect on Staphylococcus aureus. International Journal of Environmental Research and Public Health. 2013; 10(12):6687-6700. https://doi.org/10.3390/ijerph10126687
Chicago/Turabian StyleKensova, Renata, Iva Blazkova, Marie Konecna, Pavel Kopel, Dagmar Chudobova, Ondrej Zitka, Marketa Vaculovicova, David Hynek, Vojtech Adam, Miroslava Beklova, and et al. 2013. "Lead Ions Encapsulated in Liposomes and Their Effect on Staphylococcus aureus" International Journal of Environmental Research and Public Health 10, no. 12: 6687-6700. https://doi.org/10.3390/ijerph10126687




