Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of dextran magnetic fluid
2.3. Synthesis of chitosan-PAA nanospheres by polymerization
2.4. Preparation of Fe3O4/chitosan-PAA polymer magnetic microspheres
2.5. Characterization
3. Results and Discussion
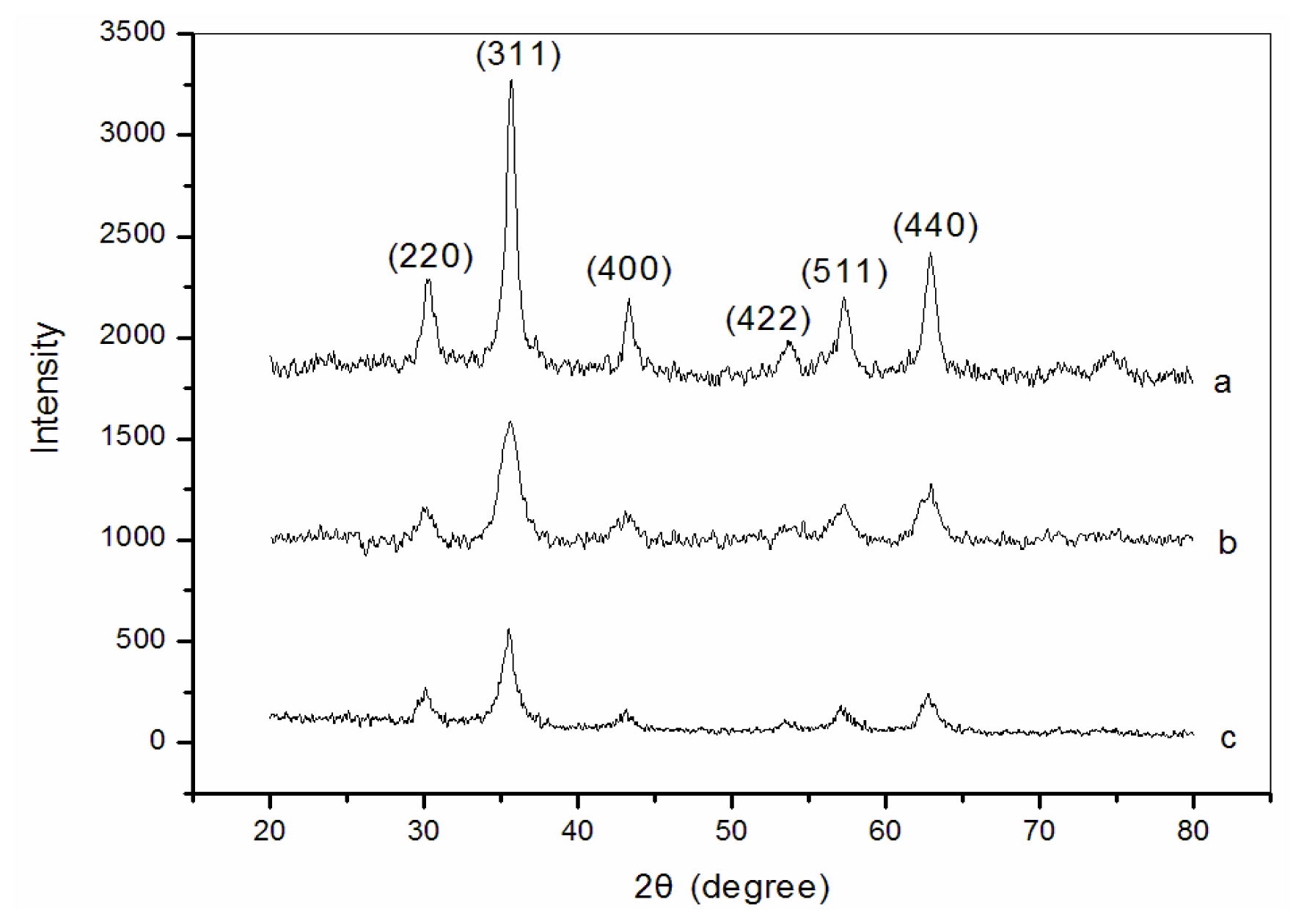
3.1. X-ray diffraction (XRD) analysis
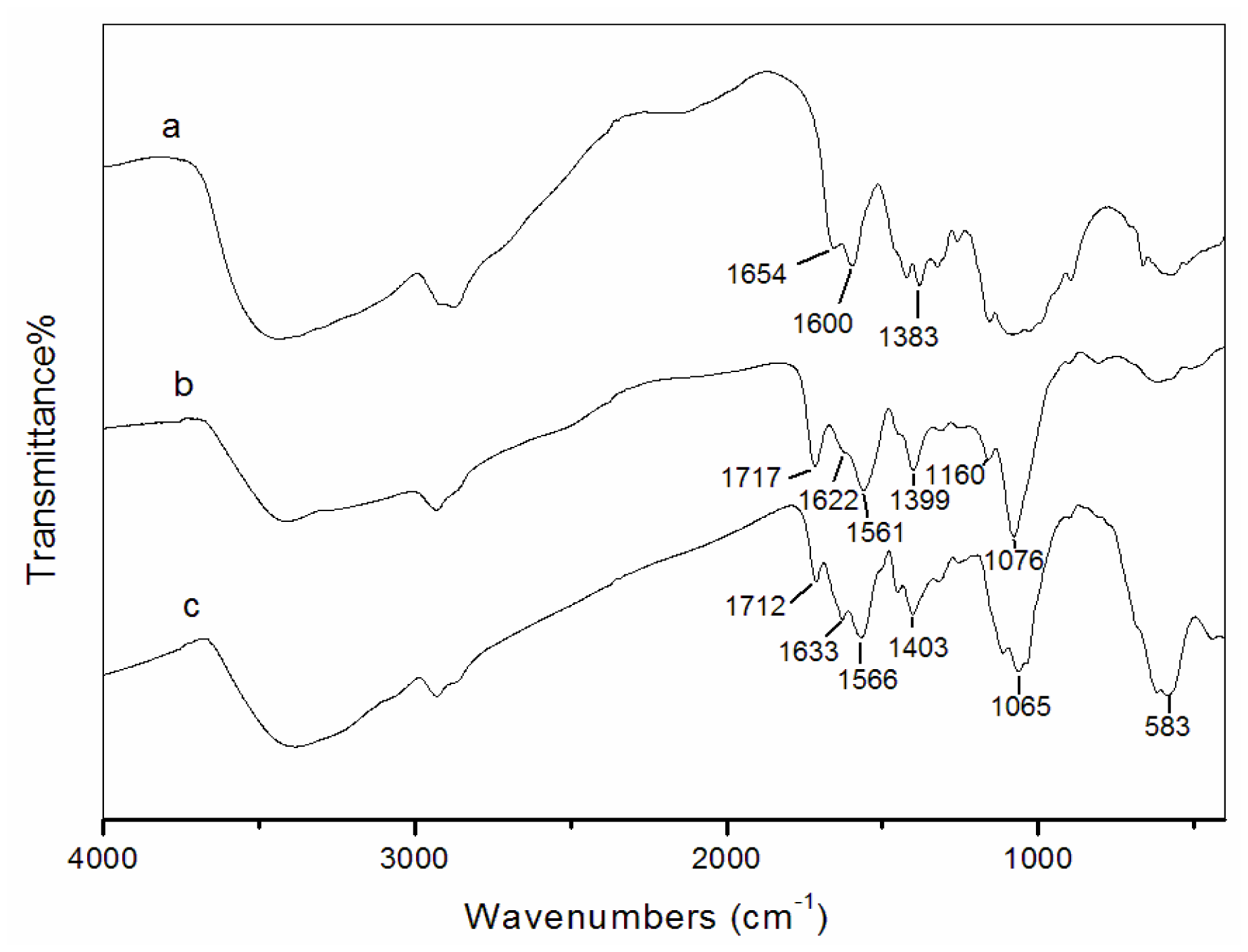
3.2. Fourier transform infrared spectroscopy (FT-IR) analysis
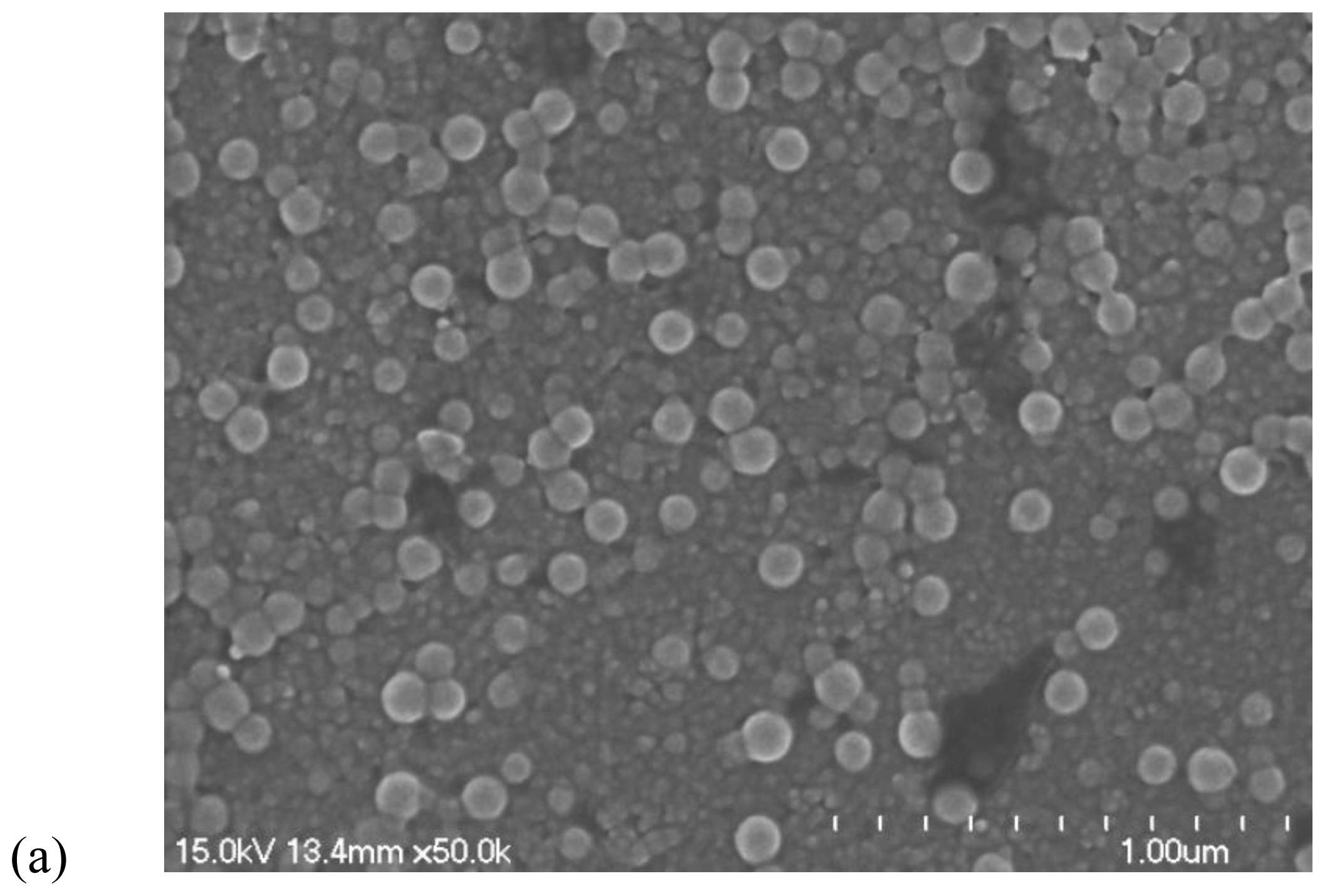
3.3. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
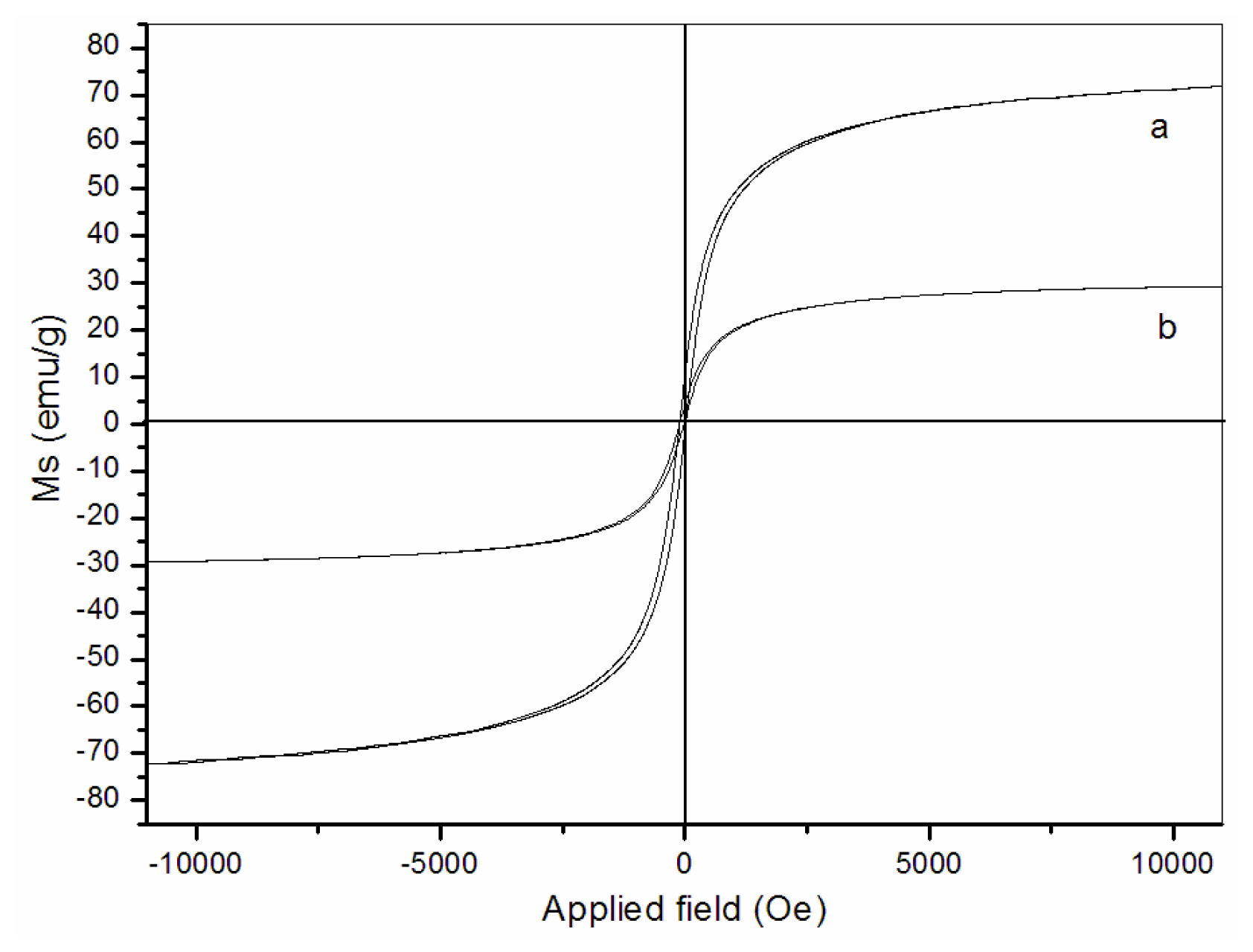
3.4. Magnetic properties
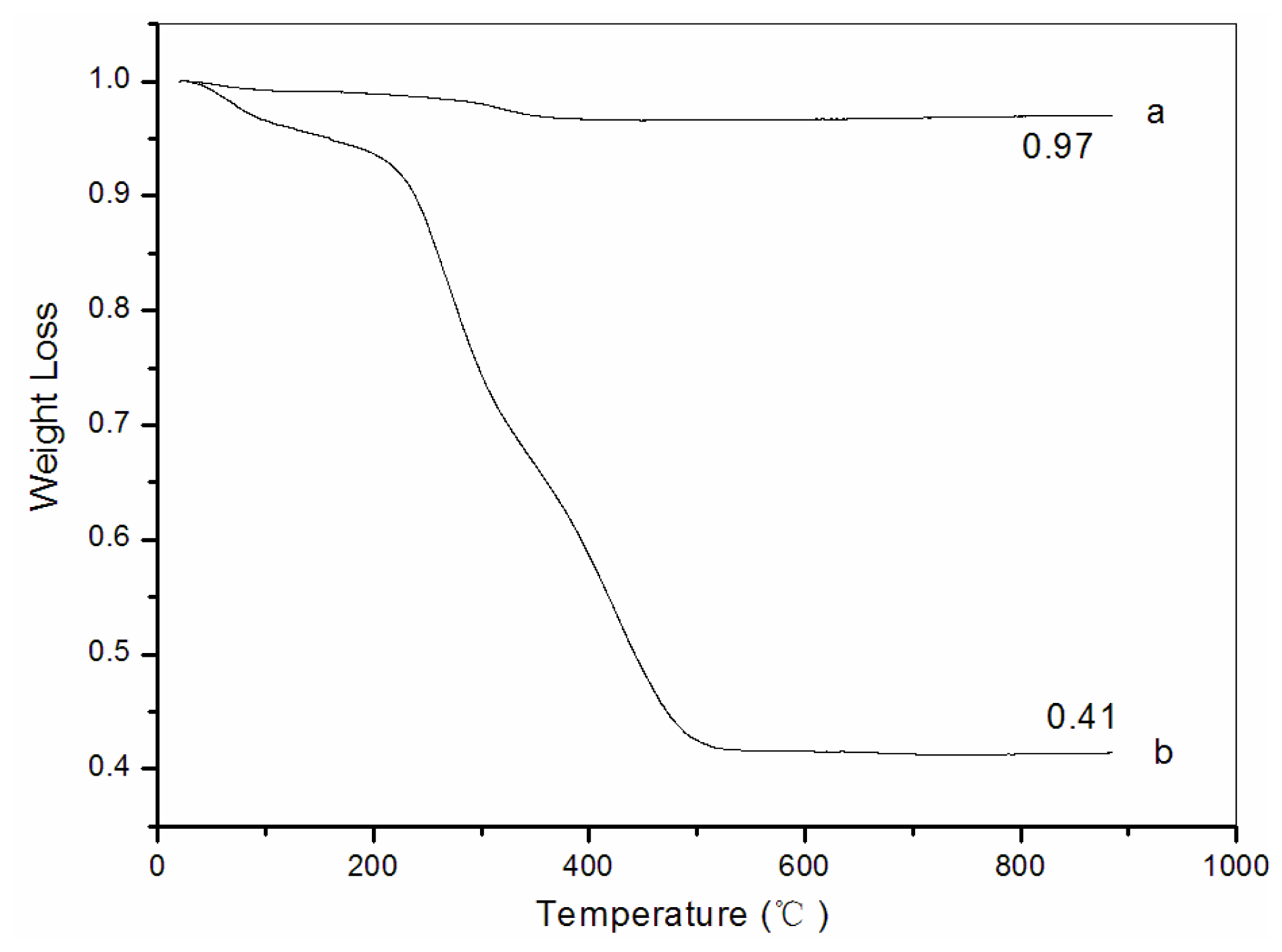
3.5. Thermogravimetric analysis (TG)
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Tartaj, P; Morales, MP; Veintemillas-Verdaguer, S; Gonzalez-Carreno, T; Serna, CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys 2003, 36, R182–R197. [Google Scholar]
- Hafeli, U; Pauer, G; Failing, S; Tapolsky, G. Radiolabeling of magnetic particles with rhenium-188 for cancer therapy. J. Magn. Magn. Mater 2001, 225, 73–78. [Google Scholar]
- Johannsen, M; Gneveckow, U; Eckelt, L; Feussner, A; Waldöfner, N; Scholz, R; Deger, S; Wust, P; Loening, SA; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperthermia 2005, 21, 637–647. [Google Scholar]
- Neuberger, T; Schöpf, B; Hofmann, H; Hofmann, M; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater 2005, 293, 483–496. [Google Scholar]
- Mornet, S; Vasseur, S; Grasset, F; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem 2004, 14, 2161–2175. [Google Scholar]
- Högemann, D; Josephson, L; Weissleder, R; Basilion, JP. Improvement of MRI Probes to Allow Efficient Detection of Gene Expression. Bioconjug. Chem 2000, 11, 941–946. [Google Scholar]
- Weiming, Z; Feng, G; Hongchen, G. Magnetic polymer nanospheres with high and uniform magnetite content. J. Magn. Magn. Mater 2005, 288, 403–410. [Google Scholar]
- Ramírez, LP; Landfester, K. Magnetic Polystyrene Nanoparticles with a High Magnetite Content Obtained by Miniemulsion Processes. Macromol. Chem. Phys 2003, 204, 22–31. [Google Scholar]
- Křížová, J; Španová, A; Rittich, B; Horák, D. Magnetic hydrophilic methacrylate-based polymer microspheres for genomic DNA isolation. J. Chromatogr. A 2005, 1064, 247–253. [Google Scholar]
- Zhang, Y; Kohler, N; Zhang, M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar]
- Berry, CC; Wells, S; Charles, S; Curtis, ASG. Dextran and albumin derivatised iron oxide nanoparticles: influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar]
- Bergemann, C; Muller-Schulte, D; Oster, J; Brassard, L; Lubbe, AS. Magnetic ion-exchange nano- and microparticles for medical, biochemical and molecular biological applications. J. Magn. Magn. Mater 1999, 194, 45–52. [Google Scholar]
- Yang, C; Liu, H; Guan, Y; Xing, J; Liu, J; Shan, G. Preparation of magnetic poly(methylmethacrylate–divinylbenzene–glycidylmethacrylate) microspheres by spraying suspension polymerization and their use for protein adsorption. J. Magn. Magn. Mater 2005, 293, 187–192. [Google Scholar]
- Horák, D; Semenyuk, N; Lednicky, F. Effect of the reaction parameters on the particle size in the dispersion polymerization of 2-hydroxyethyl and glycidyl methacrylate in the presence of a ferrofluid. J. Polym. Sci. A Polym. Chem 2003, 41, 1848–1863. [Google Scholar]
- Kondo, A; Kamura, H; Higashitani, K. Development and application of thermo-sensitive magnetic immunomicrospheres for antibody purification. Appl. Microbiol. Biotechnol 1994, 41, 99–105. [Google Scholar]
- Qiu, GH; Wang, Q; Wang, C; Lau, WL; Guo, YL. Polystyrene/Fe3O4 magnetic emulsion and nanocomposite prepared by ultrasonically initiated miniemulsion polymerization. Sonochemistry 2007, 14, 55–61. [Google Scholar]
- Zhang, QY; Xie, G; Zhang, HP; Zhang, JP; He, M. Encapsulation of magnetic particles via miniemulsion polymerization of styrene. II. Effect of some parameters on the polymerization of styrene. J. Appl. Polym. Sci 2007, 105, 3525–3530. [Google Scholar]
- Wang, PH; Pan, CY. Polymer-metal composite particles: Metal particles on poly(St-co-MAA) microspheres. J. Appl. Polym. Sci 2000, 75, 1693–1698. [Google Scholar]
- Horák, D. Magnetic Polyglycidylmethacrylate Microspheres by Dispersion Polymerization. J. Polym. Sci. B Polym. Phys 2001, 39, 3707–3715. [Google Scholar]
- Lee, YH; Rho, JN; Jung, B. Preparation of magnetic ion-exchange resins by the suspension polymerization of styrene with magnetite. J. Appl. Polym. Sci 2003, 89, 2058–2067. [Google Scholar]
- Ma, ZY; Guan, YP; Liu, XQ; Liu, HZ. Preparation and characterization of micron-sized nonporous magnetic polymer microspheres with immobilized metal affinity ligands by modified suspension polymerization. J. Appl. Polym. Sci 2005, 96, 2174–2180. [Google Scholar]
- Zaitsev, VS; Filimonow, DS; presnyakov, IA; Gambino, RJ; Chu, BJ. Physical and Chemical Properties of Magnetite and Magnetite-Polymer Nanoparticles and Their Colloidal Dispersions. J. Colloid Interface Sci 1999, 212, 49–57. [Google Scholar]
- Chang, YC; Chen, DH. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J. Colloid Interface Sci 2005, 283, 446–451. [Google Scholar]
- Kima, EH; Ahnb, Y; Lee, HS. Biomedical applications of superparamagnetic iron oxide nanoparticles encapsulated within chitosan. J. Alloys Compd 2007, 434/435, 633–636. [Google Scholar]
- Zhou, LM; Wang, YP; Liu, ZR; Huang, QW. Carboxymethyl chitosan-Fe3O4 nanoparticles: preparation and adsorption behavior toward Zn2+ ions. Acta Phys. Chim. Sin 2006, 22, 1342–1346. [Google Scholar]
- Chang, YC; Chen, DH. Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan-conjugated magnetic nanoadsorbent. Macromol. Biosci 2005, 5, 254–261. [Google Scholar]
- Cuyper, MD; Joniau, M. Magnetoliposomes. Formation and structural characterization. Eur. Biophys. J 1988, 15, 311–319. [Google Scholar]
- Ulrich, AS. Biophysical aspects of using liposomes as delivery vehicles. Biosci. Rep 2002, 22, 129–150. [Google Scholar]
- Hernandez-Borrell, J; Mas, F; Puy, J. A theoretical approach to describe monolayer-liposome lipid interaction. Biophys. Chem 1990, 36, 47–55. [Google Scholar]
- Yamaura, M; Camilo, RL; Sampaio, LC; Macêdo, MA; Nakamura, M; Toma, HE. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater 2004, 279, 210–217. [Google Scholar]
- Chen, Y; Qian, Z; Zhang, ZC. Novel preparation of magnetite/polystyrene composite particles via inverse emulsion polymerization. Colloids Surf. A Physicochem. Eng. Asp 2008, 312, 209–213. [Google Scholar]


© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, L.; Liu, G.; Hong, R.-Y.; Li, H.-Z. Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres. Mar. Drugs 2010, 8, 2212-2222. https://doi.org/10.3390/md8072212
Guo L, Liu G, Hong R-Y, Li H-Z. Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres. Marine Drugs. 2010; 8(7):2212-2222. https://doi.org/10.3390/md8072212
Chicago/Turabian StyleGuo, Liang, Guang Liu, Ruo-Yu Hong, and Hong-Zhong Li. 2010. "Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres" Marine Drugs 8, no. 7: 2212-2222. https://doi.org/10.3390/md8072212



