Polycitorols A and B, New Tricyclic Alkaloids from an Ascidian
Abstract
:Introduction
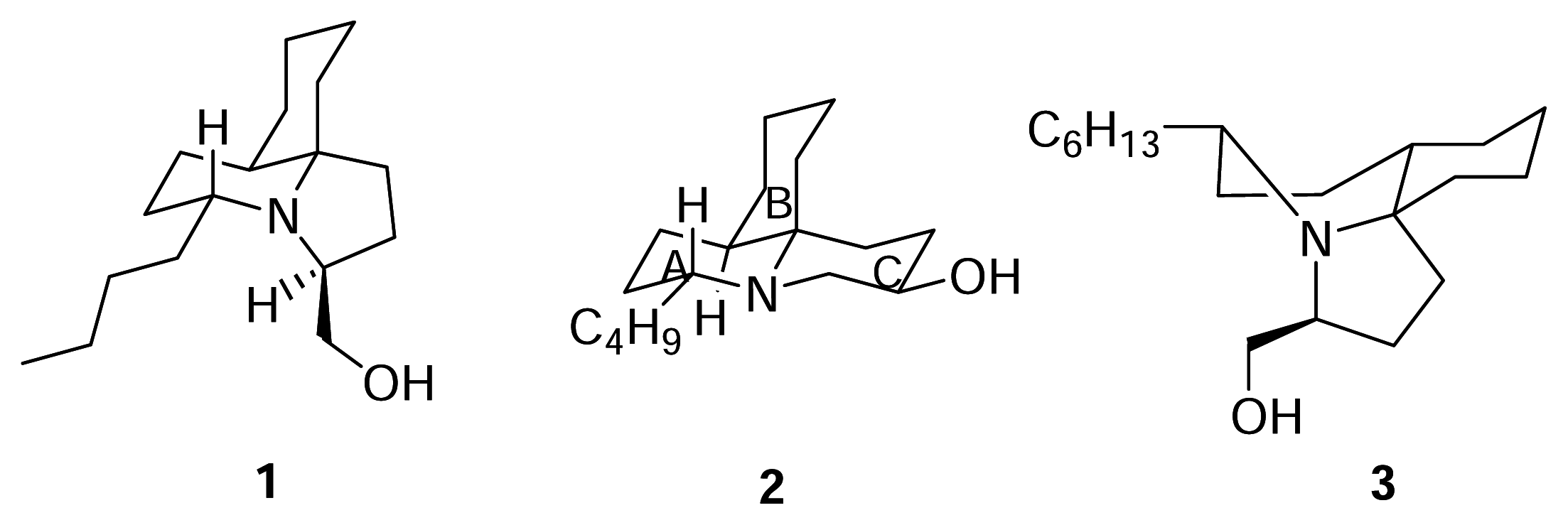
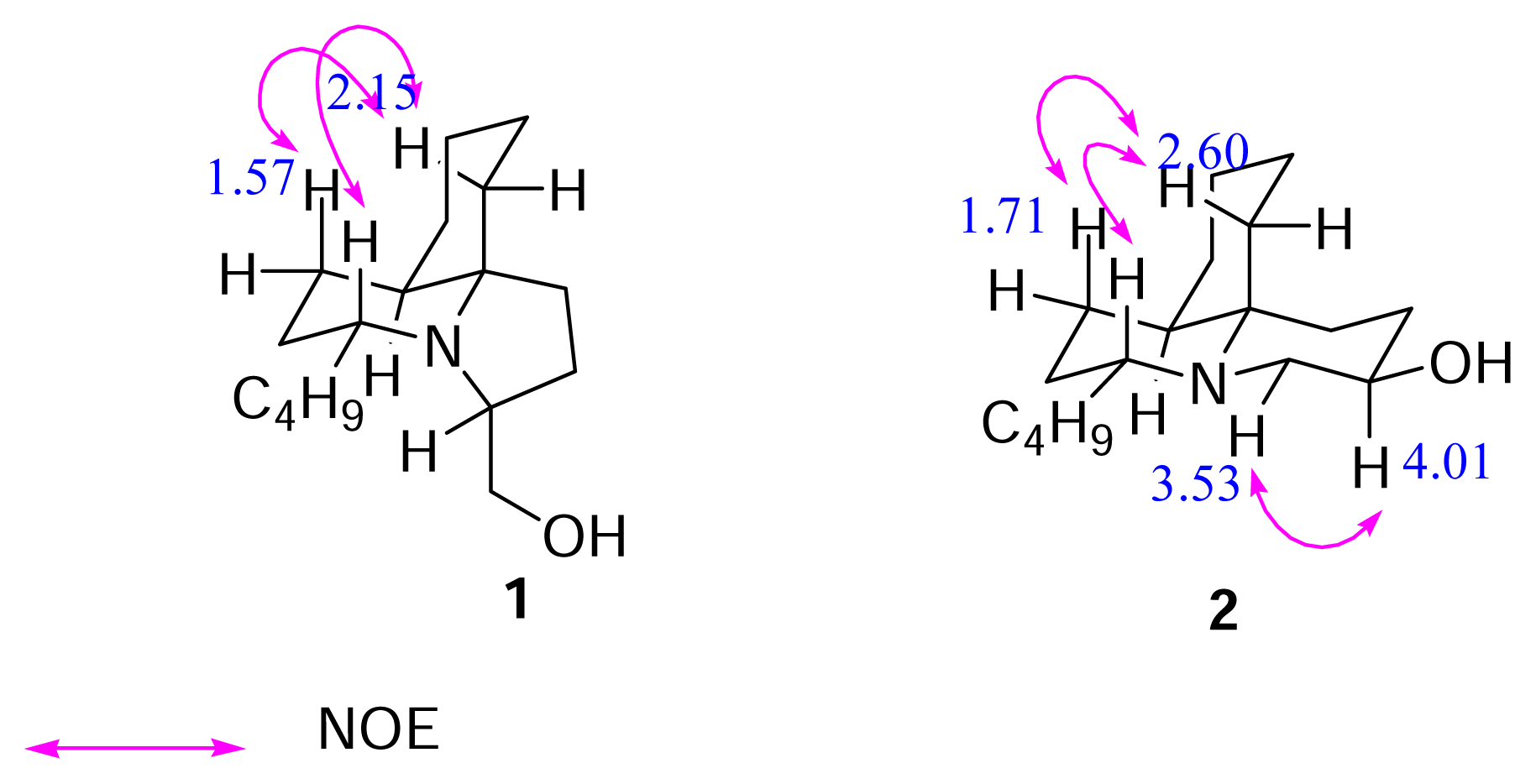
Results and Discussion
Conclusion
Experimental
General
Biological material
Extraction and Isolation
| 1a | 2b | ||||
|---|---|---|---|---|---|
| # | δC | δH | HMBC | δC | δH |
| 2 | 67.0 d | 2.97 brt, J = 9.4 Hz | C-15 | 58.3 d | 3.39 brs |
| 3 | 30.9 t | 2.10 m, 1.49 m | C-2, C-4, C-5 | 28.9 t | 1.71 m, 2.08 m |
| 4 | 24.0 t | 1.57 m, 1.74 m | C-2, C-3, C-5, C-10 | 24.9 t | 1.37 m, 1.47 m |
| 5 | 44.2 d | 1.81 m | C-10 | 43.3 d | 1.98 m |
| 6 | 32.1 t | 1.25 m, 1.60 m | C-5, C-10, C-7 | 29.5 t | 1.26 m, 1.53 m |
| 7 | 26.4 t | 1.36 m, 1.70 m | C-6 | 24.6 t | 1.70 m |
| 8 | 25.4 t | 1.47 m, 1.43 m | C-9 | 22.3 t | 1.41 m, 1.78 m |
| 9 | 32.7 t | 1.47 m, 2.15 m | C-8 | 31.2 t | 1.61 m, 2.60 brd, J = 12.5 Hz |
| 10 | 77.9 s | 66.5 s | |||
| 11 | 24.4 t | 2.09 m, 2.17 m | C-9, C-10, C-12 | 17.6 t | 1.97 m |
| 12 | 24.6 t | 1.81 m, 2.24 dt, J = 13.4, 8.2 Hz | C-10, C-11 | 26.3 t | 1.78 m, 2.11 m |
| 13 | 71.9 d | 3.76 m | C-12, C-14 | 60.5 d | 4.06 brs |
| 14 | 65.0 t | 3.72 m | C-13, C-12 | 45.5 d | 3.36 brs 3.53 m |
| 15 | 34.7 t | 1.58 m, 2.05 m | C-2, C-3 | 30.6 t | 1.60 m, 1.94 m |
| 16 | 29.5 t | 1.21 m, 1.36 m | C-15, C-17, C-18 | 27.4 t | 1.24 m, 1.38 m |
| 17 | 23.6 t | 1.31 m, 1.39 m | C-16, C-18 | 22.4 t | 1.28 m |
| 18 | 14.2 q | 0.90, t, J = 7.0 Hz | C-16, C-17 | 13.7 q | 0.90 t, J = 7.0 Hz |
Acknowledgments
- Sample availability:The authentic sample of both polycitorol A and B are deposited at Department of Chemistry, Biology, and Marine Science, University of the Ryukyus.
References and Notes
- Blackman, A. J.; Li, C.; Hockless, D. C. R.; Skelton, B. W.; White, A. H. Cyclindricines A-B, novel alkaloids from the ascidian Clavelina cylindrica. Tetrahedron 1993, 49, 8645–8656. [Google Scholar]Li, C.; Blackman, A. J. Cyclindricines C-G, perhydropyrrolo[2,1-j]quinolin-7-one alkaloids from the ascidian Clavelina cylindrica. Aust. J. Chem. 1994, 47, 1355–1361. [Google Scholar]Li, C.; Blackman, A. Cyclindricines H-K, novel alkaloids from the ascidian Clavelina cylindrica. Aust. J. Chem 1995, 48, 955–965. [Google Scholar]
- Biard, J. F.; Guyot, S.; Roussakis, C.; Verbist, J. F.; Vercauteren, J.; Weber, J. F.; Boukef, K. Lepadiformine, a new marine cytotoxic alkaloid from Clavelina lepadiformis. Tetrahedron Lett 1994, 35, 2691–2694. [Google Scholar]
- Patil, A. D.; Freyer, A. J.; Reichwein, R.; Carte, B.; Killmer, L. B.; Faucette, L.; Johnson, R. K. Fascicularin, a novel tricyclic alkaloid from the ascidian Neptheis fascicularis. Tetrahedron Lett 1997, 38, 363–364. [Google Scholar]
- Abe, H.; Aoyagi, S.; Kibayashi, C. First total synthesis of the marine alkaloid (±)-fascicularin and (±)-lepadiformine based on stereocontrolled intramolecular Acylnitroso-Diels-Alder-Reaction. J. Am. Chem. Soc 2000, 122, 4583–4592. [Google Scholar]
- Sun, P.; Sun, C.; Weinreb, S. M. Stereoselective total syntheses of racemic form and the natural enantiomer of the marine alkaloid lepadiformine via a novel N-acyliminium ion/allylsilane spirocyclization strategy. J. Org. Chem 2002, 67, 4337–4345. [Google Scholar]
- Juge, M.; Grimaud, N.; Biard, J. F.; Sauviat, M. P.; Nabil, M.; Verbist, J. F.; Petit, J. Y. Cardiovascular effects of lepadiformine, an alkaloid isolated from the ascidians Clavelina lepadiformis (Muller) and C. moluccensis (Sluiter). Toxicon 2001, 39, 1231–1237. [Google Scholar]
- Issa, H. H.; Tanaka, J.; Rachmat, R.; Higa, T. Floresolides, new metacyclophane hydroquinone lactones from an ascidian, Aplidium sp. Tetrahedron Lett 2003, 44, 1243–1245. [Google Scholar]
© 2005 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Issa, H.H.; Tanaka, J.; Rachmat, R.; Setiawan, A.; Trianto, A.; Higa, T. Polycitorols A and B, New Tricyclic Alkaloids from an Ascidian. Mar. Drugs 2005, 3, 78-83. https://doi.org/10.3390/md303078
Issa HH, Tanaka J, Rachmat R, Setiawan A, Trianto A, Higa T. Polycitorols A and B, New Tricyclic Alkaloids from an Ascidian. Marine Drugs. 2005; 3(3):78-83. https://doi.org/10.3390/md303078
Chicago/Turabian StyleIssa, Hamad H., Junichi Tanaka, Rachmaniar Rachmat, Andi Setiawan, Agus Trianto, and Tatsuo Higa. 2005. "Polycitorols A and B, New Tricyclic Alkaloids from an Ascidian" Marine Drugs 3, no. 3: 78-83. https://doi.org/10.3390/md303078




