The Kinetic Signature of Toxicity of Four Heavy Metals and Their Mixtures on MCF7 Breast Cancer Cell Line †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Culturing Reagent
2.3. Description of Exposure Studies
2.3.1. Real-Time Cell Electronic Sensing (RT-CES)
2.3.2. Testing the Kinetic Response of the Individual Metals on MCF7 Cells
2.3.3. Testing the Kinetic Response of Quaternary Mixture of Metals on MCF7 Cells
2.3.4. Testing the Kinetic Response of Individual and Mixture of Metals on LBSO Pretreated MCF7 Cells
3. Results
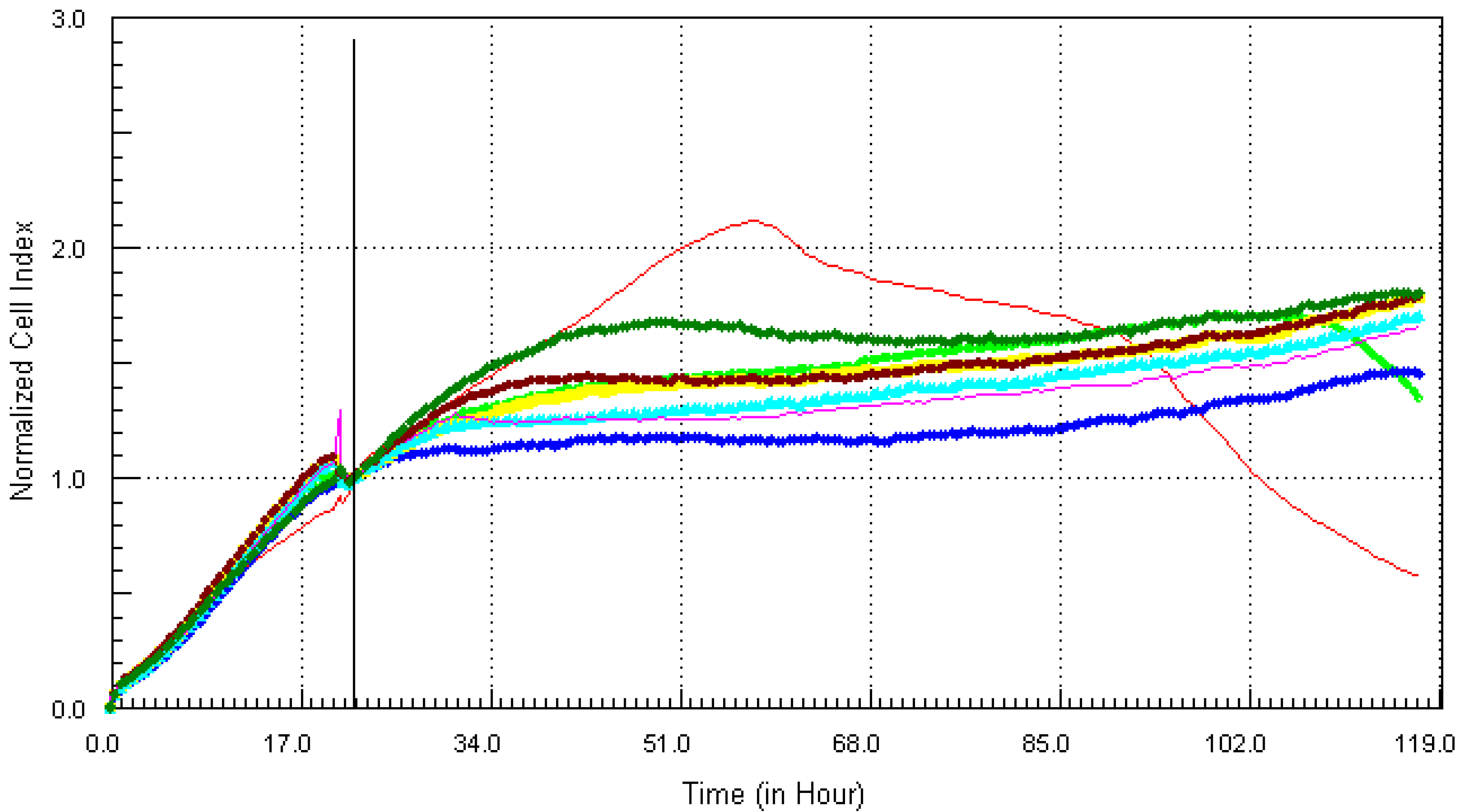
3.1. Kinetic Response of Individual and Composite Mixture of Metals on MCF7 Cells
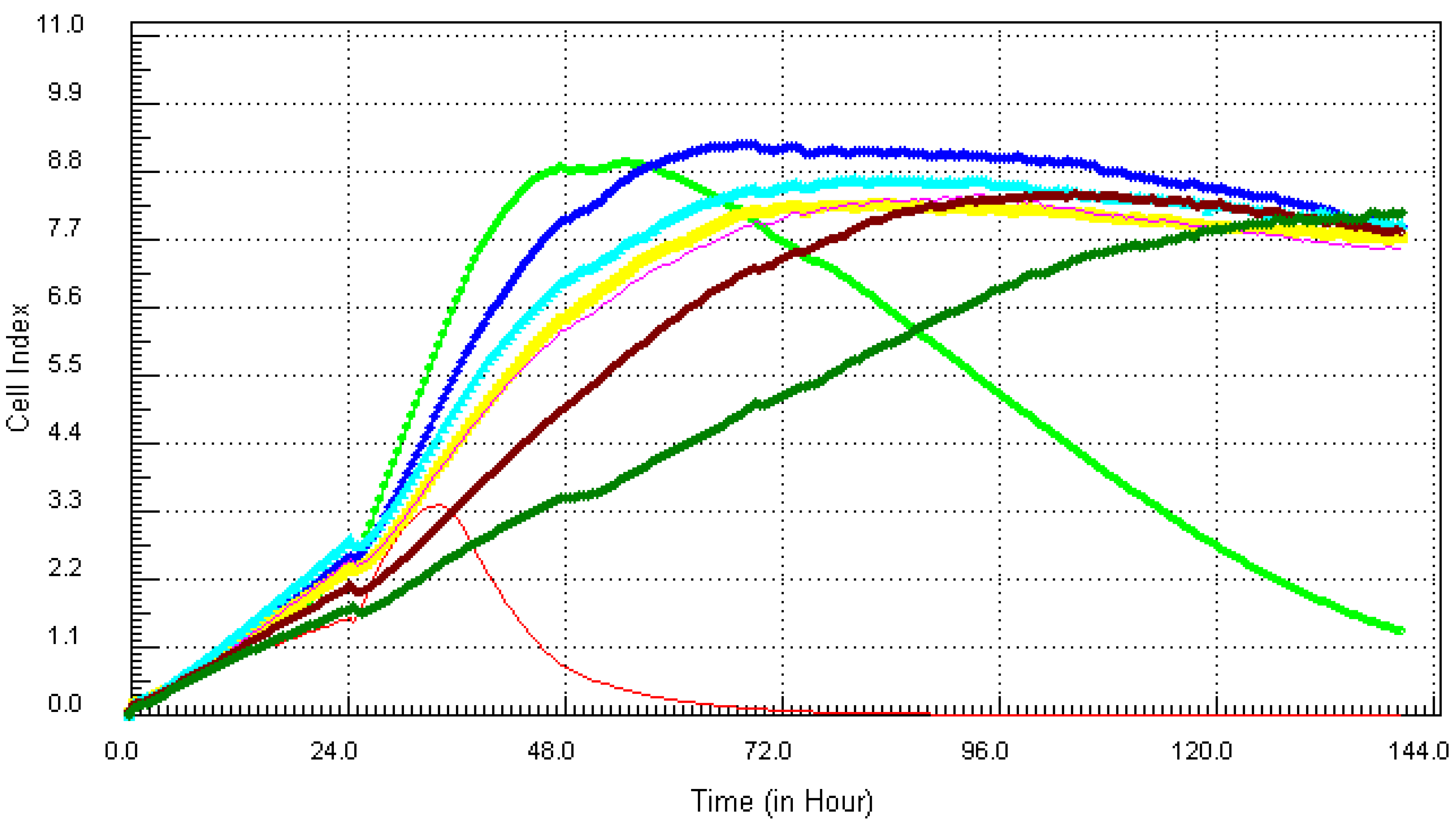
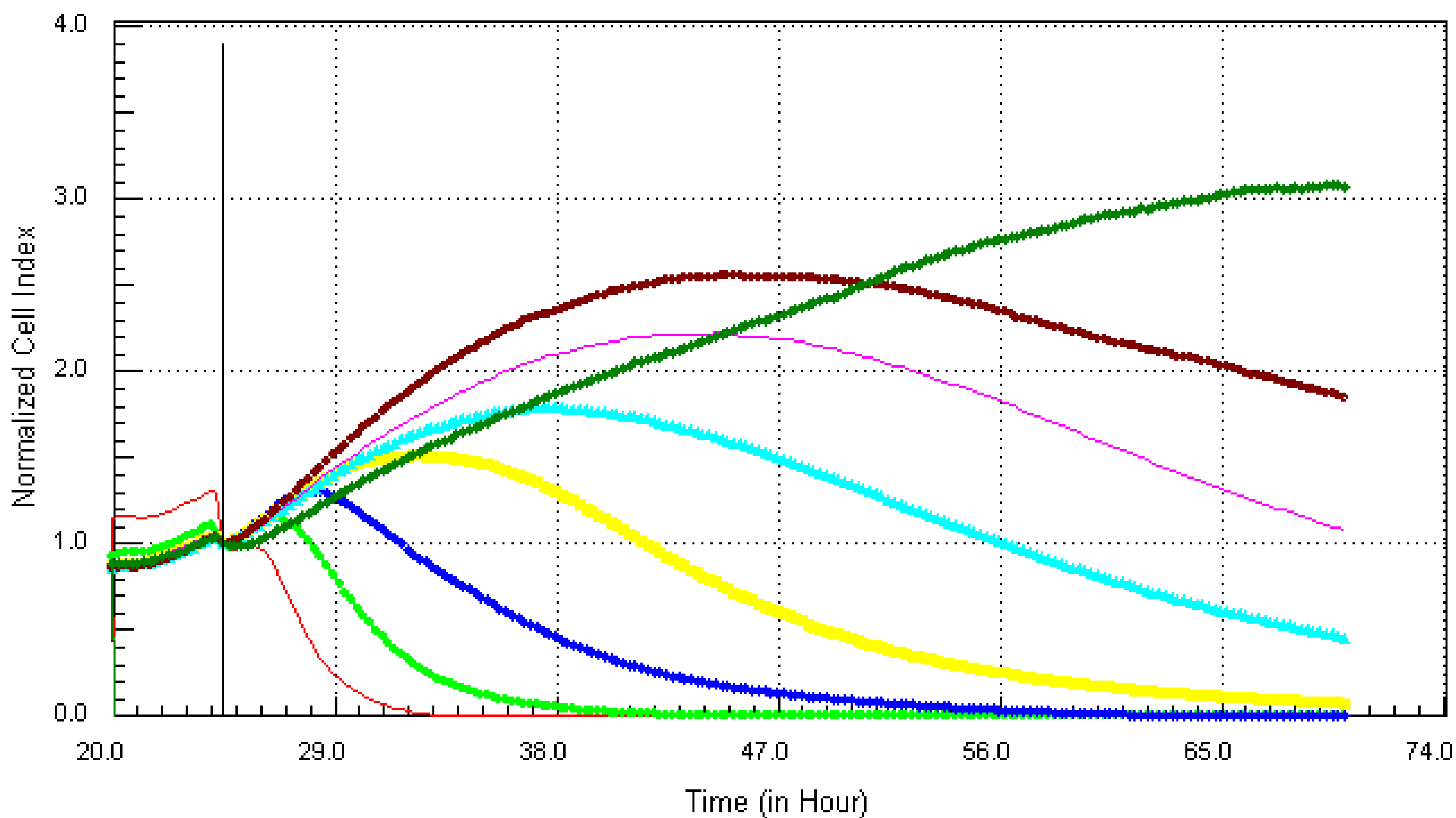
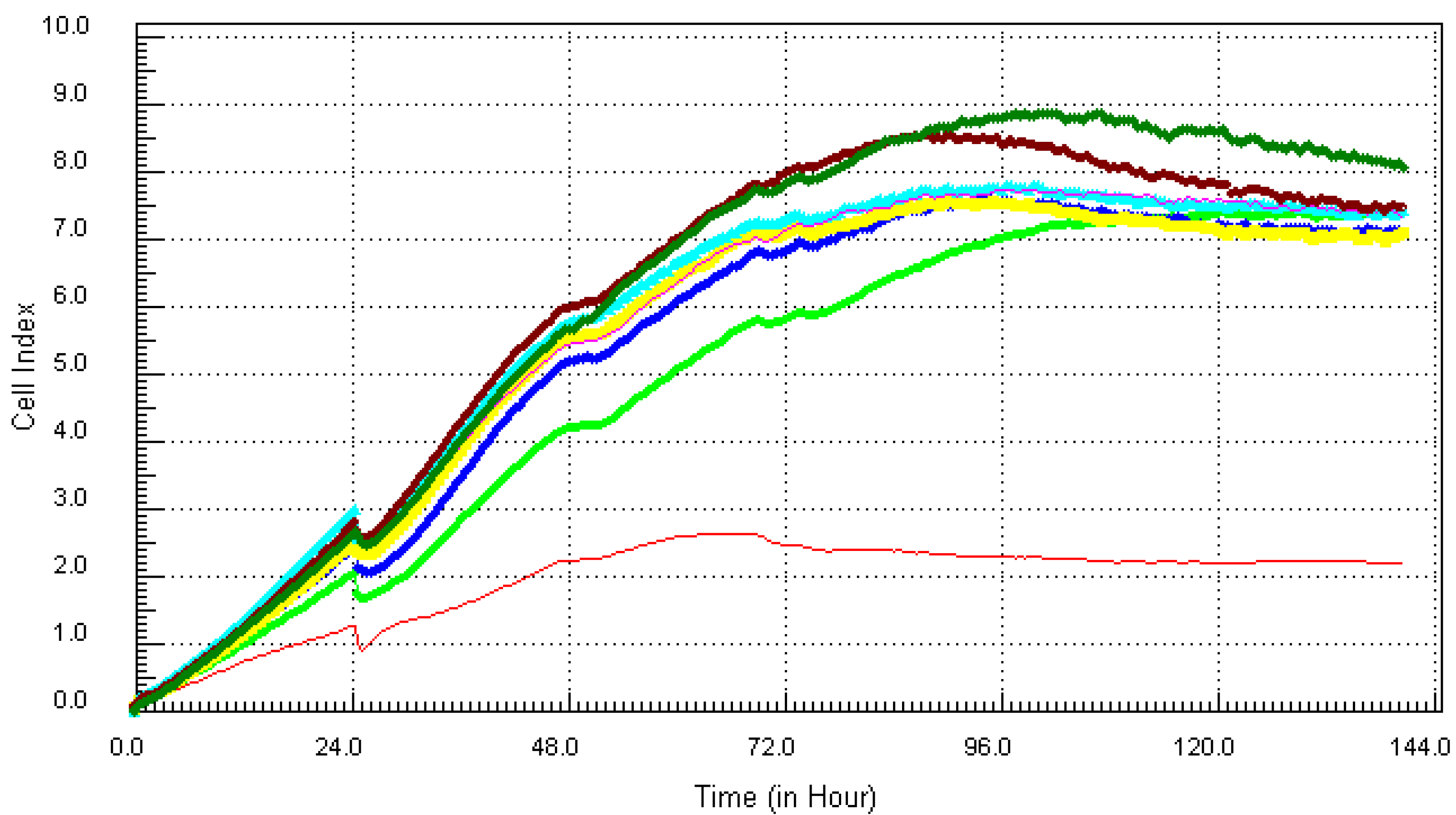
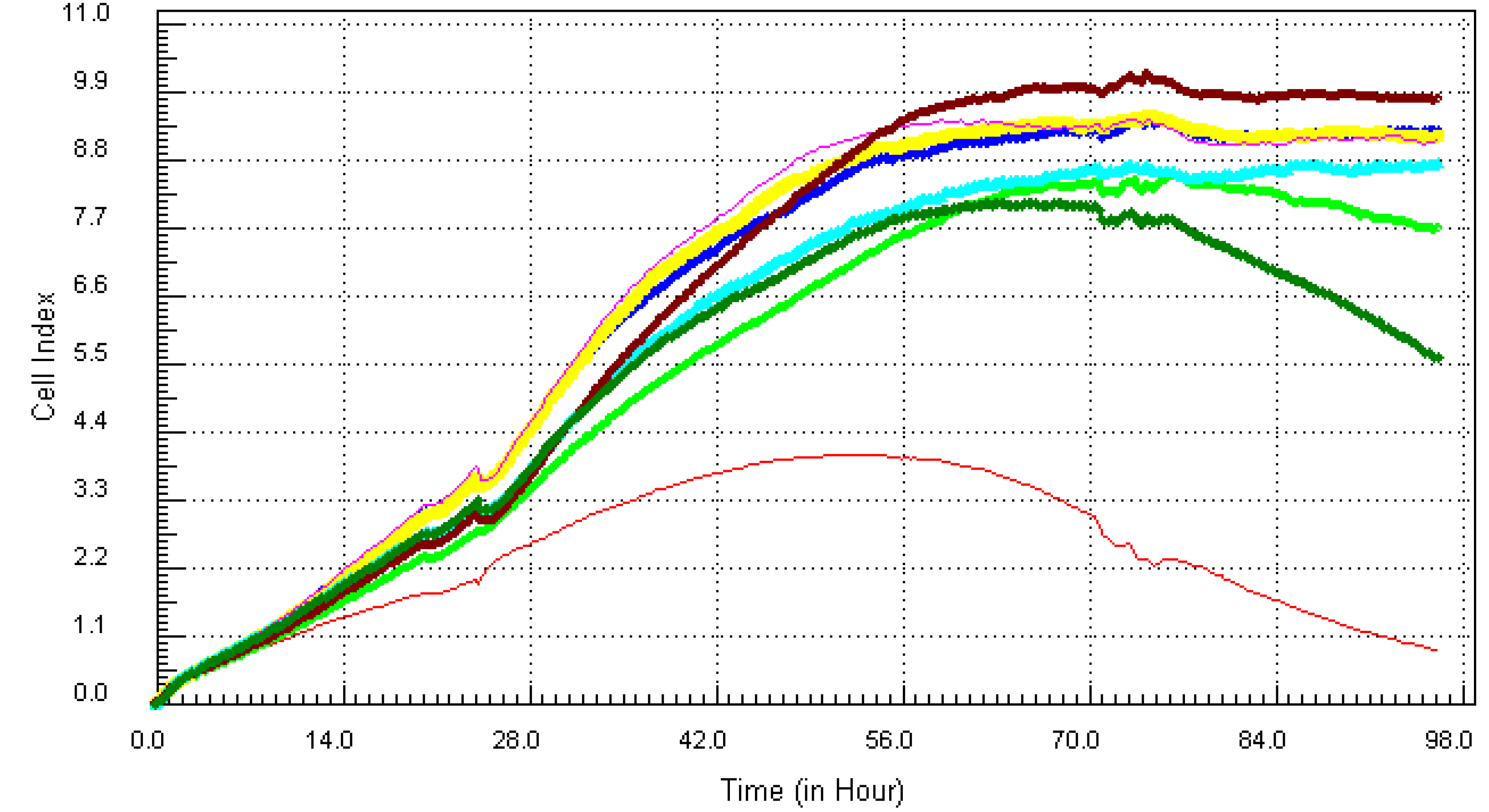
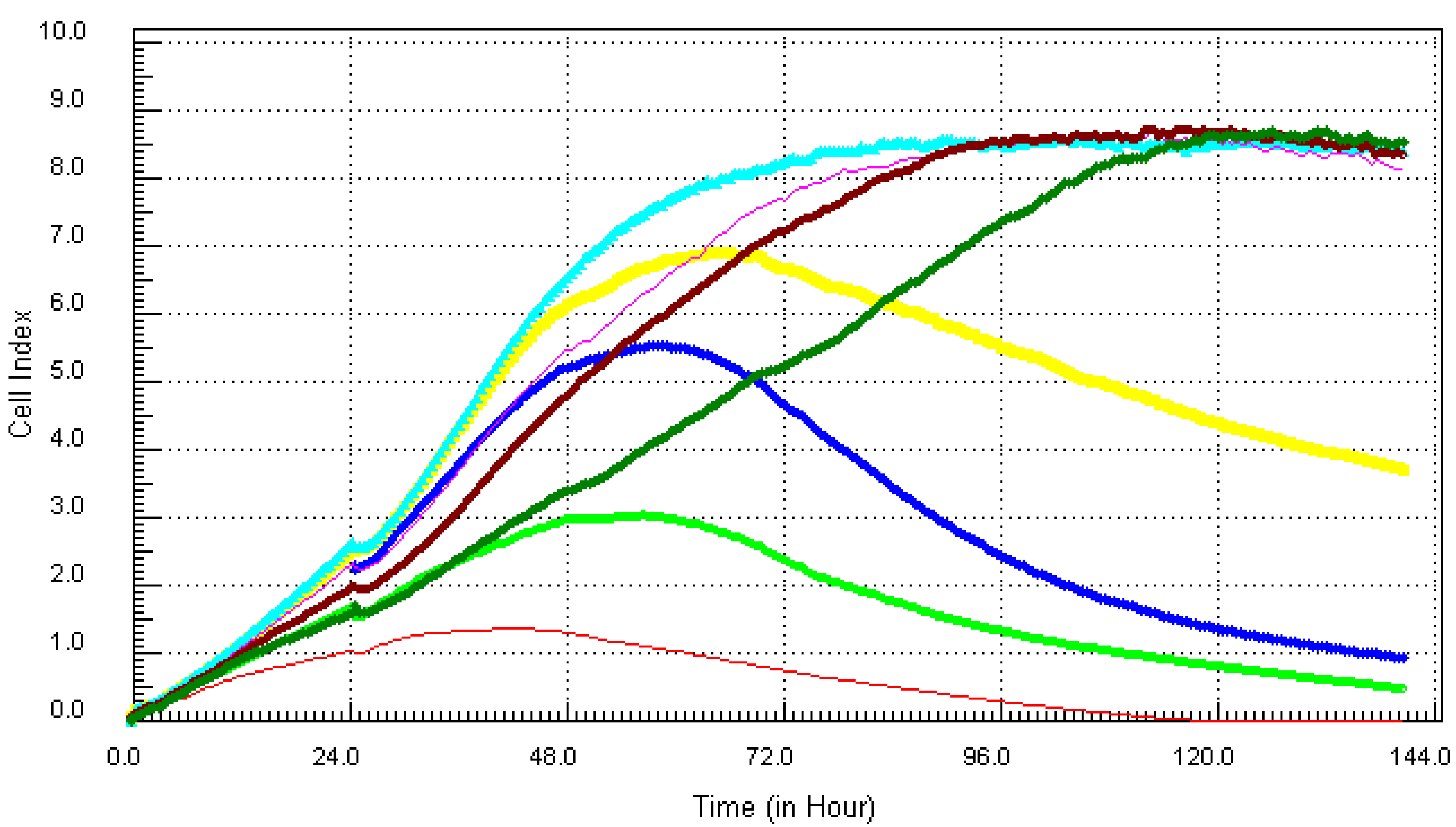
3.2. Kinetic Response of Individual and Quaternary Mixture of Metals on LBSO Pretreated MCF7 Cells
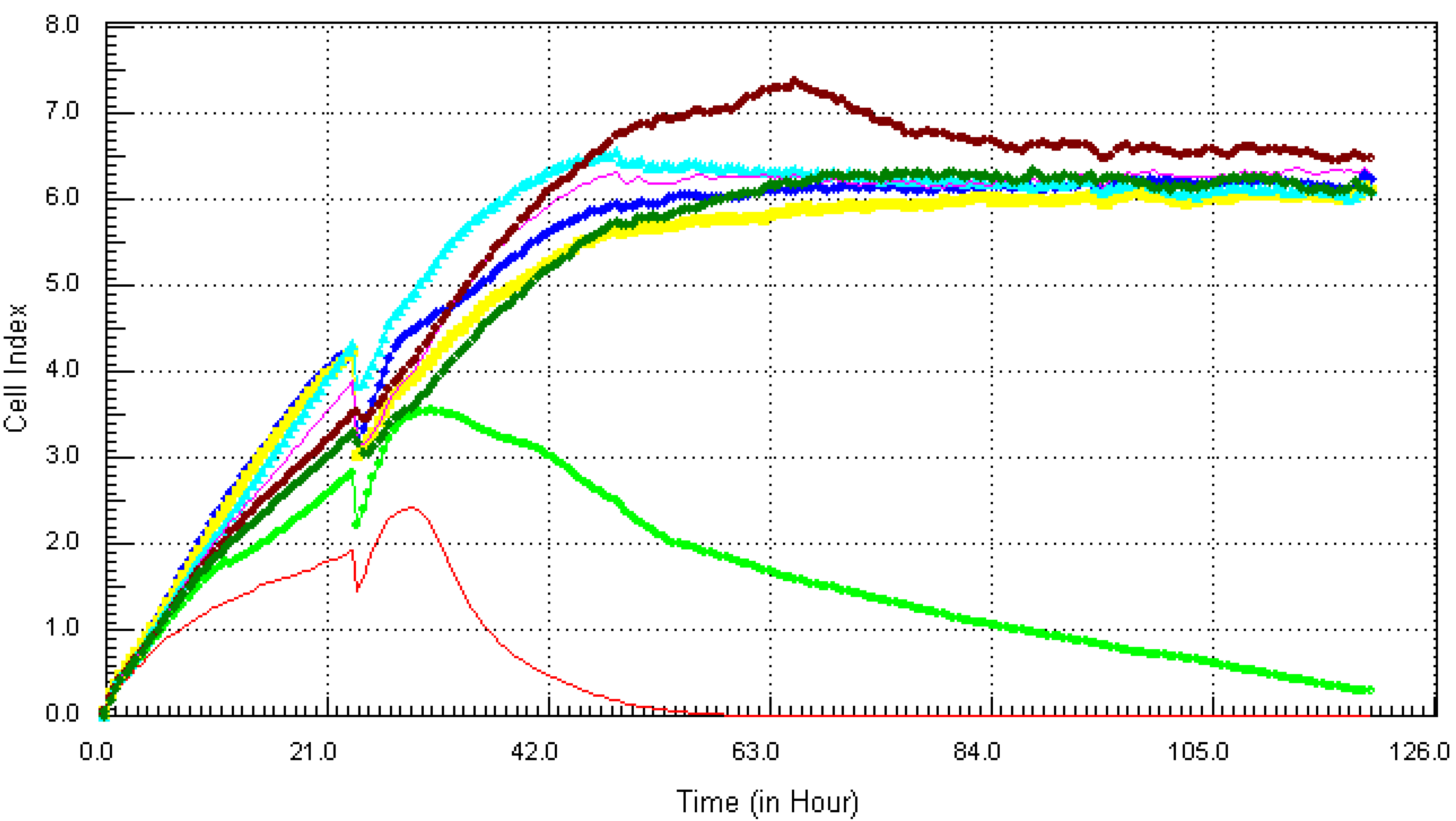
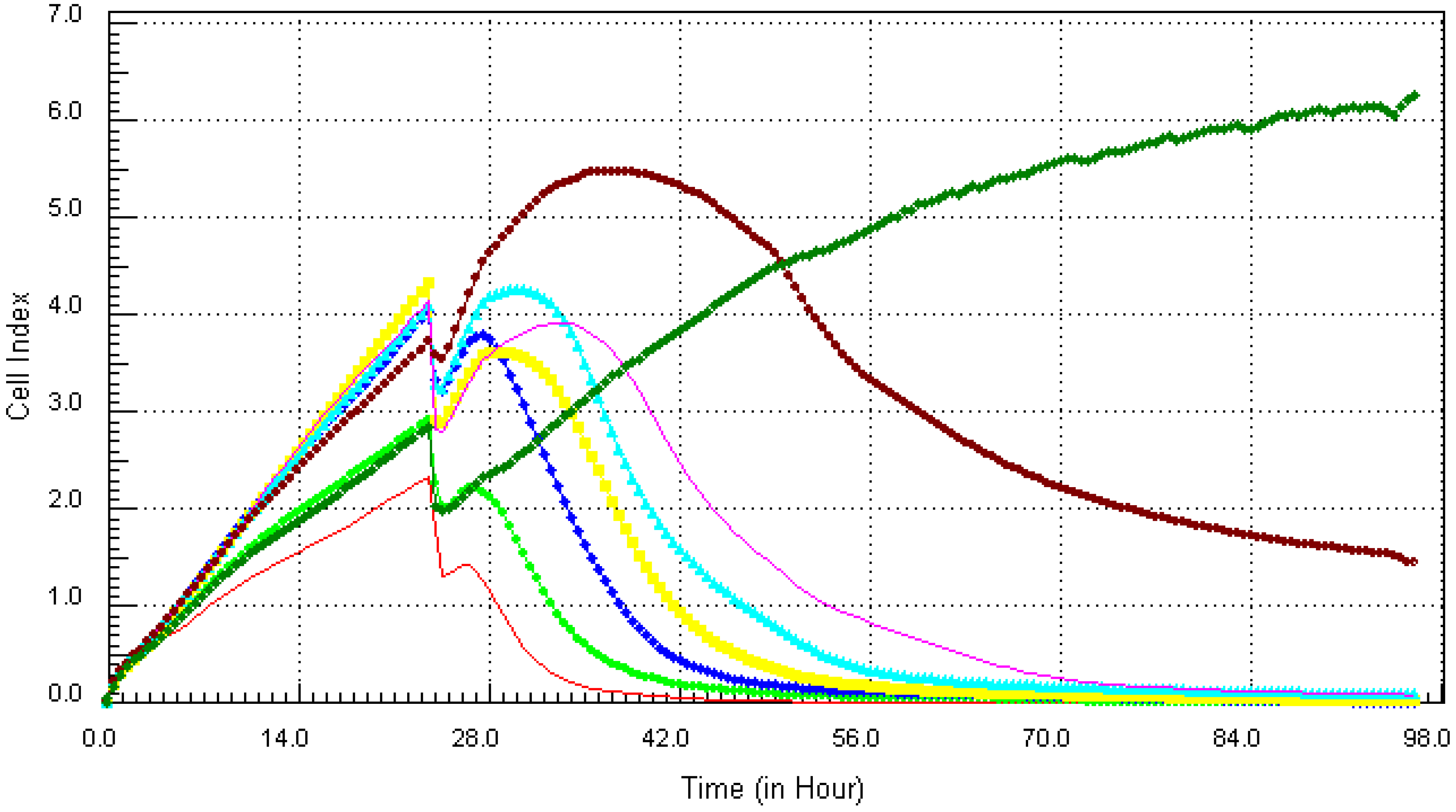
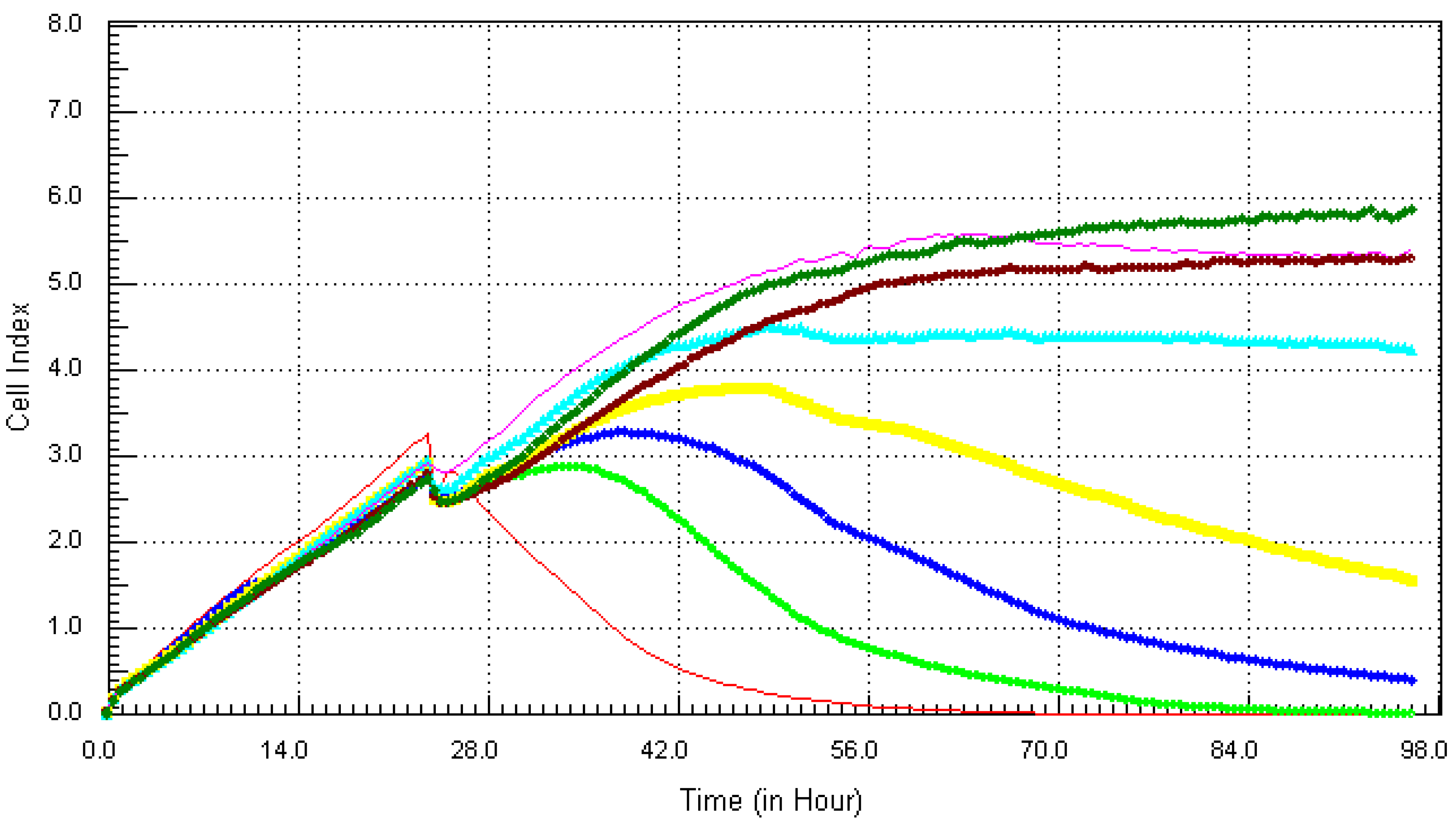

4. Discussion and Conclusions
Conflicts of Interest
References
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of heavy metals. Free. Radical Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; de Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res. 2003, 533, 227–241. [Google Scholar] [CrossRef]
- Ishaque, A.; Johnson, L.; Gerald, T.; Boucaud, D.; Okoh, J.; Tchounwou, P. Assessment of Individual and Combined Toxicities of Four Non-Essential Metals (As, Cd, Hg and Pb) in the Microtox Assay. Int. J. Environ. Res. Public Health. 2006, 3, 118–120. [Google Scholar] [CrossRef]
- Kang, Y.J.; Enger, M.D. Effect of cellular glutathione depletion on cadmium-induced cytotoxicity in human lung carcinoma cells. Cell Biol. Toxicol. 1987, 3, 347–360. [Google Scholar] [CrossRef]
- Hsu, J.M. Lead toxicity as related to glutathione metabolism. J. Nutr. 1981, 111, 26–33. [Google Scholar]
- Winston, D.W.; Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Xing, J.Z.; Zheu, L.; Jackson, J.A.; Gabos, S.; Sun, X.J.; Wang, X.B. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. [Google Scholar] [CrossRef]
- Schwartz, R.A. Arsenic and the skin. Int. J. Dermatol. 1997, 36, 241–250. [Google Scholar]
- Guo, T.L.; Miller, M.A.; Shapiro, I.M.; Shenker, B.J. Mercuric chloride induces apoptosis in human T lymphocytes: Evidence of mitochondrial dysfunction. Toxicol. Appl. Pharm. 1989, 153, 250–257. [Google Scholar]
- Flora, S.; Mittal, M.; Metha, A. Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar]
- Wang, G.; Fowler, B.A. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol. Appl. Pharmacol. 2008, 233, 92–99. [Google Scholar] [CrossRef]
- Pu, Y.; Hour, T.; Chen, J.; Huang, C.; Guan, J.; Lu, S. Cytotoxicity of arsenic to transitional carcinoma cells. Urology 2002, 60, 346–350. [Google Scholar] [CrossRef]
- Lee, T.C.; Wei, M.L.; Chang, W.J.; Ho, I.C.; Lo, J.F.; Jan, K.Y.; Huang, H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic resistant Chinese hamster ovary cells. In Vitro Cell. Dev. Biol. 1989, 25, 442–448. [Google Scholar] [CrossRef]
- Yang, J.M.; Arnush, M.; Chen, Q.Y.; Wu, X.D.; Pang, B.; Jiang, X.Z. Cadmium induced damage to primary cultures of rat Leydig cells. Reprod. Toxicol. 2003, 17, 553–560. [Google Scholar] [CrossRef]
- Masella, R.; di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological system: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Lavazza, A.; Rezzani, R.; Borsani, E.; Rodella, L.F.; Bianchi, R. Mercuric chloride-induced alterations in stress protein distribution in rat kidney. Histol. Histopathol. 2009, 19, 1209–1218. [Google Scholar]
- Geier, D.A.; King, P.G.; Geier, M.R. Mitochondrial dysfunction, impaired oxidative-reduction activity, degeneration, and cell death in human neuronal and fetal cells induced by low-level exposure to Thimerosal and other metal compounds. Toxicol. Environ. Chem. 2009, 91, 735–749. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Egiebor, E.; Tulu, A.; Abou-Zeid, N.; Aighewi, I.T.; Ishaque, A. The Kinetic Signature of Toxicity of Four Heavy Metals and Their Mixtures on MCF7 Breast Cancer Cell Line. Int. J. Environ. Res. Public Health 2013, 10, 5209-5220. https://doi.org/10.3390/ijerph10105209
Egiebor E, Tulu A, Abou-Zeid N, Aighewi IT, Ishaque A. The Kinetic Signature of Toxicity of Four Heavy Metals and Their Mixtures on MCF7 Breast Cancer Cell Line. International Journal of Environmental Research and Public Health. 2013; 10(10):5209-5220. https://doi.org/10.3390/ijerph10105209
Chicago/Turabian StyleEgiebor, Egbe, Adam Tulu, Nadia Abou-Zeid, Isoken Tito Aighewi, and Ali Ishaque. 2013. "The Kinetic Signature of Toxicity of Four Heavy Metals and Their Mixtures on MCF7 Breast Cancer Cell Line" International Journal of Environmental Research and Public Health 10, no. 10: 5209-5220. https://doi.org/10.3390/ijerph10105209



