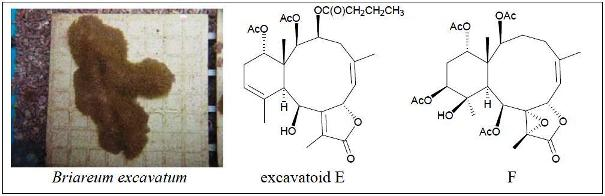
Excavatoids E and F: Discovery of Two New Briaranes from the Cultured Octocoral Briareum excavatum
Abstract
:1. Introduction
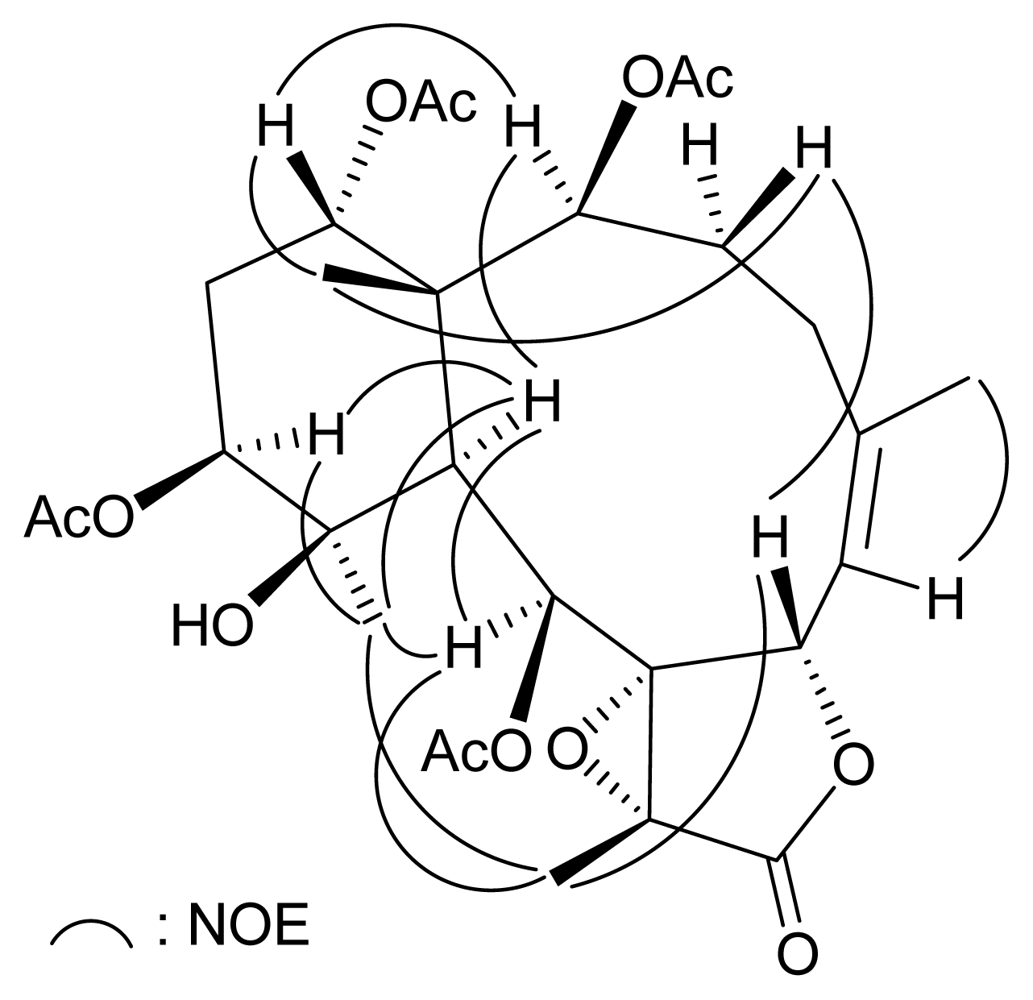
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Human Neutrophil Superoxide Anion Generation and Elastase Release
Acknowledgements
- Sample Availability: Not available.
References
- Sheu, J-H; Sung, P-J; Huang, L-H; Lee, S-F; Wu, T; Chang, B-Y; Duh, C-Y; Fang, L-S; Soong, K; Lee, T-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J Nat Prod 1996, 59, 935–938. [Google Scholar]
- Sheu, J-H; Sung, P-J; Cheng, M-C; Liu, H-Y; Fang, L-S; Duh, C-Y; Chiang, MY. Novel cytotoxic diterpenes, excavatolides A-E, isolated from the Formosan gorgonian Briareum excavatum. J Nat Prod 1998, 61, 602–608. [Google Scholar]
- Sung, P-J; Su, J-H; Wang, G-H; Lin, S-F; Duh, C-Y; Sheu, J-H. Excavatolides F-M, new briarane diterpenes from the gorgonian Briareum excavatum. J Nat Prod 1999, 62, 457–463. [Google Scholar]
- Sheu, J-H; Sung, P-J; Su, J-H; Wang, G-H; Duh, C-Y; Shen, Y-C; Chiang, MY; Chen, I-T. Excavatolides U-Z, new briarane diterpenes from the gorgonian Briareum excavatum. J Nat Prod 1999, 62, 1415–1420. [Google Scholar]
- Sheu, J-H; Sung, P-J; Su, J-H; Liu, H-Y; Duh, C-Y; Chiang, MY. Briaexcavatolides A-J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar]
- Sung, P-J; Su, J-H; Duh, C-Y; Chiang, MY; Sheu, J-H. Briaexcavatolides K-N, new briarane diterpenes from the gorgonian Briareum excavatum. J Nat Prod 2001, 64, 318–323. [Google Scholar]
- Wu, S-L; Sung, P-J; Chiang, MY; Wu, J-Y; Sheu, J-H. New polyoxygenated briarane diterpenoids, briaexcavatolides O-R, from the gorgonian Briareum excavatum. J Nat Prod 2001, 64, 1415–1420. [Google Scholar]
- Wu, S-L; Sung, P-J; Su, J-H; Sheu, J-H. Briaexcavatolides S-V, four new briaranes from a Formosan gorgonian Briareum excavatum. J Nat Prod 2003, 66, 1252–1256. [Google Scholar]
- Wu, S-L; Sung, P-J; Su, J-H; Wang, G-H; Sheu, J-H. Briaexcavatolide W, a new diterpenoid from Briareum excavatum. Heterocycles 2004, 63, 895–898. [Google Scholar]
- Sung, P-J; Hu, W-P; Wu, S-L; Su, J-H; Fang, L-S; Wang, J-J; Sheu, J-H. Briaexcavatolides X-Z, three new briarane-related derivatives from the gorgonian coral Briareum excavatum. Tetrahedron 2004, 60, 8975–8979. [Google Scholar]
- Sung, P-J; Hu, W-P; Fang, L-S; Fan, T-Y; Wang, J-J. Briarenol A, a new diterpenoid from a gorgonian Briareum sp. (Briareidae). Nat Prod Res 2005, 19, 689–694. [Google Scholar]
- Sung, P-J; Chao, C-H; Chen, Y-P; Su, J-H; Hu, W-P; Sheu, J-H. Briaexcavatins A and B, novel briaranes from the octocoral Briareum excavatum. Tetrahedron Lett 2006, 47, 167–170. [Google Scholar]
- Sung, P-J; Chen, Y-P; Hwang, T-L; Hu, W-P; Fang, L-S; Wu, Y-C; Li, J-J; Sheu, J-H. Briaexcavatins C-F, four new briarane-related diterpenoids from the Formosan octocoral Briareum excavatum (Briareidae). Tetrahedron 2006, 62, 5686–5691. [Google Scholar]
- Chen, Y-P; Wu, S-L; Su, J-H; Lin, M-R; Hu, W-P; Hwang, T-L; Sheu, J-H; Fan, T-Y; Fang, L-S; Sung, P-J. Briaexcavatins G and H, two new briaranes from the octocoral Briareum excavatum. Bull Chem Soc Jpn 2006, 79, 1900–1905. [Google Scholar]
- Su, J-H; Sung, P-J; Kuo, Y-H; Hsu, C-H; Sheu, J-H. Briarenolides A-C, briarane diterpenoids from the gorgonian coral Briareum sp. Tetrahedron 2007, 63, 8282–8285. [Google Scholar]
- Sung, P-J; Lin, M-R; Su, Y-D; Chiang, MY; Hu, W-P; Su, J-H; Cheng, M-C; Hwang, T-L; Sheu, J-H. New briaranes from the octocorals Briareum excavatum (Briareidae) and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 2596–2604. [Google Scholar]
- Sung, P-J; Lin, M-R; Hwang, T-L; Fan, T-Y; Su, W-C; Ho, C-C; Fang, L-S; Wang, W-H. Briaexcavatins M-P, four new briarane-related diterpenoids from cultured octocoral Briareum excavatum (Briareidae). Chem Pharm Bull 2008, 56, 930–935. [Google Scholar]
- Hwang, T-L; Lin, M-R; Tsai, W-T; Yeh, H-C; Hu, W-P; Sheu, J-H; Sung, P-J. New polyoxygenated briaranes from octocorals Briareum excavatum and Ellisella robusta. Bull Chem Soc Jpn 2008, 81, 1638–1646. [Google Scholar]
- Sung, P-J; Lin, M-R; Chiang, MY. The structure and absolute stereochemistry of briaexcavatin U, a new chlorinated briarane from a cultured octocoral Briareum excavatum. Chem Lett 2009, 38, 154–155. [Google Scholar]
- Sung, P-J; Lin, M-R; Chiang, MY; Hwang, T-L. Briaexcavatins V-Z, discovery of new briaranes from a cultured octocoral Briareum excavatum. Bull Chem Soc Jpn 2009, 82, 987–996. [Google Scholar]
- Sung, P-J; Su, Y-D; Li, G-Y; Chiang, MY; Lin, M-R; Huang, I-C; Li, J-J; Fang, L-S; Wang, W-H. Excavatoids A-D, new polyoxygenated briaranes from the octocoral Briareum excavatum. Tetrahedron 2009, 65, 6918–6924. [Google Scholar]
- Sung, P-J; Tsai, W-T; Chiang, MY; Su, Y-M; Kuo, J. Robustolides A-C, three new briarane-type diterpenoids from the female gorgonian coral Ellisella robusta (Ellisellidae). Tetrahedron 2007, 63, 7582–7588. [Google Scholar]
- Su, Y-M; Fan, T-Y; Sung, P-J. 11,20-Epoxybriaranes from the gorgonian coral Ellisella robusta (Ellisellidae). Nat Prod Res 2007, 21, 1085–1090. [Google Scholar]
- Sung, P-J; Chiang, MY; Tsai, W-T; Su, J-H; Su, Y-M; Wu, Y-C. Chlorinated briarane-type diterpenoids from the gorgonian coral Ellisella robusta (Ellisellidae). Tetrahedron 2007, 63, 12860–12865. [Google Scholar]
- Sung, P-J; Tsai, W-T; Lin, M-R; Su, Y-D; Pai, C-H; Chung, H-M; Su, J-H; Chiang, MY. Robustolides H and I, chlorinated briaranes from the gorgonian coral Ellisella robusta (Ellisellidae). Chem Lett 2008, 37, 88–89. [Google Scholar]
- Sung, P-J; Wu, S-L; Fang, H-J; Chiang, MY; Wu, J-Y; Fang, L-S; Sheu, J-H. Junceellolides E-G, new briarane diterpenes from the West Pacific Ocean gorgonian Junceella fragilis. J Nat Prod 2000, 63, 1483–1487. [Google Scholar]
- Sung, P-J; Fan, T-Y. 9-O-Deacetylumbraculolide A, a new diterpenoid from the gorgonian Junceella fragilis. Heterocycles 2003, 60, 1199–1202. [Google Scholar]
- Sung, P-J; Fan, T-Y; Fang, L-S; Sheu, J-H; Wu, S-L; Wang, G-H; Lin, M-R. Juncin N, a new briarane-type diterpenoid from the gorgonian coral Junceella juncea. Heterocycles 2003, 61, 587–592. [Google Scholar]
- Sung, P-J; Fan, T-Y; Fang, L-S; Wu, S-L; Li, J-J; Chen, M-C; Cheng, Y-M; Wang, G-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem Pharm Bull 2003, 51, 1429–1431. [Google Scholar]
- Sung, P-J; Fan, T-Y; Chen, M-C; Fang, L-S; Lin, M-R; Chang, P-C. Junceellin and praelolide, two briaranes from the gorgonian corals Junceella fragilis and Junceella juncea (Ellisellidae). Biochem Syst Ecol 2004, 32, 111–113. [Google Scholar]
- Sung, P-J; Lin, M-R; Fang, L-S. Briarane diterpenoids from the Formosan gorgonian coral Junceella fragilis. Chem Pharm Bull 2004, 52, 1504–1506. [Google Scholar]
- Sung, P-J; Lin, M-R; Chen, W-C; Fang, L-S; Lu, C-K; Sheu, J-H. Fragilide A, a novel diterpenoid from Junceella fragilis. Bull Chem Soc Jpn 2004, 77, 1229–1230. [Google Scholar]
- Sheu, J-H; Chen, Y-P; Hwang, T-L; Chiang, MY; Fang, L-S; Sung, P-J. Junceellolides J-L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J Nat Prod 2006, 69, 269–273. [Google Scholar]
- Sung, P-J; Chen, Y-P; Su, Y-M; Hwang, T-L; Hu, W-P; Fan, T-Y; Wang, W-H. Fragilide B, a novel briarane-type diterpenoid with a s-cis diene moiety. Bull Chem Soc Jpn 2007, 80, 1205–1207. [Google Scholar]
- Sung, P-J; Pai, C-H; Su, Y-D; Hwang, T-L; Kuo, F-W; Fan, T-Y; Li, J-J. New 8-hydroxy- briarane diterpenoids from the gorgonians Junceella juncea and Junceella fragilis (Ellisellidae). Tetrahedron 2008, 64, 4224–4232, (corrigendum in Tetrahedron 2008, 64, 9150). [Google Scholar]
- Sung, P-J; Pai, C-H; Hwang, T-L; Fan, T-Y; Su, J-H; Chen, J-J; Fang, L-S; Wang, W-H; Sheu, J-H. Junceols D-H, new polyoxygenated briaranes from sea whip gorgonian coral Junceella juncea (Ellisellidae). Chem Pharm Bull 2008, 56, 1276–1281. [Google Scholar]
- Sung, P-J; Li, G-Y; Chen, Y-P; Huang, I-C; Chen, B-Y; Wang, S-H; Huang, S-K. Fragilide E, a novel chlorinated 20-acetoxybriarane from the gorgonian coral Junceella fragilis. Chem Lett 2009, 38, 454–455. [Google Scholar]
- Sung, P-J; Wang, S-H; Chiang, MY; Su, Y-D; Chang, Y-C; Hu, W-P; Tai, C-Y; Liu, C-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull Chem Soc Jpn 2009, 82. in press. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, W-P; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244, and references cited therein. [Google Scholar]
- Berrue, F; Kerr, RG. Diterpenes from gorgonian corals. Nat Prod Rep 2009, 26, 681–710, and references cited therein. [Google Scholar]
- Hanson, JR. Diterpenoids. Nat Prod Rep 2009, 26, 1156–1171, and references cited therein. [Google Scholar]
- Sung, P-J; Sheu, J-H; Xu, J-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar]
- Sung, P-J; Chang, P-C; Fang, L-S; Sheu, J-H; Chen, W-C; Chen, Y-P; Lin, M-R. Survey of briarane-type diterpenoids-Part II. Heterocycles 2005, 65, 195–204. [Google Scholar]
- Sung, P-J; Chang, P-C; Fang, L-S; Sheu, J-H; Chen, W-C; Chen, Y-P; Lin, M-R. Survey of briarane-type diterpenoids-Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar]
- Taglialatela-Scafati, O; Deo-Jangra, U; Campbell, M; Roberge, M; Andersen, RJ. Diterpenoids from cultured Erythropodium caribaeorum. Org Lett 2002, 4, 4085–4088. [Google Scholar]
- Bayer, FM. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: anthozoa), with diagnoses of new taxa. Proc Biol Soc Wash 1981, 94, 902–947. [Google Scholar]
- Benayahu, Y; Jeng, M-S; Perkol-Finkel, S; Dai, C-F. Soft corals (Octocorallia: Alcyonacea) from southern Taiwan. II. Species diversity and distribution patterns. Zool Stud 2004, 43, 548–560. [Google Scholar]
- Fabricius, K; Alderslade, P. Soft Corals and Sea Fans–A comprehensive guide to the tropical shallow-water genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001. [Google Scholar]
- Hwang, T-L; Li, G-L; Lan, Y-H; Chia, Y-C; Hsieh, P-W; Wu, Y-H; Wu, Y-C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic Biol Med 2009, 46, 520–528. [Google Scholar]
- Hwang, T-L; Su, Y-C; Chang, H-L; Leu, Y-L; Chung, P-J; Kuo, L-M; Chang, Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J Lipid Res 2009, 50, 1395–1408. [Google Scholar]
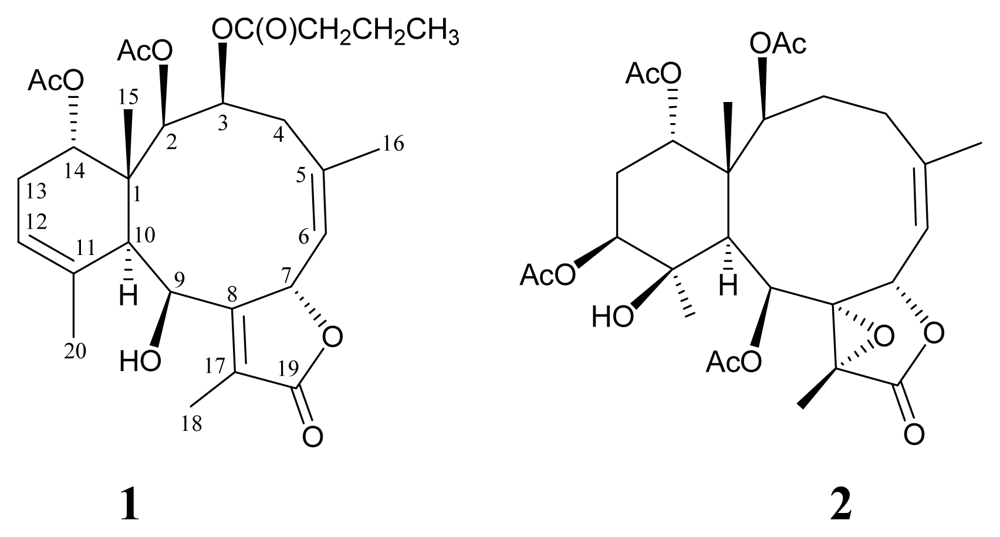
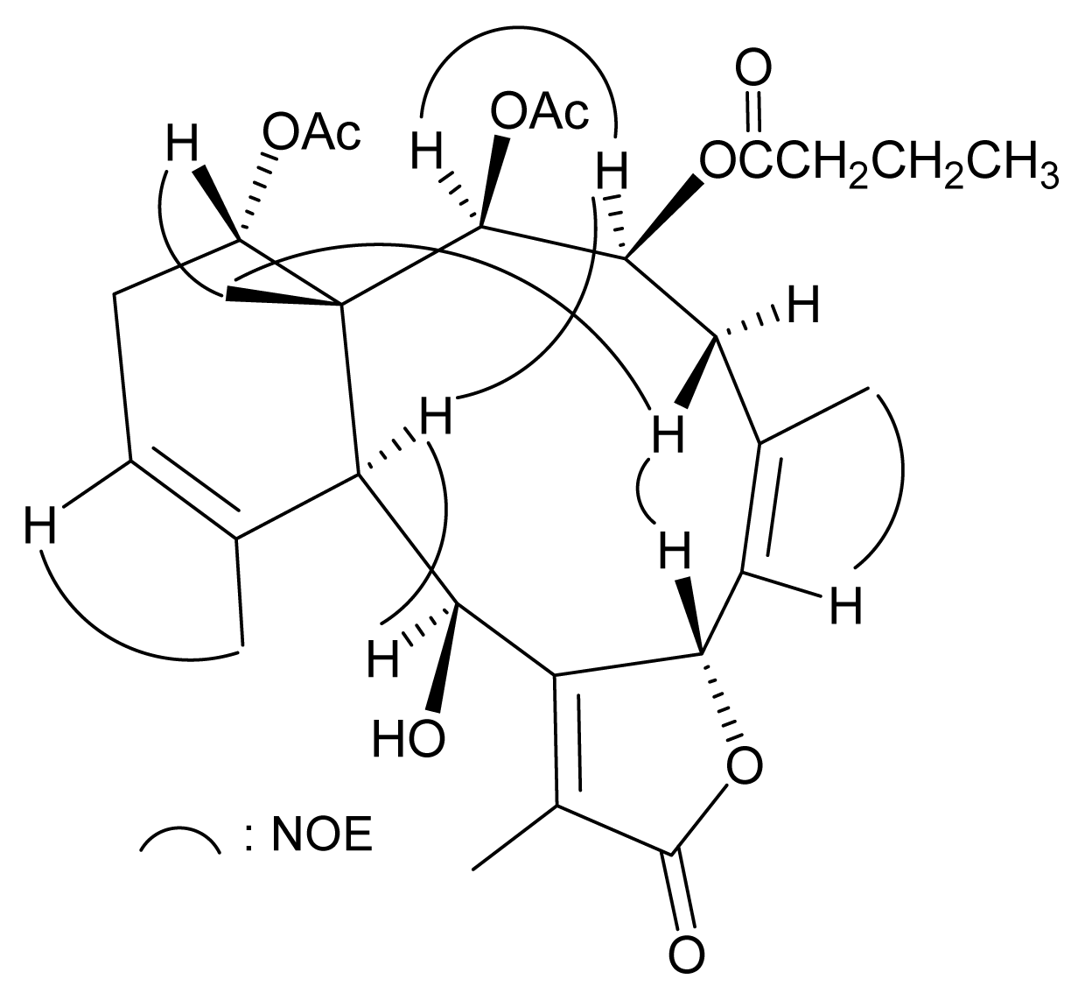
| 1 | 2 | |||
|---|---|---|---|---|
| Position | 1Ha | 13Cb | 1Ha | 13Cb |
| 1 | 41.7 (s)d | 46.3 (s) | ||
| 2 | 5.45 d (2.4)c | 74.9 (d) | 5.03 d (7.2) | 75.3 (d) |
| 3α | 4.98 dd (6.0, 2.4) | 72.2 (d) | 1.69 m | 32.5 (t) |
| β | 2.65 ddd (15.6, 15.6, 6.0) | |||
| 4α | 2.04 d (15.6) | 34.4 (t) | 1.97 m | 28.7 (t) |
| β | 3.46 dd (15.6, 6.0) | 2.50 m | ||
| 5 | 141.9 (s) | 146.2 (s) | ||
| 6 | 5.21 d (8.8) | 123.9 (d) | 5.25 d (9.2) | 117.6 (d) |
| 7 | 6.07 d (8.8) | 79.2 (d) | 5.32 d (9.2) | 74.9 (d) |
| 8 | 160.8 (s) | 70.6 (s) | ||
| 9 | 4.98 br s | 68.4 (d) | 5.86 d (2.0) | 67.8 (d) |
| 10 | 3.25 br s | 45.2 (d) | 2.20 d (2.0) | 47.8 (d) |
| 11 | 130.5 (s) | 75.7 (s) | ||
| 12 | 5.58 br s | 123.4 (d) | 4.90 dd (11.6, 5.2) | 73.1 (d) |
| 13α | 2.15 m | 28.0 (t) | 1.89–1.97 m (2H) | 25.6 (t) |
| β | 2.57 br d (18.4) | |||
| 14 | 5.08 dd (6.0, 6.0) | 75.6 (d) | 4.87 dd (4.8, 2.0) | 75.6 (d) |
| 15 | 1.36 s | 16.7 (q) | 1.30 s | 15.6 (q) |
| 16 | 1.85 s | 23.0 (q) | 2.02 s | 27.0 (q) |
| 17 | 127.6 (s) | 64.5 (s) | ||
| 18 | 2.01 s | 10.2 (q) | 1.73 s | 10.3 (q) |
| 19 | 173.9 (s) | 170.9 (s) | ||
| 20 | 1.64 s | 22.1 (q) | 1.24 s | 27.9 (q) |
| 2-OAc | 169.1 (s) | 170.5 (s) | ||
| 2.12 s | 20.9 (q) | 2.00 s | 21.3 (q) | |
| 9-OAc | 168.1 (s) | |||
| 2.19 s | 21.5 (q) | |||
| 12-OAc | 169.5 (s) | |||
| 2.07 s | 21.1 (q) | |||
| 14-OAc | 170.4 (s) | 170.6 (s) | ||
| 1.99 s | 21.2 (q) | 2.05 s | 21.3 (q) | |
| 3-OCOPr | 172.3 (s) | |||
| 2.22 t (7.6) | 35.9 (t) | |||
| 1.61 m | 18.0 (t) | |||
| 0.94 t (7.6) | 13.6 (q) | |||
| Position | 1 | 2 | ||
|---|---|---|---|---|
| 1H-1H COSY | HMBC | 1H-1H COSY | HMBC | |
| H-2 | H-3 | C-1, −3, −4, −14, −15, acetate carbonyl | H2-3 | C-1, −3, −4, −10, −14, −15, acetate carbonyl |
| H-3 | H-2, H2-4 | C-1 | H-2, H2-4 | C-1, −4 |
| H-4 | H-3 | C-3, −5, −6, −16 | H2-3, H-6 | C-5, −6 |
| H-6 | H-7, H3-16 | C-4, −16 | H-4, H-7, H3-16 | C-4 |
| H-7 | H-6 | C-5, −6, −8 | H-6 | C-5, −6, −19 |
| H-9 | H-10 | C-1, −8 | H-10 | C-1, −7, −8, −10, −17, acetate carbonyl |
| H-10 | H-9 | C-9, −11 | H-9 | C-1, −2, −8, −9, −11, −14, −15 |
| H-12 | H2-13, H3-20 | n.o.a | H2-13 | acetate carbonyl |
| H-13 | H-12, H-14 | n.o. | H-12, H-14 | C-1, −11, −14 |
| H-14 | H2-13 | C-1, −2, −13, −15, acetate carbonyl | H2-13 | C-10, acetate carbonyl |
| H-15 | C-1, −2, −10, −14 | C-1, −2, −14 | ||
| H-16 | H-6 | C-4, −5, −6 | H-6 | C-4, −5, −6 |
| H-18 | C-8, −17, −19 | C-8, −17, −19 | ||
| H-20 | H-12 | C-10, −11, −12 | C-10, −11, −12 | |
| Elastase | Superoxide Anion | |
|---|---|---|
| Compound | Inh.% | Inh.% |
| 1 | 26.22 ± 0.50 *** | 12.95 ± 6.99 |
| 2 | 30.63 ± 4.68 * | 2.57 ± 1.11 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sung, P.-J.; Chen, B.-Y.; Lin, M.-R.; Hwang, T.-L.; Wang, W.-H.; Sheu, J.-H.; Wu, Y.-C. Excavatoids E and F: Discovery of Two New Briaranes from the Cultured Octocoral Briareum excavatum. Mar. Drugs 2009, 7, 472-482. https://doi.org/10.3390/md7030472
Sung P-J, Chen B-Y, Lin M-R, Hwang T-L, Wang W-H, Sheu J-H, Wu Y-C. Excavatoids E and F: Discovery of Two New Briaranes from the Cultured Octocoral Briareum excavatum. Marine Drugs. 2009; 7(3):472-482. https://doi.org/10.3390/md7030472
Chicago/Turabian StyleSung, Ping-Jyun, Bo-Yuan Chen, Mei-Ru Lin, Tsong-Long Hwang, Wei-Hsien Wang, Jyh-Horng Sheu, and Yang-Chang Wu. 2009. "Excavatoids E and F: Discovery of Two New Briaranes from the Cultured Octocoral Briareum excavatum" Marine Drugs 7, no. 3: 472-482. https://doi.org/10.3390/md7030472