Antiproliferative and Antiangiogenic Activities of Smenospongine, a Marine Sponge Sesquiterpene Aminoquinone
Abstract
:1. Introduction
2. Results and Discussion
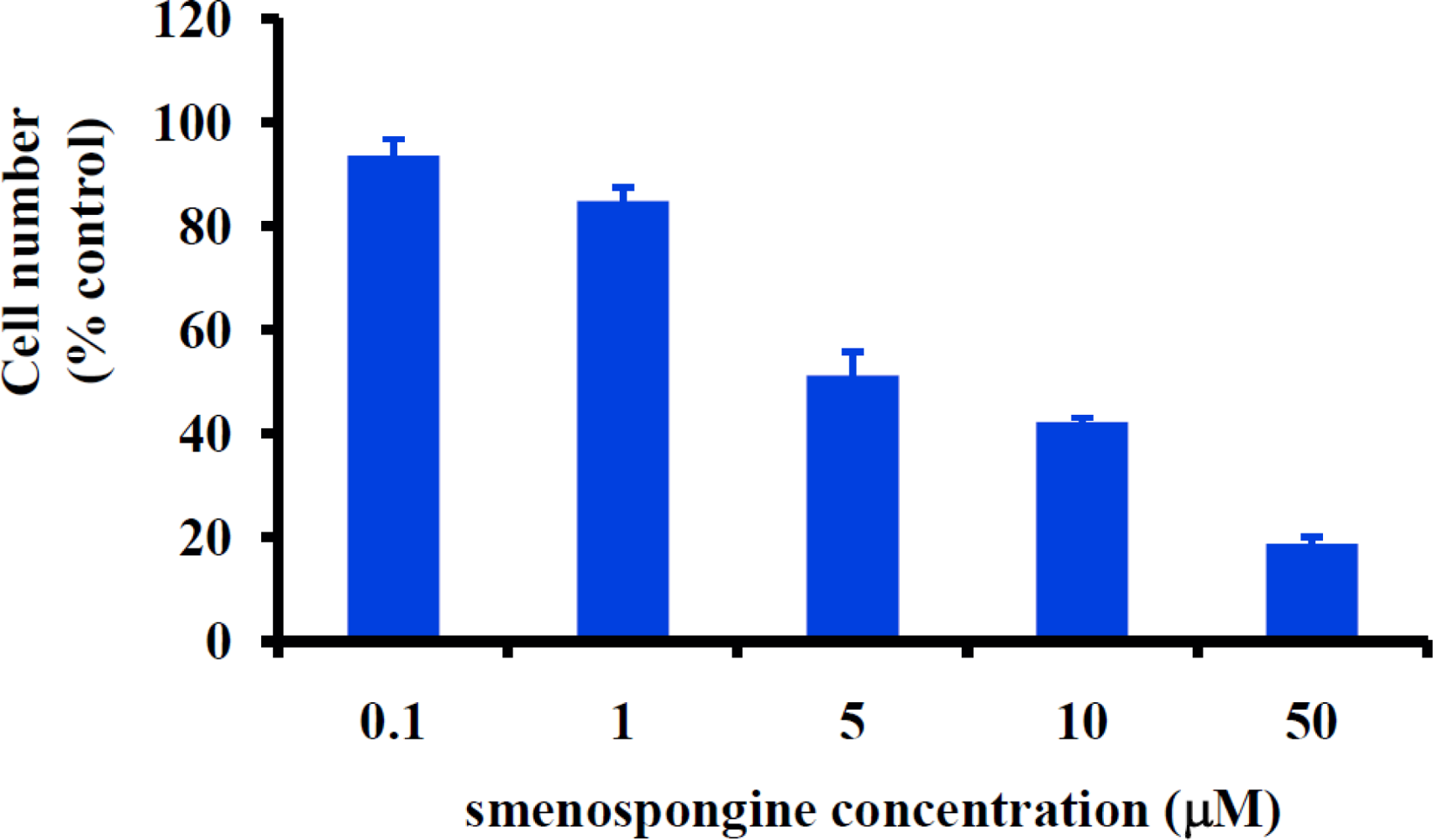
2.1. Smenospongine Inhibits Proliferation of Human Umbilical Vein Endothelial Cells (HUVECs)
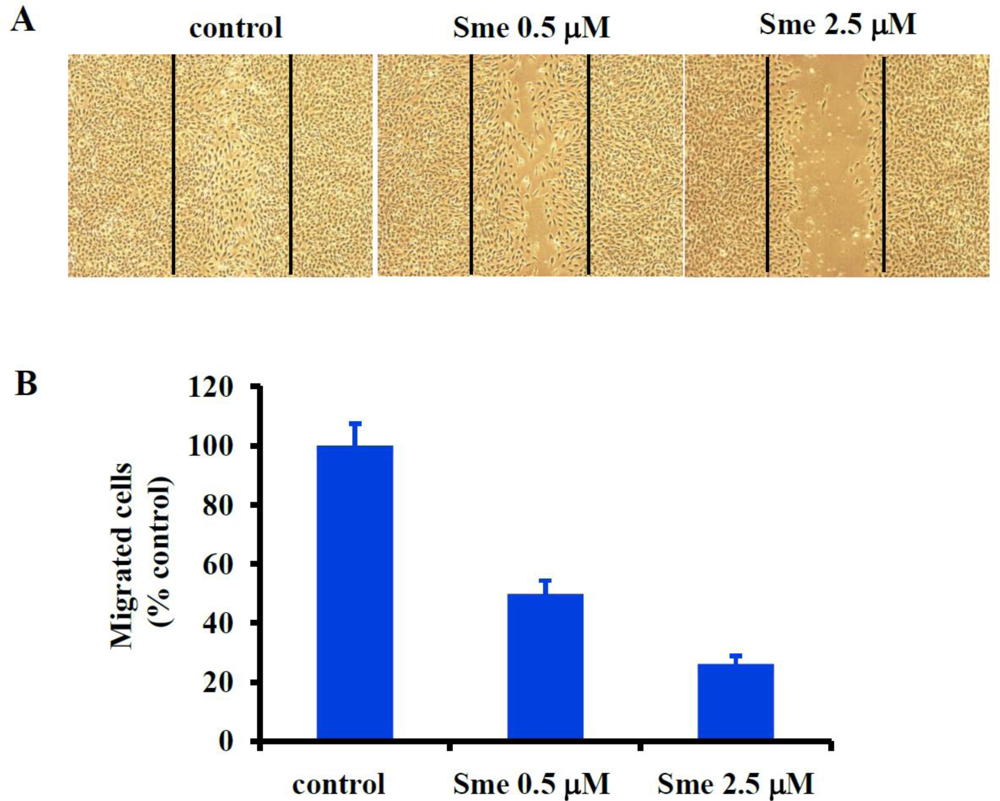
2.2. Smenospongine Blocks HUVEC Migration
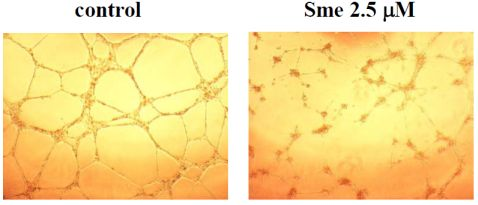
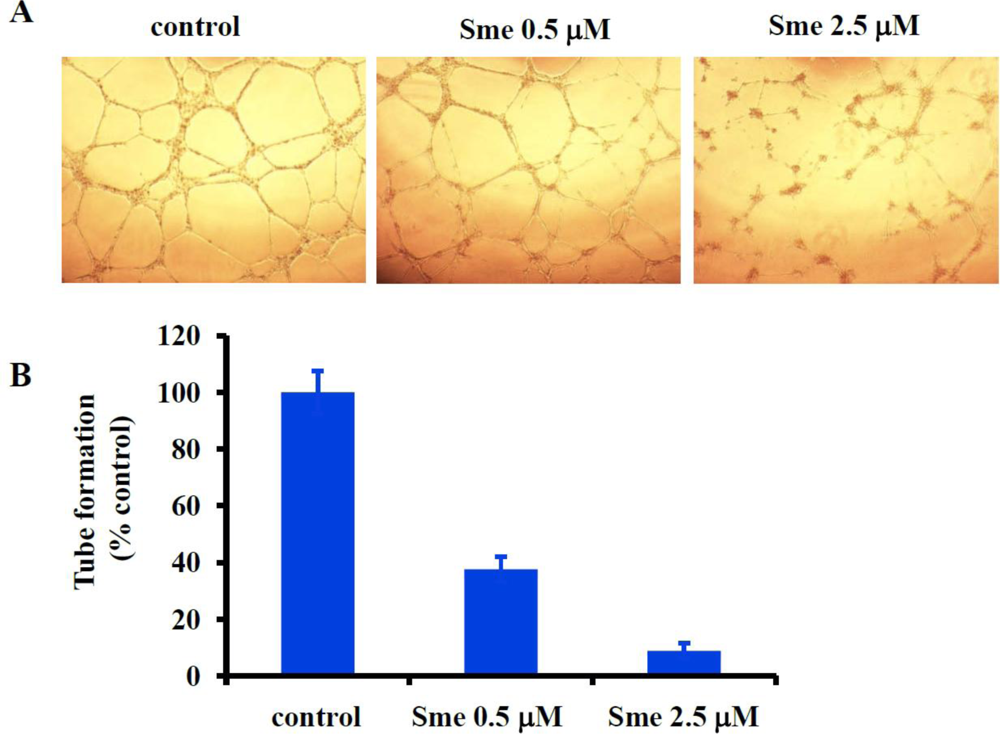
2.3. Smenospongine Inhibits Capillary-like Tube Formation by HUVECs
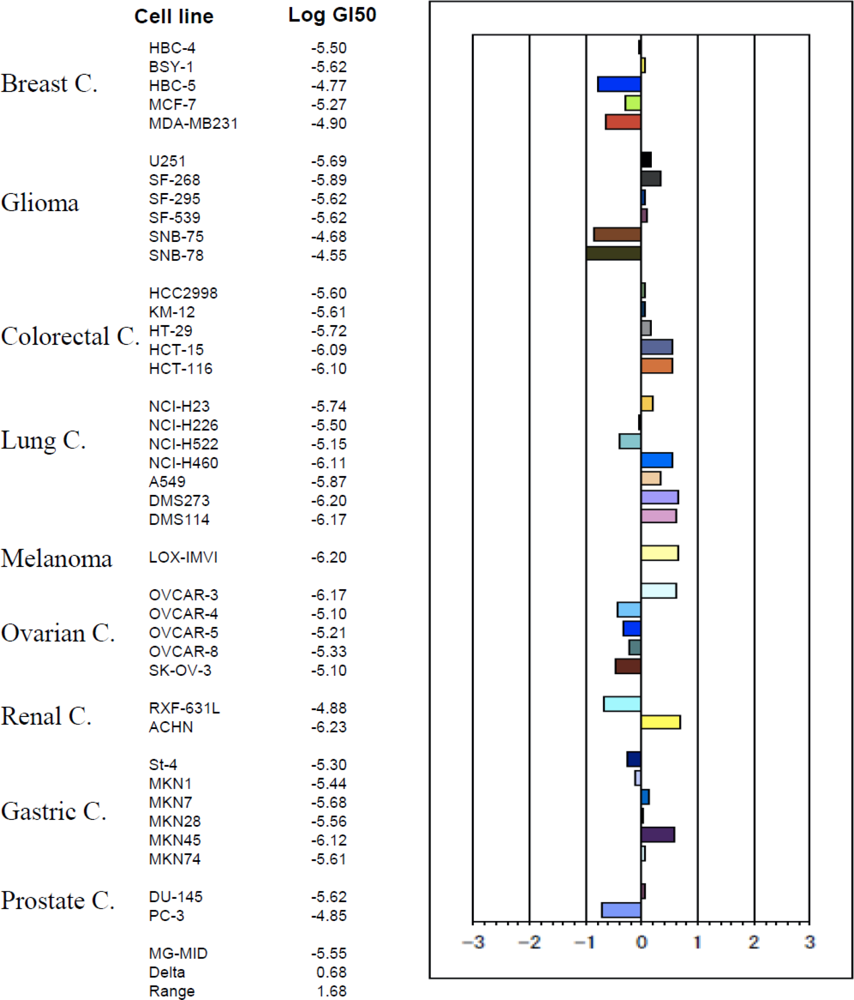
2.4. Smenospongine Inhibits Growth of Various Solid Tumor Cells
3. Experimental Section
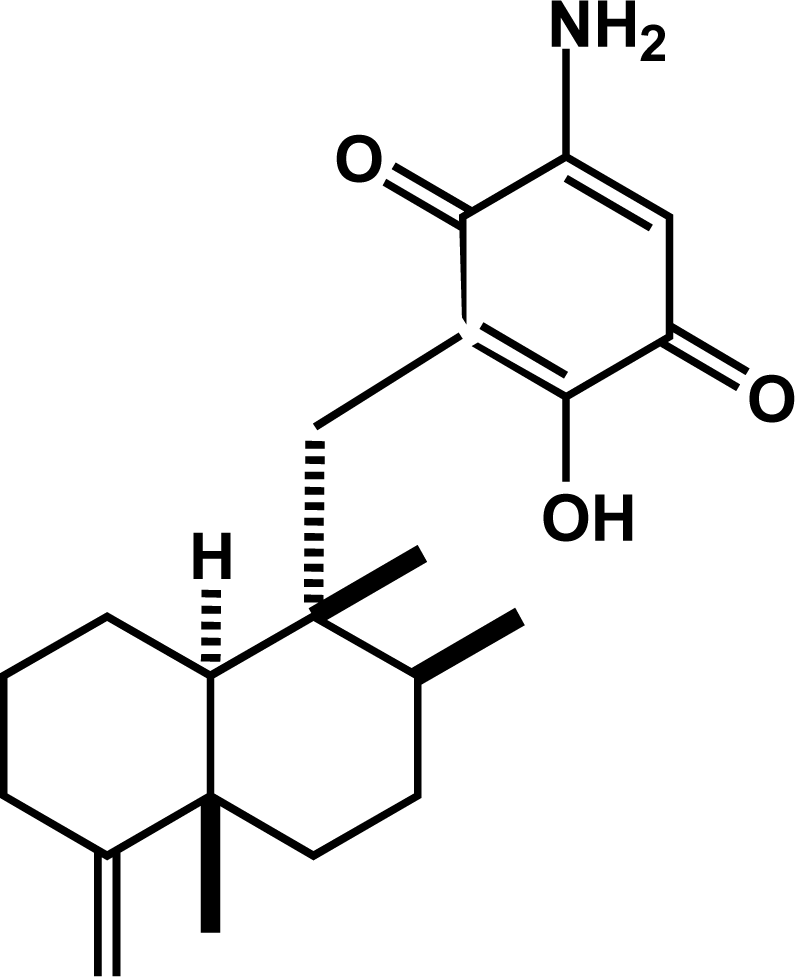
3.1. Isolation and Identification of Smenospongine
3.2. Cell Lines and Cell Culture
3.3. WST-8 Assay
3.4. Wound Healing Assay for Cell Migration
3.5. In Vitro Assay for Capillary-like Tube Formation
3.6. Determination of Inhibitory Activity on Cell Growth of 39 Cancer Cell Lines and Plotting of JFCR39 Fingerprint
4. Conclusions
Acknowledgments
- Samples Availability: Not available.
References
- Folkman, J. Angiogenesis. Annu Rev Med 2006, 57, 1–18. [Google Scholar]
- Folkman, J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov 2007, 6, 273–286. [Google Scholar]
- Kong, D; Okamura, M; Yoshimi, H; Yamori, T. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur J Cancer 2009, 45, 857–865. [Google Scholar]
- Aoki, S; Kong, D; Matsui, K; Kobayashi, M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in K562 cells. Anticancer Drugs 2004, 15, 363–369. [Google Scholar]
- Aoki, S; Kong, D; Matsui, K; Rachmat, R; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem Pharm Bull 2004, 52, 935–937. [Google Scholar]
- Kong, D; Aoki, S; Sowa, Y; Sakai, T; Kobayashi, M. Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces G1 arrest or apoptosis in different leukemia cells. Mar Drugs 2008, 6, 480–488. [Google Scholar]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar]
- Kong, D; Yamori, T. ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor identified using the JFCR39 drug discovery system. Acta Pharmacol Sin 2010, 31, 1189–1197. [Google Scholar]
- Xiao, D; Singh, SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res 2007, 67, 2239–2346. [Google Scholar]
- Kong, D; Dan, S; Yamazaki, K; Yamori, T. Inhibition profiles of phosphatidylinositol 3-kinase inhibitors against PI3K superfamily and human cancer cell line panel JFCR39. Eur J Cancer 2010, 46, 1111–1121. [Google Scholar]
- Yamori, T; Matsunaga, A; Sato, S; Yamazaki, K; Komi, A; Ishizu, K; Mita, I; Edatsugi, H; Matsuba, Y; Takezawa, K; Nakanishi, O; Kohno, H; Nakajima, Y; Komatsu, H; Andoh, T; Tsuruo, T. Potent antitumor activity of MS-247, a novel DNA minor groove binder, evaluated by an in vitro and in vivo human cancer cell line panel. Cancer Res 1999, 59, 4042–4049. [Google Scholar]
- Kondracki, ML; Guyot, M. Smenospongine: a cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett 1987, 28, 5815–5818. [Google Scholar]
- Gordaliza, M. Cytotoxic terpene quinones from marine Songes. Mar Drugs 2010, 8, 2849–2870. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and Antiangiogenic Activities of Smenospongine, a Marine Sponge Sesquiterpene Aminoquinone. Mar. Drugs 2011, 9, 154-161. https://doi.org/10.3390/md9020154
Kong D, Yamori T, Kobayashi M, Duan H. Antiproliferative and Antiangiogenic Activities of Smenospongine, a Marine Sponge Sesquiterpene Aminoquinone. Marine Drugs. 2011; 9(2):154-161. https://doi.org/10.3390/md9020154
Chicago/Turabian StyleKong, Dexin, Takao Yamori, Motomasa Kobayashi, and Hongquan Duan. 2011. "Antiproliferative and Antiangiogenic Activities of Smenospongine, a Marine Sponge Sesquiterpene Aminoquinone" Marine Drugs 9, no. 2: 154-161. https://doi.org/10.3390/md9020154