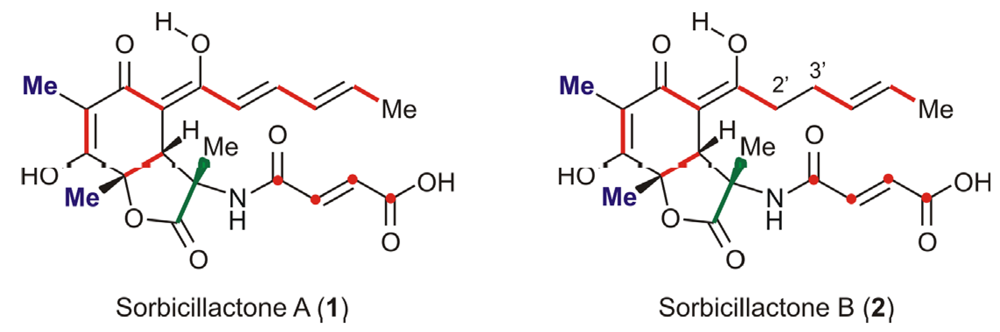
Large-Scale Biotechnological Production of the Antileukemic Marine Natural Product Sorbicillactone A
Abstract
:Introduction
Results and Discussion
Biotechnological Production of Sorbicillactone A (1)
Media Composition and Growth Conditions
Production of the First 100 Grams of Substance
Improvement of the Culture Conditions
Preparation of Spore Suspension for Inoculation
Screening for New Sorbicillactone A Producers
Selection and Improvement of Production of Sorbicillactone A (1) by Strain KIPB33
Extraction of Sorbicillactone A (1) from the Culture Media
Extraction of Sorbicillactone A (1) from the XAD Eluate
Purification of the Crude Extract by Fast Centrifugal Partition Chromatography (FCPC)
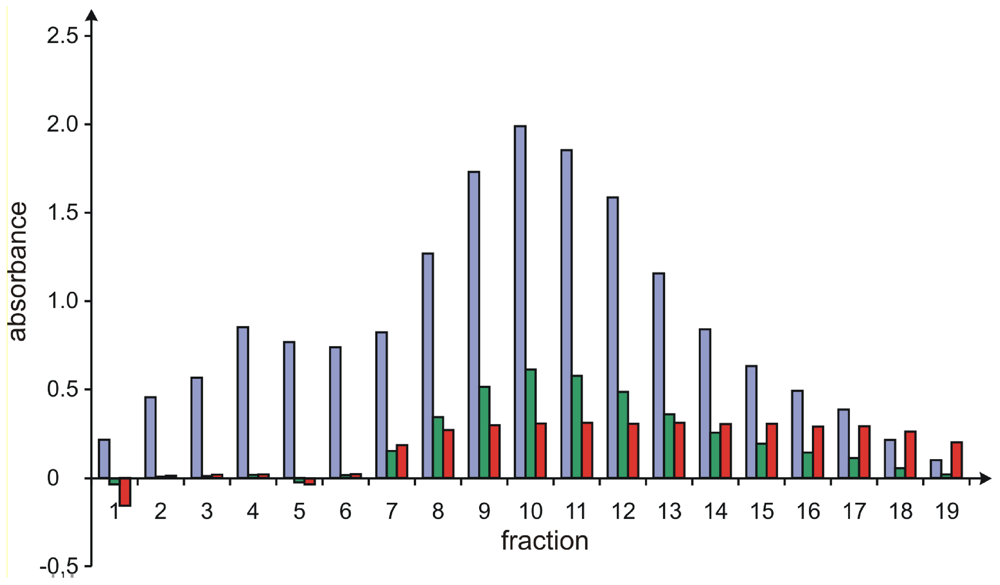
Resolution of 1 and 2 by Gel Chromatography on Sephadex LH20
Analysis of the Purity of Sorbicillactone A (1) Containing Fractions
Conclusions
Acknowledgement
- Dedicated to Prof. Dr. W.E.G. Müller on the occasion of his 65th birthday
- Samples Availability: Available from the authors.
References and Notes
- Bringmann, G; Lang, G; Mühlbacher, J; Schaumann, K; Steffens, S; Rytik, PG; Hentschel, U; Morschhäuser, J; Brun, R. Müller, WEG, Ed.; Sponges (Porifera); Springer: Berlin, 2003; Volume 1, pp. 231–253. [Google Scholar]
- Bringmann, G; Lang, G; Gulder, TAM; Hideyuki, H; Mühlbacher, J; Maksimenka, K; Steffens, S; Schaumann, K; Stöhr, R; Wiese, J; Imhoff, JF; Perovi-Ottstadt, S; Boreiko, O; Müller, WEG. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar]
- Bringmann, G; Lang, G; Mühlbacher, J; Schaumann, K; Steffens, S; Müller, WEG. Sorbicillactone A derivatives for the treatment of tumor and viral diseases. International Patent Application WO 2004/026854; 1.4.2004,
- Bringmann, G; Lang, G; Gulder, TAM; Schaumann, K; Müller, WEG; Perovi-Ottstadt, S; Stöhr, R; Wiese, J; Schmaljohan, R; Imhoff, JF. Method for producing sorbicillactone A. International Patent Application WO 2005/072711; 11.8.2005,
- Wickerham, L. J. Taxonomy of yeasts. US Dept Technol Bull 1951, 1029, 1–56. [Google Scholar]
- For more information on FCPC from KROMATON TECHNOLOGIES, see www.kromaton.com
- Ito, Y. High Speed Countercurrent Chromatography. Crit Rev Anal Chem 1986, 17, 65–143. [Google Scholar]
- The amount of extract purified per run was easily scaled up in a linear manner by using a 1–l rotor in the same FCPC system.
- Henke, H. Präparative Gelchromatographie an Sephadex LH-20; Obernburg, 1994. [Google Scholar]
| Strain | Source | Quantity of 1 produced [mg l−1 ] | Conditions of cultivation | |
|---|---|---|---|---|
| E01–10/3* | Ircinia spec., Elba | 25 | Modified Wickerham medium (0.5% NaCl, pH 6.6) (inoculated with spore suspension type 1) | |
| E01–10/3* | Ircinia spec., Elba | 102 |  | Modified Wickerham medium, 0.5% NaCl, pH 7.0 (inoculated with spore suspension type 2) |
| KIPB25 | Tubulipora spec., Baltic Sea | 99 | ||
| KIPB11 | Tubulipora spec., Baltic Sea | 97 | ||
| KIPB10 | Tubulipora spec., Baltic Sea | 96 | ||
| E99–2/174* | Unidentified sponge, Elba | 77 | ||
| E00–7/22* | Hamigera hamigera, Elba | 58 | ||
| E01–12/1* | Ircinia spec., Elba | 43 | ||
| R02-S2 | marine sediment, Rovinj | 26 | ||
| R02-S8 | marine sediment, Rovinj | 9 | ||
| H269 | Halichondria panicea, Baltic Sea | 9 | ||
| R03–9/5 | Cacospongia scalaris, Rovinj | 6 | ||
| H259 | Halichondria panicea, Baltic Sea | 5 | ||
| L122 | Laminaria saccharina, Baltic Sea | 5 | ||
| R02–13/2 | Acanthella spec., Rovinj | <1 | ||
| R03–8/4 | Tethya aurantium, Rovinj | <1 | ||
| H236 | Halichondria panicea, Baltic Sea | <1 | ||
| KIPB33 | Callopora aurita, Baltic Sea | 295 |  | WSP medium (inoculated with spore suspension type 3) |
| E01–10/3* | Ircinia spec., Elba | 157 | ||
| KIPB25 | Tubulipora spec., Baltic Sea | 178 | ||
| KIPB11 | Tubulipora spec., Baltic Sea | 192 | ||
| KIPB10 | Tubulipora spec., Baltic Sea | 111 | ||
| H269 | Halichondria panicea, Baltic Sea | 11 | ||
| L122 | Laminaria saccharina, Baltic Sea | 17 | ||
| KIPB33 | Callopora aurita, Baltic Sea | 505 | Modified WSP medium (2.5 g glucose and 7.5 g lactose) (inoculated with spore suspension type 3) | |
Share and Cite
Bringmann, G.; Gulder, T.A.M.; Lang, G.; Schmitt, S.; Stöhr, R.; Wiese, J.; Nagel, K.; Imhoff, J.F. Large-Scale Biotechnological Production of the Antileukemic Marine Natural Product Sorbicillactone A. Mar. Drugs 2007, 5, 23-30. https://doi.org/10.3390/md502023
Bringmann G, Gulder TAM, Lang G, Schmitt S, Stöhr R, Wiese J, Nagel K, Imhoff JF. Large-Scale Biotechnological Production of the Antileukemic Marine Natural Product Sorbicillactone A. Marine Drugs. 2007; 5(2):23-30. https://doi.org/10.3390/md502023
Chicago/Turabian StyleBringmann, Gerhard, Tobias A. M. Gulder, Gerhard Lang, Stefanie Schmitt, Rüdiger Stöhr, Jutta Wiese, Kerstin Nagel, and Johannes F. Imhoff. 2007. "Large-Scale Biotechnological Production of the Antileukemic Marine Natural Product Sorbicillactone A" Marine Drugs 5, no. 2: 23-30. https://doi.org/10.3390/md502023




