4-Acetoxydolastane Diterpene from the Brazilian Brown Alga Canistrocarpus cervicornis as Antileishmanial Agent
Abstract
:1. Introduction
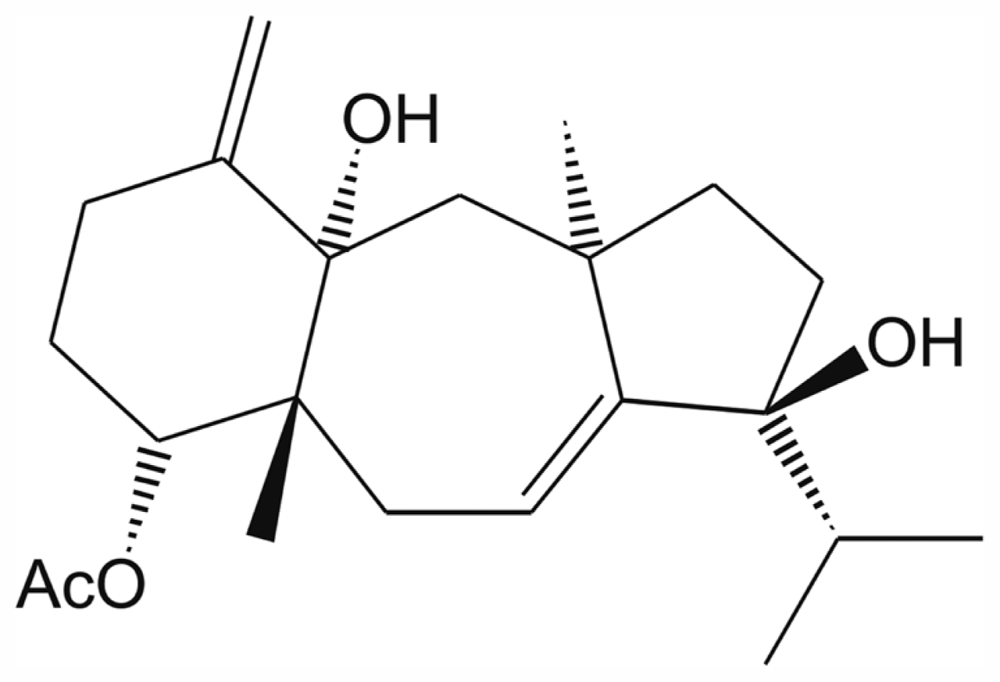
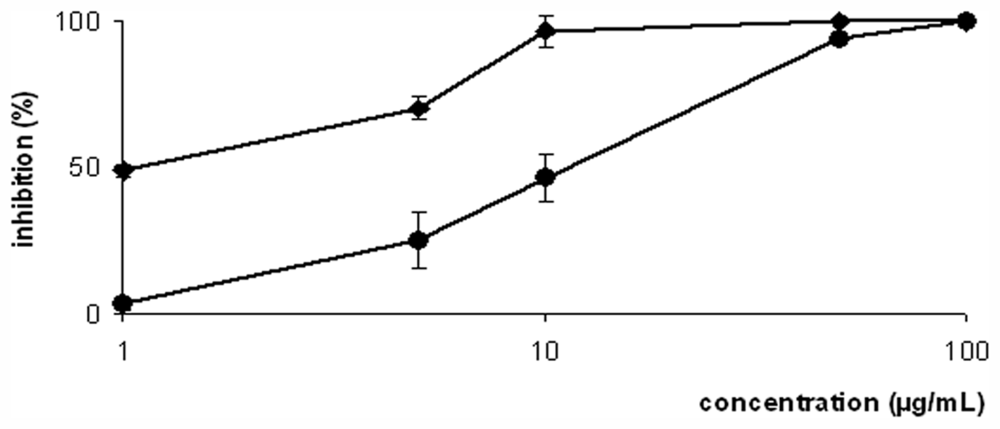
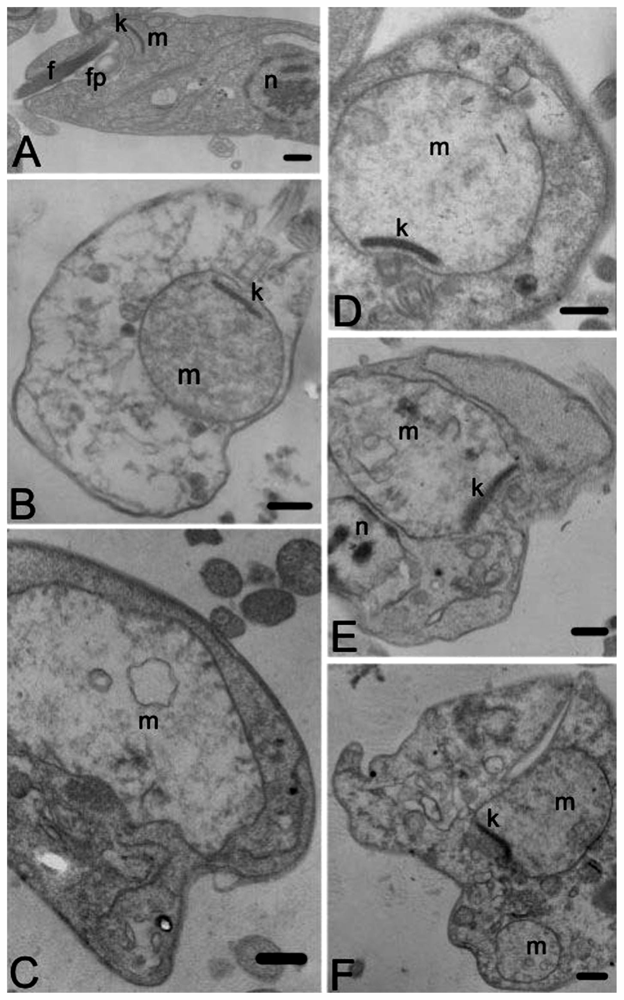
2. Results and Discussion
3. Experimental Section
3.1. Algal Collection
3.2. Chemical Extraction
3.3. Fractionation and Compound Isolation
3.4. Spectroscopic Data
3.5. Parasites
3.6. Macrophage J774G8 Cells
3.7. Antileishmania Activity in Vitro against Promastigotes and Axenic Amastigotes
3.8. Activity against Intracellular Amastigotes
3.9. Cytotoxicity Assay
3.10. Transmission Electron Microscopy
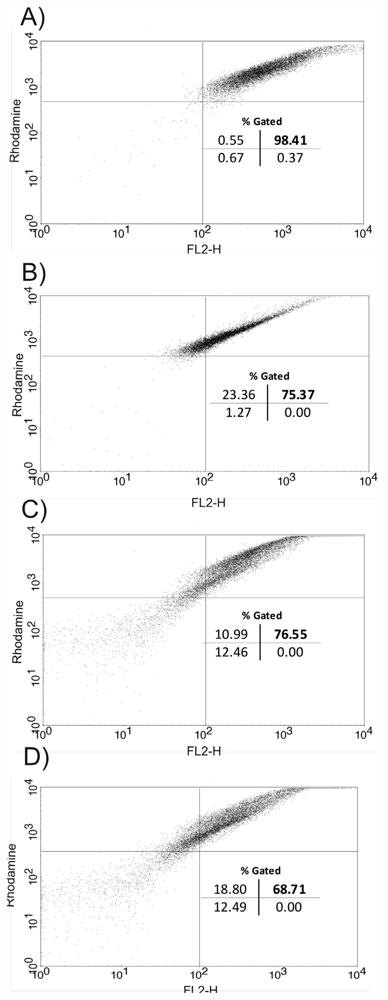
3.11. Flow Cytometry
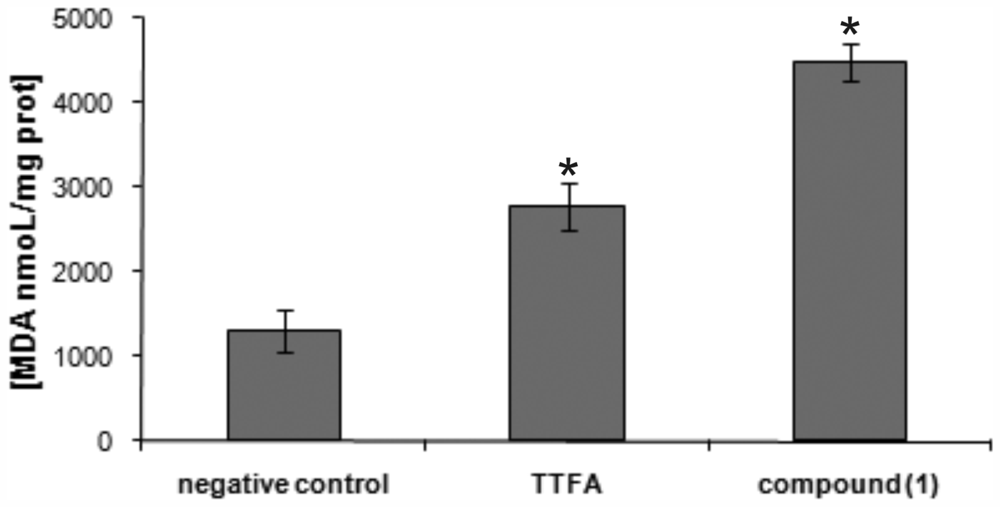
3.12. Measurement of Lipid Peroxidation Product
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- World Health Organization. Leishmaniasis: Disease Burden and Epidemiological Trends; WHO: Geneva, Switzerland, 2009. Avaiable online: http://www.who.int/tdr/diseases/leish/files/direction.pdf (accessed on 30 July 2011).
- Mitropoulos, P.; Konidas, P.; Durkin-Konidas, M. New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment. J. Am. Acad. Dermatol 2010, 6, 309–322. [Google Scholar]
- Zucca, M.; Savoia, D. Current developments in the therapy of protozoan infections. Open Med. Chem. J 2011, 5, 4–10. [Google Scholar]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Broker, S. Cutaneous leishmaniasis. Lancet Infect. Dis 2007, 7, 581–596. [Google Scholar]
- Chawla, B.; Madhubala, R. Drug targets in Leishmania. J. Parasit. Dis 2010, 34, 1–13. [Google Scholar]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis 2011. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharm. Sci 2010, 31, 255–265. [Google Scholar]
- Pereira, R.C.; Bianco, E.M.; Bueno, L.B.; Oliveira, M.A.L.; Pamplona, O.S.; Da-Gama, B.A.P. Associational defense against herbivory between brown seaweeds. Phycologia 2010, 49, 424–428. [Google Scholar]
- Barbosa, J.P.; Fleury, B.G.; Da-Gama, B.A.P.; Teixeira, V.L.; Pereira, R.C. Natural products as antifoulants in the Brazilian brown alga Dictyota pfaffii (Phaeophyta, Dictyotales). Biochem. Syst. Ecol 2007, 35, 549–553. [Google Scholar]
- Bianco, E.M.; Rogers, R.; Teixeira, V.L.; Pereira, R.C. Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J. Appl. Phycol 2009a, 21, 341–346. [Google Scholar]
- Engel, S.; Jensen, P.R.; Fenical, W. Chemical ecology of marine microbial defense. J. Chem. Ecol 2002, 28, 1971–1985. [Google Scholar]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rabello, M.A.; Frugulhetti, I.C.; Teixeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffii. Planta Med 2004, 70, 856–860. [Google Scholar]
- Veiga-Santos, P.; Rocha, K.J.P.; Santos, A.O.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Silva, S.O.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. In vitro anti-trypanosomal activity of elatol isolated from red seaweed Laurencia dendroidea. Parasitology 2010, 137, 1661–1670. [Google Scholar]
- Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 29, 2733–2743. [Google Scholar]
- Morales, J.L.; Cantillo-Ciau, Z.O.; Sánchez-Molina, I.; Mena-Rejón, G.J. Screening of antibacterial and antifungal activities of six marine macroalgae from coasts of Yucatán Península. Pharm. Biol 2006, 44, 632–635. [Google Scholar]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, México. J. Appl. Phycol 2007, 19, 449–458. [Google Scholar]
- Medeiros, V.P.; Queiroz, K.C.S.; Cardoso, M.L.; Monteiro, G.R.; Oliveira, F.W.; Chavante, S.F.; Guimaraes, L.A.; Rocha, H.A.; Leite, E.L. Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry 2008, 73, 1018–1024. [Google Scholar]
- Teixeira, V.L.; Tomassini, T. Dolastane and secodolastane diterpenes from the marine brown alga Dictyota cervicornis. J. Nat. Prod 1986, 49, 570–575. [Google Scholar]
- Bianco, E.M.; Teixeira, V.L.; Pereira, R.C.; De-Souza, A.M.; Nucci, P.; Afonso, I.F.; Rodrigues, C.R.; Castro, H.C. Brown seaweed defensive chemicals: A structure-activity relationship approach for the marine environment. Nat. Prod. Commun 2009b, 4, 173–178. [Google Scholar]
- Moura, L.A.; Sanchez, E.F.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Antiophidian properties of a dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. Biol. Med. Prev. Nutr 2011, 1, 61–66. [Google Scholar]
- Moura, L.A.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Anticoagulation and antiplatelet effects of a dolastane diterpene isolated from the marine brown alga Canistrocarpus. J. Thromb. Thrombolysis 2011, 31, 235–240. [Google Scholar]
- Pereira, R.C.; Pinheiro, M.D.; Teixeira, V.L.; Da-Gama, B.A.P. Feeding preferences of the endemic gastropod Astraea latispina in relation to chemical defenses of brazilian tropical seaweeds. Braz. J. Biol 2002, 62, 33–40. [Google Scholar]
- Vallim, M.A.; Barbosa, J.E.; Cavalcanti, D.N.; De-Paula, J.C.; Silva, V.A.G.G.; Teixeira, V.L.; Paixão, I.C.N.P. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J. Med. Plant Res 2010, 4, 2379–2382. [Google Scholar]
- Calvalcanti, D.N.; Rezende, C.M.; Pinto, A.C.; Pereira, R.C.; Teixeira, V.L. Diterpenoid constituents from the brown alga Dictyota menstrualis (Dictyotaceae, Phaeophyta). Nat. Prod. Commun 2006, 8, 609–611. [Google Scholar]
- Freitas, O.S.P.; Oliveira, A.S.; Paula, J.C.; Pereira, R.C.; Cavalcanti, D.N.; Teixeira, V.L. Chemical variation in the diterpenes from the Brazilian Brown Alga Dictyota mertensii (Dictyotaceae, Phaeophyta). Nat. Prod. Commun 2007, 2, 13–15. [Google Scholar]
- Bianco, E.M.; Teixeira, V.L.; Pereira, R.C. Chemical defenses of the tropical marine seaweed Canistrocarpus cervicornis against herbivory by sea urchin. Braz. J. Ocean 2010, 58, 213–218. [Google Scholar]
- Demunshi, Y.; Chugh, A. Role of traditional knowledge in marine bioprospecting. Biodivers. Conserv 2010, 19, 3015–3033. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep 2011, 28, 196–268. [Google Scholar]
- Kima, P.E. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol 2007, 37, 1087–1096. [Google Scholar]
- Rodrigues, J.C.F.; Bernardes, C.F.; Visbal, G.; Urbina, J.A.; Vercesi, A.E.; Souza, W. Sterol methenyl tranferase inhibitors alter the ultrastructure and function of the Leishmania amazonensis mitochondrion leading to potent growth inhibition. Protist 2007, 158, 447–456. [Google Scholar]
- Ghosh, S.; Debnath, S.; Hazra, S.; Hazra, S.; Hartung, A.; Thomale, K.; Schulthesis, M.; Kapkova, P.; Schurigt, U.; Moll, H.; et al. Valeriana wallichii root extracts and fractions with activity against Leishmania spp. Parasitol. Res 2010, 108, 861–871. [Google Scholar]
- Santos, A.O.; Ueda-Nakamura, T.; Dias-Filho, B.P.; Veiga-Junior, V.F.; Nakamura, C.V. Copaiba oil: An alternative to development of new drugs against Leishmaniais. Evid. Based Complement. Alternat. Med 2012. [Google Scholar] [CrossRef]
- Santa-Rita, R.M.; Barbosa, H.S.; de Castro, S.L. Ultrastructural analysis of edelfosine-treated trypomastigotes and amastigotes of Trypanosoma cruzi. Parasitol. Res 2006, 100, 187–190. [Google Scholar]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Gangyly, S.; Saha, P.; Chatterjee, M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J. Med. Microbiol 2007, 56, 1213–1218. [Google Scholar]
- Roy, A.; Ganguly, A.; BoseDasgupta, S.; Das, B.B.; Pal, C.; Jaisanka, P.; Majumder, H.K. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol. Pharmacol 2008, 74, 1292–1307. [Google Scholar]
- Kaur, J.; Singh, N.; Singh, B.K.; Dube, A.; Tripathi, R.P.; Singh, P.; Singh, N. Leishmania donovani: Oral therapy with glycosyl 1,4-dihydropyridine analogue showing apoptosis like phenotypes targeting pteridine reductase 1 in intracellular amastigotes. Exp. Parasitol 2010, 125, 310–314. [Google Scholar]
- Sen, N.; Das, B.B.; Ganguly, A.; Mukherjee, T.; Tripathi, G.; Bandyopadhyay, S.; Rakshit, S.; Sen, T.; Majumder, H.K. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ 2004, 11, 924–936. [Google Scholar]
- Das, R.; Roy, A.; Dutta, N.; Majumder, H.K. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis 2008, 13, 867–882. [Google Scholar]
- Garcia, D.G.; Bianco, E.M.; Santos, M.C.B.; Pereira, R.C.; Faria, M.V.C.; Teixeira, V.L.; Burth, P. Inhibition of Mammal Na+K+-ATPase by diterpenes extracted from the Brazilian brown alga Dictyota cervicornis. Phytother. Res 2009, 23, 943–947. [Google Scholar]
- Ueda-Nakamura, T.; Attias, M.; Souza, W. Megasome biogenesis in Leishmania amazonensis: A morphometric and cytochemical study. Parasitol. Res 2001, 87, 89–97. [Google Scholar]
- Gadelha, F.R.; Thomson, L.; Fagian, M.M.; Costa, A.D.; Radi, R.; Vercesi, A.E. Ca2+-independent permeabilization of the inner mitochondrial membrane by peroxynitrite is mediated by membrane protein thiol cross-linking and lipid peroxidation. Arch. Biochem. Biophys 1997, 15, 243–250. [Google Scholar]
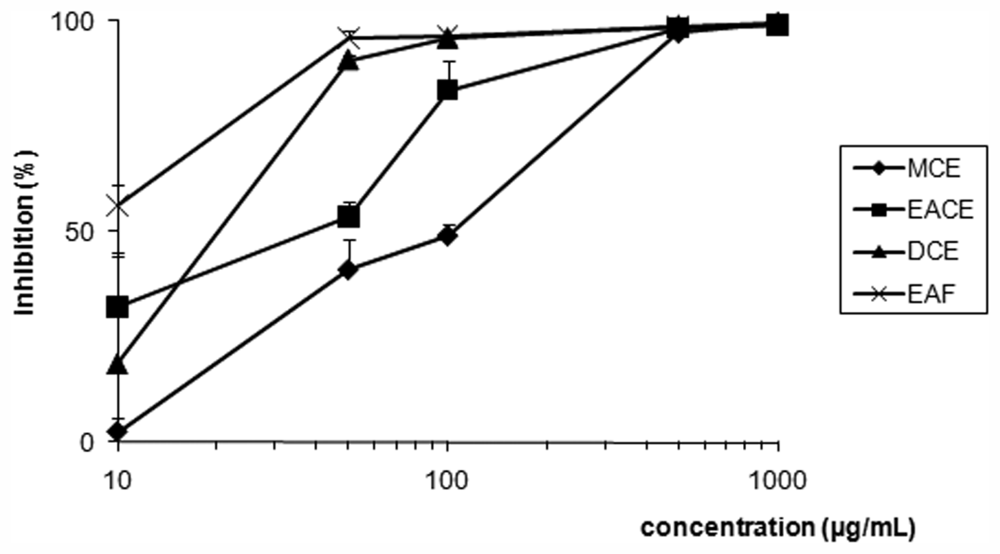

| Compound | CC50 (μg/mL) a | Promastigote forms | |
|---|---|---|---|
| IC50 (μg/mL) | SI | ||
| EACE | 50.0 ± 0.08 | 50.0 ± 2.12 | 1.00 |
| MCE | 51.0 ± 0.20 | 100.0 ± 4.50 | 0.51 |
| DCE | 46.0 ± 0.46 | 20.0 ± 1.55 | 2.30 |
| EAF | 47.0 ± 0.75 | 8.0 ± 0.55 | 5.88 |
| 1 | 186.0 ± 3.29 | 2.0 ± 0.37 | 93.00 |
| Amphotericin-B | nd | 0.06 ± 0.00 | nd |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Santos, A.O.d.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane Diterpene from the Brazilian Brown Alga Canistrocarpus cervicornis as Antileishmanial Agent. Mar. Drugs 2011, 9, 2369-2383. https://doi.org/10.3390/md9112369
Santos AOd, Britta EA, Bianco EM, Ueda-Nakamura T, Filho BPD, Pereira RC, Nakamura CV. 4-Acetoxydolastane Diterpene from the Brazilian Brown Alga Canistrocarpus cervicornis as Antileishmanial Agent. Marine Drugs. 2011; 9(11):2369-2383. https://doi.org/10.3390/md9112369
Chicago/Turabian StyleSantos, Adriana Oliveira dos, Elizandra Aparecida Britta, Everson Miguel Bianco, Tania Ueda-Nakamura, Benedito Prado Dias Filho, Renato Crespo Pereira, and Celso Vataru Nakamura. 2011. "4-Acetoxydolastane Diterpene from the Brazilian Brown Alga Canistrocarpus cervicornis as Antileishmanial Agent" Marine Drugs 9, no. 11: 2369-2383. https://doi.org/10.3390/md9112369




