Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast
Abstract
:1. Introduction
2. Results and Discussion
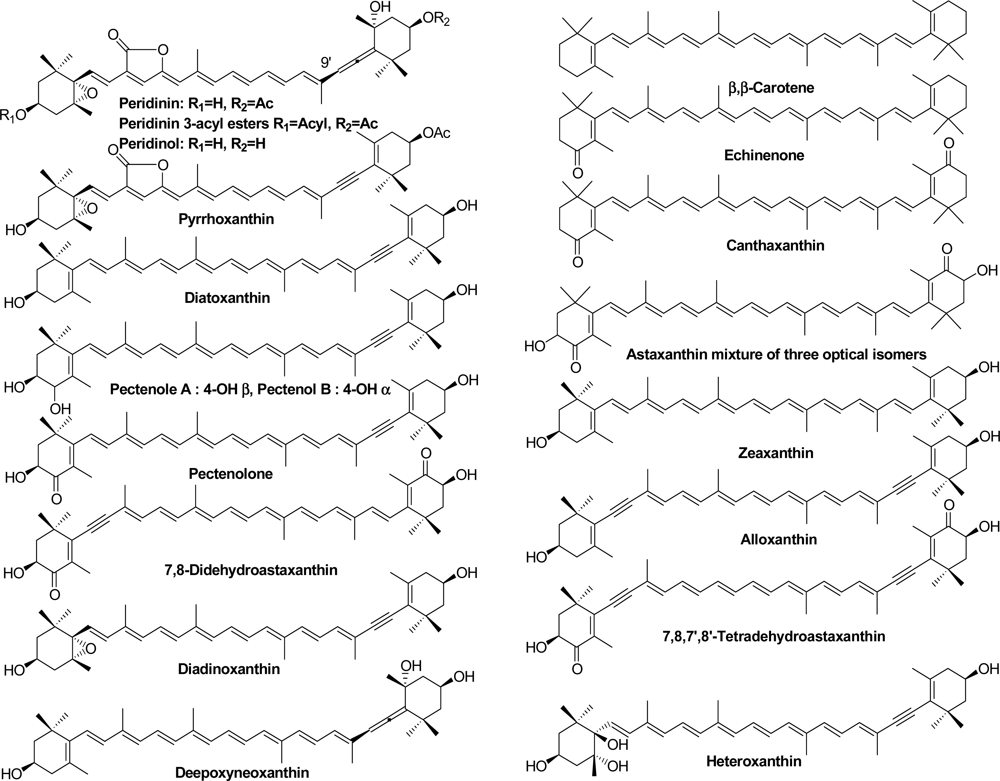
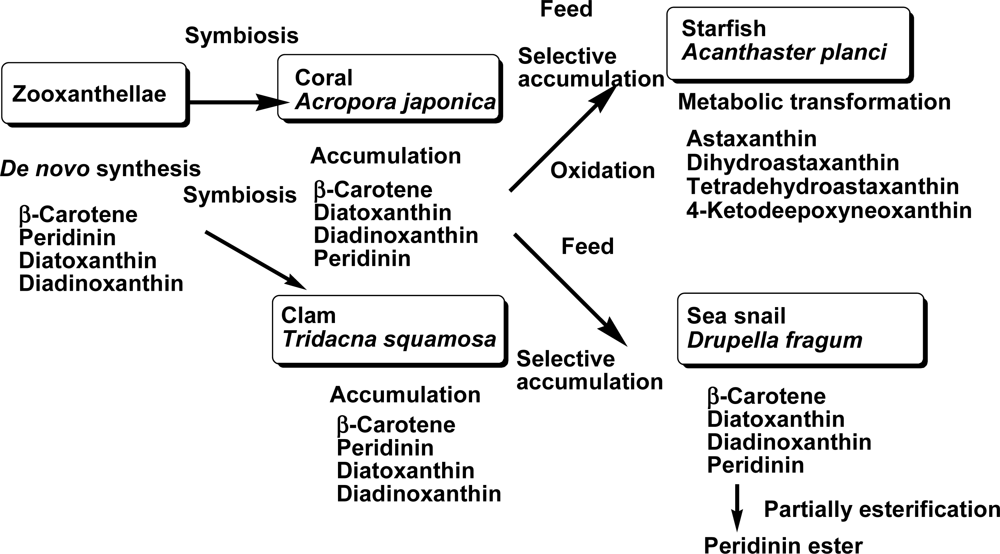
2.1. Carotenoids of Corals and the Tridacnid Clam
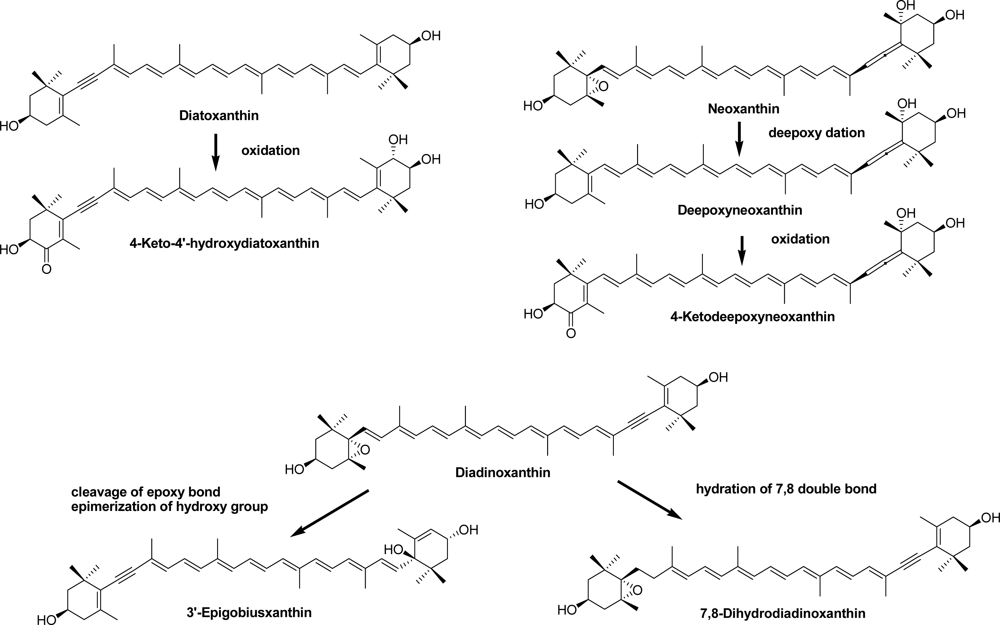
2.2. Carotenoids of the Crown-of-Thorns Starfish
2.3. Carotenoids of the Sea Snail D. fragum
3. Experimental Section
3.1. General
3.2. Animal Material
3.3. Analysis of Carotenoids
3.4. Identification of Carotenoids
3.5. Caracterization of Peridinin-3-acyl Esters
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Britton, G; Liaaen-Jensen, S; Pfander, H. Carotenoids Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Liaaen-Jensen, S. Biosynthesis and Metabolism. In Carotenoids in Food Chain; Britton, G, Liaaen-Jensen, S, Pfander, H, Eds.; Birkhäuser: Basel, Switzerland, 1998; Volume 3, pp. 359–371. [Google Scholar]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci 2001, 67, 771–789. [Google Scholar]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys 2009, 483, 191–195. [Google Scholar]
- Skjenstad, T; Haxo, FT; Liaaen-Jensen, S. Carotenoids of clam, coral and nudibranch zooxanthellae in aposymbiotic culture. Biochem. Syst. Ecol 1984, 12, 149–153. [Google Scholar]
- Liaaen-Jensen, S. Biosynthesis and Metabolism. In Carotenoids in Chemosystematics; Britton, G, Liaaen-Jensen, S, Pfander, H, Eds.; Birkhäuser: Basel, Switzerland, 1998; Volume 3, pp. 217–247. [Google Scholar]
- Bjerkeng, B. Natural functions. In Carotenoids in Aquaculture: Fish and Crustaceans; Britton, G, Liaaen-Jensen, S, Pfander, H, Eds.; Birkhäuser: Basel, Switzerland, 2008; Volume 4, pp. 237–254. [Google Scholar]
- Daigo, K; Nakano, Y; Casareto, BE; Suzuki, Y; Shioi, Y. High-performance liquid chromatographic analysis of photosynthetic pigments in corals: An existence of a variety of epizoic, endozoic and endolithic algae. Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008; pp. 123–127.
- Maoka, T; Fujiwara, Y; Hashimoto, K; Akimoto, N. Characterizatoin of fucoxanthin and fucoxanthinol esters in the chinese surf clam, Mactra chinensis. J. Agric. Food Chem 2007, 55, 1563–1567. [Google Scholar]
- Maoka, T; Akimoto, N; Yim, M-J; Hosokawa, M; Miyashita, K. A new C37-skeletal carotenoid from the clam, Paphia amabillis. J. Agric. Food Chem 2008, 56, 12069–12072. [Google Scholar]
- Maoka, T; Akimoto, N; Murakoshi, M; Sugiyama, K; Nishino, H. Carotenoids in clams, Ruditapes philippinarum and Meretrix petechialis. J. Agric. Food Chem 2010, 58, 5784–5788. [Google Scholar]
- Bjerkeng, B; Hertberg, S; Liaaen-Jensen, S. Carotenoids in food chain studies-V. Carotenoids of the bivalves Modiolus modiolus and Pecten maximus—structural, metabolic and food chain aspects. Comp. Biochem. Physiol 1993, 106B, 243–250. [Google Scholar]
- Maoka, T; Tsushima, M; Matsuno, T. New acetylenic carotenoids from the starfishes Asterina pectinifera and Asterias amurensis. Comp. Biochem. Physiol 1989, 93B, 829–834. [Google Scholar]
- Maoka, T; Akimoto, N; Terada, Y; Komemushi, S; Harada, R; Sameshima, N; Sakagami, Y. Structure of minor carotenoids from crown-of-thorns starfish, Acanthaster planci. J. Nat. Prod 2010, 73, 675–678. [Google Scholar]
- Englert, G. NMR Spectroscopy. In Carotenoids; Britton, G, Liaaen-Jensen, S, Pfander, H, Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; Volume 1B, pp. 147–260. [Google Scholar]
- Haugan, JA; Englert, G; Aakermann, T; Glinz, E; Liaaen-Jensen, S. Algal carotenoids 58. Isomerization studies of peridinin. Acta Chem. Scand 1994, 48, 769–779. [Google Scholar]
- Sugawara, T; Yamashita, K; Asai, A; Nagao, A; Shiraishi, T; Imai, I; Hirata, T. Esterification of xanthophylls by human intestinal Caco-2 cells. Arch. Biochem. Biophys. 2009, 483, 205–212. [Google Scholar]
- Maoka, T; Komori, T; Matsuno, T. Direct resolution of diastereomeric carotenoid-I 3-oxo-β-end group. J. Chromatogr. A 1985, 318, 122–124. [Google Scholar]
- Maoka, T; Akimoto, N. Natural product chemistry in carotenoid, some experimental techniques for structural elucidation and analysis of natural carotenoids. Carotenoid Sci. 2008, 13, 10–17. [Google Scholar]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids; Britton, G, Liaaen-Jensen, S, Pfander, H, Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; Volume 1B, pp. 13–62. [Google Scholar]
| Acropora japonica | A. secale | A. hyacinthus | Tridacna squamosa | ||
|---|---|---|---|---|---|
| Whole | Egg | Whole | Whole | Mantle and foot | |
| Carotenoid content | 3.3 (mg/100 g) | 0.02 | 2.4 | 2.9 | 10 |
| composition (%) | % | % | % | % | |
| β,β-Carotene | 15.5 | 5.0 | 12.0 | 13.4 | 5.1 |
| Diatoxanthin | 5.5 | 15.0 | 4.5 | 5.2 | 0.9 |
| Diadinoxanthin | 4.5 | 5.0 | 5.0 | 5.5 | 9.2 |
| Pyrrhoxanthin | 45.5 | 20.0 | 50.6 | 40.5 | 10.1 |
| Peridinin | 13.0 | 50.0 | 10.0 | 16.0 | 44.1 |
| 9′Z-Peridinin | 16.0 | 5.0 | 17.9 | 19.4 | 30.6 |
| Whole | Gonad | |
|---|---|---|
| 0.46 mg/100 g | 6.6 mg/100 g | |
| β,β-Carotene | 2.1 | 1.4 |
| Echinenone | 1.3 | 1.3 |
| Canthaxanthin | 1.6 | 1.6 |
| 7,8,7′,8′-Tetradehydroastaxanthin | 2.0 | 1.6 |
| 7,8-Didehydroastaxanthin | 35.6 | 35.3 |
| Astaxanthin | 9.8 | 5.8 |
| Pectenolone | 3.2 | 3.0 |
| Diatoxanthin | 3.2 | 15.8 |
| Alloxanthin | 3.2 | 11.8 |
| Diadinoxanthin | 3.0 | 8.6 |
| 7,8-Dihydrodiadinoxanthin | 4.0 | 1.0 |
| 3′-Epigobiusxanthin | 2.0 | 1.0 |
| Pectenol A | 2.0 | 1.6 |
| Pectenol B | 4.0 | 3.2 |
| 4-Keto-4′-hydroxydiatoxanthin | 5.5 | 1.3 |
| 4-Ketodeepoxyneoxanthin | 1.8 | 1.8 |
| Deepoxyneoxanthin | 1.0 | 0.3 |
| Heteroxanthin | 1.2 | 0.6 |
| Peridinol | 13.5 | 3.0 |
| Carotenoid content | 4.03 mg/100 g |
|---|---|
| composition (%) | |
| β,β-Carotene | 10.0 |
| Peridinin-3-acyl esters | 25.0 |
| Zeaxanthin | 15.0 |
| Diatoxanthin | 18.3 |
| Diadinoxanthin | 9.2 |
| Pyrrhoxanthin | 5.8 |
| Peridinin | 16.7 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maoka, T.; Akimoto, N.; Tsushima, M.; Komemushi, S.; Mezaki, T.; Iwase, F.; Takahashi, Y.; Sameshima, N.; Mori, M.; Sakagami, Y. Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast. Mar. Drugs 2011, 9, 1419-1427. https://doi.org/10.3390/md9081419
Maoka T, Akimoto N, Tsushima M, Komemushi S, Mezaki T, Iwase F, Takahashi Y, Sameshima N, Mori M, Sakagami Y. Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast. Marine Drugs. 2011; 9(8):1419-1427. https://doi.org/10.3390/md9081419
Chicago/Turabian StyleMaoka, Takashi, Naoshige Akimoto, Miyuki Tsushima, Sadao Komemushi, Takuma Mezaki, Fumihito Iwase, Yoshimitsu Takahashi, Naomi Sameshima, Miho Mori, and Yoshikazu Sakagami. 2011. "Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast" Marine Drugs 9, no. 8: 1419-1427. https://doi.org/10.3390/md9081419




