Marine Natural Products from Indonesian Waters
Abstract
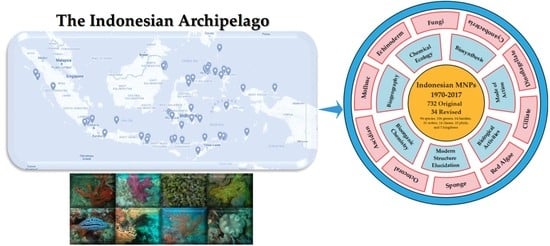
:1. Introduction
2. New MNPs Discovered from Indonesian Waters in the Period 1970–2017
2.1. General
2.2. Terpenoids
2.2.1. Sesquiterpenoids
2.2.2. Diterpenoids
2.2.3. Sesterterpenoids
2.2.4. Triterpenoids
2.2.5. Steroids
2.2.6. Saponins
2.2.7. Meroterpenoids
2.3. Alkaloids
2.3.1. Piperidine Alkaloids
2.3.2. Pyridine Alkaloids
2.3.3. Indole Alkaloids
2.3.4. Acridine Alkaloids
2.3.5. Quinoline and Isoquinoline Alkaloids
2.3.6. Tyrosine Alkaloids
2.3.7. Pyrrole Alkaloids
2.3.8. Imidazole Alkaloids
2.3.9. Polysulfur Aromatic Alkaloids
2.3.10. Serine-Derived Alkaloids
2.3.11. Other Marine Alkaloids
2.4. Peptides
2.5. Fatty Acids and Linear Molecules
2.6. Polyketides
2.7. Carbohydrates
3. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolau, K.C.; Montagnon, T. Molecules that Changed the World; Willey-VCH: Weinheim, Germany, 2008; pp. 9–319. ISBN 978-3-527-30983-2. [Google Scholar]
- Hoffmann, R.W. Classical Methods in Structure Elucidation of Natural Products; Willey-VCHA: Zürich, Switzerland, 2018; pp. 1–259. ISBN 978-3-906390-79-6. [Google Scholar]
- Blunt, J.; Buckingham, J.; Munro, M. Taxonomy and Marine Natural Products Research. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Tagliatela-Scafati, O., Eds.; Springer: New York, NY, USA, 2012; Volume 1, pp. 3–54. ISBN 978-90-481-3833-3. [Google Scholar]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Appeltans, W.; Ahyong, A.T.; Anderson, G.; Angel, M.V.; Artois, T.; Baily, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The magnitude of global marine species diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F. All natural: The renaissance of natural product chemistry. Org. Lett. 2014, 16, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Le, J.-Y.; Orlikova, B.; Diederich, M. Signal transducers and activators of transcription (STAT) regulatory networks in marine organisms: From physiological observations toward marine drug discovery. Mar. Drugs 2015, 13, 4967–4984. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Schofield, M.M.; Jain, S.; Porat, D.; Dick, G.J.; Sherman, D.H. Identification and analysis of the bacterial endosymbiont specialized for production of the chemotherapeutic natural product ET-743. Environ. Microbiol. 2015, 17, 3964–3975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Nobel Prize in Chemistry 2008 and 2010. Available online: https://www.nobelprize.org/prizes/chemistry/ (accessed on 26 February 2019).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.; Cruz-Trinidad, A.; Geronimo, R.; Aliño, P. Opportunities and challenges in the coral triangle. Environ. Sci. Technol. 2012, 46, 7930–7931. [Google Scholar] [CrossRef] [PubMed]
- Provinces of Indonesia. Available online: https://en.wikipedia.org/wiki/ Provinces_of_Indonesia (accessed on 10 July 2017).
- Glaser, M.; Baitoningsih, W.; Ferse, S.C.A.; Neil, M.; Deswandi, R. Whose sustainability? Top-down participation and emergent rule in marine protected area management in Indonesia. Mar. Policy. 2010, 34, 1215–1225. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, E.; Gonzales, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Visbeck, M. Ocean science research is key for a sutainable future. Nat. Commun. 2018, 9, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J. Marine natural product chemistry: Past, present, and future. Aust. J. Chem. 2010, 63, 851–854. [Google Scholar] [CrossRef]
- Uemura, D. Chemistry of biologically and physiologically intriguing phenomena. Tetrahedron 2004, 60, 6969. [Google Scholar] [CrossRef]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provide insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [PubMed]
- Adam, J. Collaborations: The rise of research networks. Nature 2012, 490, 335–336. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Bonn, A.; Guerra, C.A. Recognizing the quiet extinction of invertebrates. Nat. Commun. 2019, 10, 1–3. [Google Scholar] [CrossRef]
- Dewi, A.S.; Tarman, K.; Uria, A.R. Marine natural products: Prospects and impacts on the sustainable development in Indonesia. In Proceedings of the Indonesian Students’ Scientific Meeting, Delft, The Netherlands, 13–15 May 2008; pp. 54–63. [Google Scholar]
- Chasanah, E. Marine biodiscovery research in Indonesia: Challenges and rewards. J. Coastal Dev. 2008, 12, 1–12. [Google Scholar]
- Putra, M.Y.; Muniarsih, T. Distribution and diversity of marine natural products from Indonesian marine organisms. J. Coastal Life Med. 2016, 4, 104–107. [Google Scholar] [CrossRef]
- Scheuer, P.J. Toxins from fish and other marine organisms. Adv. Food Res. 1970, 18, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, J.P.; Tursch, B.; Djerassi, C. A new sterol from an alcyonarian. Steroids 1972, 20, 121–126. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactivities. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades−where and what are we bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [PubMed]
- White, K.N.; Amagata, T.; Oliver, A.G.; Tenney, K.; Wenzel, P.J.; Crews, P. Structure revision of spiroleucettadine, a sponge alkaloid with a bicylic core meager in H-atoms. J. Org. Chem. 2008, 73, 8719–8722. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, N.; Murata, M. NMR studies on natural product: Stereochemical determination and conformational analysis in solution and in membrane. In Experimental Approaches of NMR Spectroscopy; Naito, A., Asakura, T., Shimada, I., Takegoshi, K., Yamamoto, Y., Eds.; Springer Nature: Singapore, 2018; pp. 383–414. ISBN 978-981-10-5966-7. [Google Scholar]
- Seco, J.M.; Quiñoá, E.; Riguera, R. Assignment of the absolute configuration of polyfunctional compounds by NMR using chiral derivatizing agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.P.; Li, K.; Maue, M.; Zografos, A.L.; Baran, P.S. Total synthesis of dimeric pyrolle-imidazole alkaloids; sceptrin, ageliferin, nagelamide E, oxysceptrin, nakamuric acid, and the axinellamine carbon skeleton. J. Am. Chem. Soc. 2007, 129, 4762–4775. [Google Scholar] [CrossRef]
- Sun, Y.-T.; Lin, B.; Li, S.-G.; Liu, M.; Zhou, Y.-J.; Xu, Y.; Hua, H.-M.; Lin, H.-W. New bromopyrrole alkaloids from the marine sponge Agelas sp. Tetrahedron 2017, 73, 2786–2792. [Google Scholar] [CrossRef]
- Guella, G.; Dini, F.; Pietra, F. Metabolites with a novel C30 backbone from marine ciliates. Angew. Chem. Int. Ed. 1999, 38, 1134–1136. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Zhang, H.; Ortiz, A.; Dagneau, P. Total synthesis of the originally assigned structure of vannusal B. Angew. Chem. Int. Ed. 2008, 47, 8605–8610. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Zhang, H.; Ortiz, A. The true structure of the vannusals, part 1: Initial forays into suspected and intelegence gathering. Angew. Chem. Int. Ed. 2009, 48, 5642–5647. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Ortiz, A.; Zhang, H. The true structures of the vannusals, part 2: Total synthesis and revised structure of vannusal B. Angew. Chem. Int. Ed. 2009, 48, 5648–5652. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, K.C.; Ortiz, A.; Zhang, H.; Dagneau, P.; Lanver, A.; Jennings, M.P.; Arseniyadis, S.; Faraoni, R.; Lizos, D.E. Total synthesis and structural revision of vannusals A and B: Synthesis of the originally assigned structure of vannusal B. J. Am. Chem. Soc. 2010, 132, 7138–7152. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, K.C.; Ortiz, A.; Zhang, H.; Guella, G. Total synthesis and structural revision of vannusals A and B: Synthesis of the true structures of vannusals A and B. J. Am. Chem. Soc. 2010, 132, 7153–7176. [Google Scholar] [CrossRef] [PubMed]
- Saielli, G.; Nicolaou, K.C.; Ortiz, A.; Zhang, H.; Bagno, A. Addressing the stereochemistry of complex organic molecules by density functional theory-NMR: Vannusal B in retrospective. J. Am. Chem. Soc. 2011, 133, 6072–6077. [Google Scholar] [CrossRef] [PubMed]
- Guella, G.; Skropeta, D.; Di Giuseppe, G.; Dini, F. Structures, biological activities and phylogenetic relationships of terpenoids from marine ciliates of the genus Euplotes. Mar. Drugs 2010, 87, 2080–2116. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Koyama, T.; Yamada, K.; Kita, M.; Uemura, D. Karatungiols A and B, two novel antimicrobial polyol compounds, from the symbiotic marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006, 47, 2521–2525. [Google Scholar] [CrossRef]
- Linnington, R.G.; Williams, D.E.; Tahir, A.; van Soest, R.; Andersen, R.J. Latonduines A and B, new alkaloids isolated from the marine sponge Stylissa carteri: Structure elucidation, synthesis, and biogenetic implications. Org. Lett. 2003, 5, 2735–2738. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.-D.; Weber, H.; Hartmann, R.; Wray, V.; Lin, W.; Lai, D.; Proksch, P. New cytotoxic 1,2,4-thiadiazole alkaloids from the ascidian Polycarpa aurata. Org. Lett. 2013, 15, 2230–2233. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and cytotoxic compound from marine organisms. In Marine Biotechnology: Pharmaceutical and Bioactive Natural Products; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 197–308. ISBN 0-306-44174-8. [Google Scholar]
- Gerwick, W.H. The face of a molecule. J. Nat. Prod. 2017, 80, 2583–2588. [Google Scholar] [CrossRef]
- Birch, A.M.; Pattenden, G. Capnellane sesquiterpenes. Total synthesis of epiprecapnelladiene and Δ8(9)-capnellane. J. Chem. Soc. Perkin Trans. I. 1983, 1913–1917. [Google Scholar] [CrossRef]
- Inouye, Y.; Shirai, M.; Michino, T.; Kakisawa, H. Preparation of an 8-membered ring via intramolecular [2+2] photocycloadduct: Formal total synthesis of (±)-precapnelladiene. Bull. Chem. Soc. Jpn. 1993, 66, 324–326. [Google Scholar] [CrossRef]
- Maeda, K.; Inouye, Y. Preparation of (R)-(2-cyclopentyl)methanol and the first total synthesis of (8R, 11R)-precapnelladiene. Bull. Chem. Soc. Jpn. 1994, 67, 2880–2882. [Google Scholar] [CrossRef]
- Takenaka, Y.; Ito, H.; Iguchi, K. Enantioselective formal synthesis of (+)-precapnelladiene by chiral copper-catalyzed asymmetric [2+2]-cycloaddition reaction. Tetrahedron 2007, 63, 510–513. [Google Scholar] [CrossRef]
- Stefinovic, M.; Snieckus, V. Connecting directed ortho metalation and olefin metathesis strategies. Benzene-fused multiring-sized oxygen heterocyles. First synthesis of radulanin A and helianane. J. Org. Chem. 1998, 63, 2808–2809. [Google Scholar] [CrossRef]
- Sabui, S.K.; Venkateswaran, R.V. Synthesis of helianane, an unusual marine sesquiterpene employing ring-expansion by flash vacuum thermolysis. Tetrahedron Lett. 2004, 45, 9653–9655. [Google Scholar] [CrossRef]
- Soga, K.; Kanematsu, M.; Yoshida, M.; Shishido, K. Efficient enantioselective total synthesis of (+)-helianane. Synlett 2011, 1171–1173. [Google Scholar] [CrossRef]
- Quartieri, F.; Mesiano, L.E.; Borghi, D.; Desperati, V.; Gennari, C.; Papeo, G. Total synthesis of (+)-7, 11-helianane and (+)-5-chloro-7,11-helianane through stereoselective aromatic Claisen rearrangement. Eur. J. Org. Chem. 2011, 6794–6801. [Google Scholar] [CrossRef]
- Green, J.; Jiménez-Alonso, S.; Brown, E.R.; Pettus, T.R.R. Total synthesis and repudiation of the helianane family. Org. Lett. 2011, 13, 5500–5503. [Google Scholar] [CrossRef] [PubMed]
- Asai, R.; Mitsuhashi, S.; Shigetomi, K.; Miyamoto, T.; Ubukata, M. Absolute configurations of (–)-hirsutanol A and (–)-hirsutanol C produced by Gloestereum incarnatum. J. Antibiot. 2011, 64, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R. Measurement of Bijvoet differences. Structure and absolute configuration of africanol, a sesquiterpene. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1976, B32, 2609–2614. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ham, W.H. Total synthesis of africanol. Tetrahedron Lett. 1986, 27, 2341–2344. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ham, W.H. Total synthesis of the marine sesquiterpenes dactylol and africanol. De novo construction of a cyclooctanoid natural product from cycloheptane precursors. J. Am. Chem. Soc. 1987, 109, 3025–3036. [Google Scholar] [CrossRef]
- Tursch, B. Some recent developments in the chemistry of alcyonaceans. Pure Appl. Chem. 1976, 48, 1–6. [Google Scholar] [CrossRef]
- Kato, H.; Nehira, T.; Matsuo, K.; Kawabata, T.; Kobashigawa, Y.; Morioka, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; et al. Niphateolide A: Isolation from the marine sponge Niphates olemda and determination of its absolute configuration by an ECD analysis. Tetrahedron 2015, 71, 6956–6960. [Google Scholar] [CrossRef]
- Aoki, S.; Watanabe, Y.; Sanagawa, M.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge, Corticium simplex. J. Am. Chem. Soc. 2006, 128, 3148–3149. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aoki, S.; Tanabe, D.; Setiawan, A.; Kobayashi, M. Cortistatins E, F, G, and H, four novel steroidal alkaloids from marine sponge Corticium simplex. Tetrahedron 2007, 63, 4074–4079. [Google Scholar] [CrossRef]
- Aoki, S.; Watanabe, Y.; Tanabe, D.; Setiawan, A.; Arai, M.; Kobayashi, M. Cortistatins J, K, L, novel abeo-9(10-19)-androstane-type steroidal alkaloids with isoquinoline unit, from marine sponge Corticium simplex. Tetrahedron Lett. 2007, 48, 4485–4488. [Google Scholar] [CrossRef]
- Shenvi, R.A.; Guerrero, C.A.; Shi, J.; Li, C.-C.; Baran, P.S. Synthesis of (+)-cortistatin A. J. Am Chem. Soc. 2008, 130, 7241–7243. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Sun, Y.-P.; Peng, X.-P.; Polet, D.; Chen, D.Y.K. Total synthesis of (+)-cortistatin A. Angew. Chem. Int. Ed. 2008, 47, 7310–7313. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Peng, X.-S.; Sun, Y.-P.; Polet, D.; Zhou, B.; Lim, C.S.; Chen, D.Y.-K. Total synthesis and biological evaluation of cortistatins A and J and analogues thereof. J. Am Chem. Soc. 2009, 131, 10587–10597. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Watanabe, Y.; Tanabe, D.; Arai, M.; Suna, H.; Miyamoto, K.; Tsujibo, H.; Tsujikawa, K.; Yamamoto, H.; Kobayashi, M. Structure-activity relationship and biological property of cortistatins, anti-angiogenic spongean steroidal alkaloids. Bioorg. Med. Chem. 2007, 15, 6758–6762. [Google Scholar] [CrossRef] [PubMed]
- Czako, B.; Kurti, L.; Mammoto, A.; Ingber, D.E.; Corey, E.J. Discovery of potent and practical antiangiogenic agents inspired by cortistatin A. J. Am. Chem. Soc. 2009, 131, 9014–9019. [Google Scholar] [CrossRef] [PubMed]
- Cee, V.J.; Chen, D.Y.-K.; Lee, M.R.; Nicolau, K.C. Cortistatin A is a high-affinity ligand of protein kinase ROCK, CDK8, CDK11. Angew. Chem. Int. Ed. 2009, 48, 8952–8957. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hu, J.-F.; Kazi, A.B.; Li, Z.; Avery, M.; Peraud, O.; Hill, R.; Franzblau, F.; Zhang, F.; Schinazi, R.F.; et al. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against Mycobacteria and HIV-1. J. Am. Chem. Soc. 2003, 125, 13382–13386. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, S.O.; Larghi, E.L.; Bracca, A.B.J.; Kaufman, T.S. Synthesis of the unique angular tricyclic chromone structure proposed fro aspergillitine, and its relationship with alkaloid TMC-120B. Org. Biomol. Chem. 2012, 10, 4124–4134. [Google Scholar] [CrossRef] [PubMed]
- Copp, B.R.; Jompa, J.; Tahir, A.; Ireland, C.M. Styelsamines A–D: New tetracyclic pyridoacridine alkaloids from the Indonesian ascidian Eusynstyela latericius. J. Org. Chem. 1988, 63, 8024–8026. [Google Scholar] [CrossRef]
- Skyler, D.; Heathcock, C.H. A simple biomimetic synthesis of styelsamine B. Org. Lett. 2001, 3, 4323–4324. [Google Scholar] [CrossRef]
- Nakahara, S.; Kubo, A. Total synthesis of styelsamine C, a cytotoxic fused tetracyclic aromatic alkaloid. Heterocyles 2003, 60, 2017–2018. [Google Scholar] [CrossRef]
- Fong, H.K.H.; Copp, B.R. Synthesis, DNA binding and antitumor evaluation of styelsamine and cystodytin analgues. Mar. Drugs 2013, 11, 274–299. [Google Scholar] [CrossRef]
- Skyler, D.; Heathcock, C.H. The pyridoacridine family tree: A useful scheme for designing synthesis and predicting undiscovered natural products. J. Nat. Prod. 2002, 65, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Proksch, P.; Wray, V.; van Soest, R.W.M.; Ferdinandus, E.; Pattisina, L.A.; Sudarsono. New bromopyrrole alkaloids from the Indopacific sponge Agelas nakamurai. J. Nat. Prod. 1999, 62, 1295–1297. [Google Scholar] [CrossRef]
- Mokhlesi, A.; Hartman, R.; Achten, E.; Chaidir; Hartmann, T.; Lin, W.; Daletos, G.; Proksch, P. Lissodendrins A and B: 2-Aminoimidazole alkaloids from the marine sponge Lissodendroryx (Acanthodoryx) fibrosa. Eur. J. Org. Chem. 2016, 639–643. [Google Scholar] [CrossRef]
- Liu, H.; Pratasik, S.B.; Nishikawa, T.; Shida, T.; Fujiwara, K.; Nagai, H.; Kobayashi, H.; Namikoshi, M. Lissoclibadin 1, a novel trimeric sulfur-bridged dopamine derivative, from the tropical ascidian Lissoclinum cf. badium. Tetrahedron Lett. 2004, 45, 7015–7017. [Google Scholar] [CrossRef]
- Knoth, T.; Warburg, K.; Katzka, C.; Rai, A.; Wolf, A.; Brockmeyer, A.; Janning, P.; Reubold, T.F.; Eschenburg, S.; Manstein, D.J.; et al. The ras pathway modulator melophlin A target dynamins. Angew. Chem. Int. Ed. 2009, 48, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B.; Diestel, R.; Jagusch, C.; Sasse, F.; Schobert, R. Metal complexes of natural melophlins and their cytotoxic and antibiotic activities. J. Inorg. Biochem. 2009, 103, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yamano, Y.; Kamiya, K.; Setiawan, A.; Kobayashi, M. Anti-dormant mycobacterial activity and target molecule of melophlins, tetramic acid derivatives isolated from a marine sponge of Melophlus sp. J. Nat. Med. 2016, 70, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hasegawa, M.; Harada, K.; Kobayashi, H.; Nagai, H.; Namikoshi, M. Melophlins P, Q, R, and S: Four new tetramic acid derivatives, from two Palauan marine sponges of the genus Melophlus. Chem. Pharm. Bull. 2006, 54, 852–854. [Google Scholar] [CrossRef]
- Oda, T.; Fujita, A.; Xu, J.; Mochizuki, M.; Ukai, K.; Namikoshi, M. Effects of melophlins on colony formation of Chinese hamster V79 cells and IL-8 production in PMA-stimulated HL-60 cells. Mar. Drugs 2007, 5, 1–5. [Google Scholar] [CrossRef]
- Putra, M.Y.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C.; Fattorusso, E.; Tagliatela-Scafati, O. Sinularioside, a triacetylated glycolipid from the Indonesian soft coral Sinularia sp., is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012, 22, 2723–2725. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950, 70, 2809–2810. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I.1. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Wakimoto, T.; Abe, I. Labile natural products. Med. Chem. Commun. 2012, 3, 866–870. [Google Scholar] [CrossRef]
- Lallier, L.E.; McMeel, O.; Greiber, T.; Vanagt, T.; Dobson, A.D.W.; Jaspars, M. Access to and use of marine genetic resources: Understanding the legal framework. Nat. Prod. Rep. 2014, 31, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Katz, F.; Newman, D.J.; Rosenthal, J. Legal and ethical issues involving marine biodiscovery and development. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Tagliatela-Scafati, O., Eds.; Springer: New York, NY, USA, 2012; Volume 1, pp. 1315–1342. ISBN 978-90-481-3833-3. [Google Scholar]
- Torii, M.; Kato, H.; Hitora, Y.; Angkouw, E.D.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Lamellodysidines A and B, sesquiterpenes isolated from the marine sponge Lamellodysidea herbacea. J. Nat. Prod. 2017, 80, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Tursch, B.; Colin, M.; Daloze, D.; Losman, D.; Karlsson, R. Chemical studies of marine invertebrates. XII. Lemnacarnol, a novel sesquiterpene from the soft coral Lemnalia carnosa. Bull. Soc. Chim. Belg. 1975, 84, 81–82. [Google Scholar] [CrossRef]
- Karlsson, R.; Losman, D. Structure and absolute configuration of the sesquiterpene lemnacarnol. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1977, B33, 1614–1616. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Nemorin, J.L.E.; Sternhell, S. Studies of Australian soft corals. XXIII The co-occurence of bicyclogermacrene and lemnacarnol derivatives in Parerythropodium fulvum. Tetrahedron Lett. 1980, 21, 3105–3108. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Kaisin, M. Terpenoids from coelenterates. In Marine Natural Products: Chemical and Biological Perspectives; Scheuer, P.J., Ed.; Academic Press: New York, NY, USA, 1978; Volume 2, pp. 247–296. ISBN 978-0-12-624002-3. [Google Scholar]
- Daloze, D.; Braekman, J.C.; Georget, P.; Tursch. Chemical studies of marine invertebrates. XXII. Two novel sesquiterpenes from soft corals of of the genera Lemnalia and Paralemnalia (Coelenterate, Octocorallia, Alcyonacea). Bull. Soc. Chim. Belg. 1977, 86, 47–54. [Google Scholar] [CrossRef]
- Kapojos, M.; Mangindaan, R.E.P.; Nakazawa, T.; Oda, T.; Ukai, K.; Namikoshi, M. Three new nardosinane type sesquiterpenes from an Indonesian soft coral Nephthea sp. Chem. Pharm. Bull. 2008, 56, 332–334. [Google Scholar] [CrossRef]
- Ayanoglu, E.; Gebreyesus, T.; Beechan, C.M.; Djerassi, C. Terpenoids LXXVI. Precapnelladiene, a possible biosynthetic precursor of the capnellane skeleton. Tetrahedron. 1979, 35, 1035–1039. [Google Scholar] [CrossRef]
- Grote, D.; Hänel, F.; Dahse, H.-M.; Seifert, K. Capnellenes from the soft coral Dendronephthyta rubeola. Chem. Biodivers. 2008, 5, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Ofwegen, L.; Kunzmann, A. New oxygenated sesquiterpenes from the Indonesian soft coral Nephthea chabrolii. J. Nat. Prod. 1997, 60, 716–718. [Google Scholar] [CrossRef]
- Hanif, N.; Murni, A.; Yamauchi, M.; Higashi, M.; Tanaka, J. A new trinor-guaiane sesquiterpene from an Indonesian soft coral Anthelia sp. Nat. Prod. Commun. 2015, 10, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.; Crews, P. The structure and probable biogenesis of helianane, a heterocyclic sesquiterpene, from the Indo-Pacific Sponge Haliclona? fascigera. J. Org. Chem. 1997, 62, 2646–2648. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Patrick, B.O.; Tahir, A.; van Soest, R.; Roberge, M.; Andersen, R.J. Boneratamides A–C, new sesquiterpenoids isolated from the marine sponge Axinyssa aplysinoides. J. Nat. Prod. 2004, 67, 1752–1754. [Google Scholar] [CrossRef]
- Ayanoglu, E.; Gebreyesus, T.; Beechan, C.M.; Djerassi, C. Terpenoids LXXV. Δ9(12)-capnellane, a new sesquiterpene hydrocarbon from the softcoral Capnella imbricata. Tetrahedron Lett. 1978, 19, 1671–1674. [Google Scholar] [CrossRef]
- Morris, L.A.; Jaspars, M.; Adamson, K.; Woods, S.; Wallace, H.M. The capnellenes revisited: New structures and new biological activity. Tetrahedron 1998, 54, 12953–12958. [Google Scholar] [CrossRef]
- Chang, C.-H.; Wen, Z.-H.; Wang, S.-K.; Duh, C.-Y. Capnellenes from the Formosan soft coral Capnella imbricata. J. Nat. Prod. 2008, 71, 619–621. [Google Scholar] [CrossRef]
- Sheikh, Y.M.; Singy, G.; Kaisin, M.; Eggert, H.; Djerassi, C.; Tursch, B.; Daloze, D.; Braekman, J.C. Terpenoids-LXXI: Chemical studies of marine invertebrates-XIV. Four representatives of a novel sesquiterpene class-the capnellane skeleton. Tetrahedron 1976, 32, 1171–1178. [Google Scholar] [CrossRef]
- Jean, Y.-H.; Chen, W.-F.; Sung, C.-S.; Duh, C.-Y.; Huang, S.-Y.; Lin, C.-S.; Tai, M.-H.; Tzeng, S.-F.; Wen, Z.-H. Capnellene, a natural marine compound derived soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Kaisin, M.; Sheikh, Y.M.; Durham, D.J.; Djerassi, C.; Tursch, B.; Daloze, D.; Braekman, J.C.; Losman, D.; Karlsson, R. Capnellane-a new tricyclic sesquiterpene skeleton. Tetrahedron Lett. 1974, 15, 2239–2242. [Google Scholar] [CrossRef]
- Sheikh, Y.M.; Djerassi, C.; Braeekman, J.C.; Daloze, D.; Kaisin, M.; Tursch, B.; Karlsson, R. Terpenoids-LXXII. Chemical studies of marine invertebrates-XXVI-Δ9(12)-capnellene-3β,8β,10α,14-tetrol, a novel polyoxygenated sesquiterpene from the Alyconarian Capnella imbricata. Tetrahedron 1977, 33, 2115–2117. [Google Scholar] [CrossRef]
- Wang, G.Y.S.; Abrell, L.M.; Avelar, A.; Borgeson, B.M.; Crews, P. New hirsutane based sesquiterpenes from salt water cultures of a marine sponge-derived fungus and the terrestrial fungus Coriulus consors. Tetrahedron 1998, 54, 7335–7342. [Google Scholar] [CrossRef]
- Li, H.-J.; Lan, W.-J.; Lam, C.-K.; Yang, F.; Zhu, X.-F. Hirsutane sesquiterpenoids from the marine-derived fungus Chondrostereum sp. Chem. Biodiversity. 2011, 8, 317–324. [Google Scholar] [CrossRef]
- Yang, F.; Chen, W.-D.; Deng, R.; Li, D.-D.; Wu, K.-W.; Feng, G.-K.; Li, H.-J.; Zhu, X.-F. Hirsutanol A induces apoptosis and autophagy via reactive oxygen species accumulation in breast cancer MCF-7 cells. J. Pharmacol. Sci. 2012, 119, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Fritz, P.; Kelecom, A.; Karlsson, R.; Losman, D. Chemical studies of marine invertebrate. VIII. Africanol, an unusual sesquiterpene from Lemnalia africana (Coelenterata, Octocorallia, Alcyonacea). Tetrahedron Lett. 1974, 15, 747–750. [Google Scholar] [CrossRef]
- Beechan, C.M.; Djerassi, C.; Eggert, H. Terpenoids-LXXIV. The sesquiterpenes from the soft coral Sinularia mayi. Tetrahedron 1978, 34, 2503–2508. [Google Scholar] [CrossRef]
- Beechan, C.M.; Djerassi, C.; Finer, J.S.; Clardy, J. Terpenoids LXXIII. Sinularene, a sesquiterpene hydrocarbon based on a novel skeleton from the soft coral, Sinularia mayi. Tetrahedron Lett. 1977, 18, 2395–2398. [Google Scholar] [CrossRef]
- Yasman; Edrada, R.A.; Wray, V.; Proksch, P. New 9-thiocyanatopupukeanane sesquiterpenes from the nudibranch Phyllidia varicosa and its sponge-prey Axinyssa aculeata. J. Nat. Prod. 2003, 66, 1512–1514. [Google Scholar] [CrossRef]
- Putra, M.Y.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C.; Fattorusso, E.; Taglialatela-Scafati, O. Sinulasulfoxide and sinulasulfone, sulfur-containing alkaloids from the Indonesian soft coral Sinularia sp. Tetrahedron Lett. 2012, 53, 3937–3939. [Google Scholar] [CrossRef]
- Salmoun, M.; Braekman, J.C.; Dewelle, J.; Darro, F.; Kiss, R.; de Voogd, N.J.; van Soest, R.W.M. New terpenoids from two Indonesian marine sponges. Nat. Prod. Res. 2007, 21, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Ebel, R.; Wray, V.; Müller, W.E.G.; Edrada-Ebel, R.A.; Proksch, P. Diacarperoxides, norterpene cyclic peroxides from the sponge Diacarnus megaspinorhabdosa. J. Nat. Prod. 2008, 71, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Muller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Nieto, R.M.; Jiménez, C. New briarane stecholide diterpenes from the Indonesian gorgonian Briareum sp. J. Nat. Prod. 1998, 61, 313–317. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briarane diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Rodríguez, J.; Jiménez, C. Absolute structures of new briarane diterpenoids from Junceella fragilis. J. Nat. Prod. 1999, 62, 257–260. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Williams, G.C. Malayenolides A–D, novel diterpenes from the Indonesian sea pen Veretillum malayense. J. Nat. Prod. 1999, 62, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Okano, M.; Matsui, K.; Itoh, T.; Satari, R.; Akiyama, S.; Kobayashi, M. Brianthein A, a novel briarane-type diterpene reversing multidrug resistance in human carcinoma cell line, from the gorgonian Briareum excavatum. Tetrahedron 2001, 57, 8951–8957. [Google Scholar] [CrossRef]
- Tanaka, C.; Yamamoto, Y.; Otsuka, M.; Tanaka, J.; Ichiba, T.; Marriot, G.; Rachmat, R.; Higa, T. Briarane diterpenes from two species of octocorals, Ellisella sp. and Pteroeides sp. J. Nat. Prod. 2004, 67, 1368–1373. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Hwang, T.-L.; Weng, C.-F.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Cladielloides A and B: New eunicellin-type diterpenoid from an Indonesian octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs 2011, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-Y.; Chen, Y.-H.; Hwang, T.-L.; Fang, L.-S.; Wang, W.-H.; Liu, M.-C.; Su, J.-H.; Wu, Y.-C.; Sung, P.-J. Cladielloides C and D: Novel eunicellin-based diterpenoids from an Indonesian Octocoral Cladiella sp. Bull. Chem. Soc. Jpn. 2011, 84, 531–536. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hwang, T.-L.; Su, Y.-D.; Chang, Y.-C.; Chen, Y.-H.; Hong, P.-H.; Hu, L.-C.; Yen, W.-H.; Hsu, H.-Y.; Huang, S.-J.; et al. New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 2012, 60, 160–163. [Google Scholar] [CrossRef]
- Murni, A.; Hanif, N.; Tanaka, J. A new cytotoxic dolabellane from the Indonesian soft coral Anthelia sp. Indones. J. Chem. 2013, 13, 216–220. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Bavestrello, G.; Bonelli, P.; Calcinai, B. Coelodiol and coeloic acid, ent-isocopalane diterpenes from the Indonesian sponge Coelocarteria cfr. singaporensis. Tetrahedron Lett. 2006, 47, 2197–2200. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Angkouw, E.D.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Ceylonamides A–F, nitrogenous spongian diterpenes that inhibit RANKL-induced osteoclastogenesis, from the marine sponge Spongia ceylonensis. J. Nat. Prod. 2016, 79, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Kato, H.; Kagiyama, I.; Hitora, Y.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Ceylonins A–F, spongian diterpene derivatives that inhibit RANKL-induced formation of multinuclear osteoclasts, from the marine sponge Spongia ceylonensis. J. Nat. Prod. 2017, 80, 90–95. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Tsukamoto, S. Ceylonins G-I: Spongian diterpenes from the marine sponge Spongia ceylonensis. J. Nat. Med. 2017, 71, 765–769. [Google Scholar] [CrossRef]
- Parrish, S.M.; Yoshida, W.Y.; Kondratyuk, T.P.; Park, E.-J.; Pezzuto, J.M.; Kelly, M.; Williams, P.G. Spongiapyridine and related spongians isolated from an Indonesian Spongia sp. J. Nat. Prod. 2014, 77, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Hérin, M.; Tursch, B. Chemical studies of marine invertebrates. XXV. Flexibilene, an unprecedented fifteen-membered ring diterpene hydrocarbon from the soft coral Sinularia flexibilis (Coelenterata, Octocorallia). Bull. Soc. Chim. Belg. 1976, 85, 801–803. [Google Scholar] [CrossRef]
- McMurry, J.; Matz, J.R.; Kees, K.L.; Bock, P.A. Synthesis of flexibilene, a naturally occuring 15-membered-ring diterpene. Tetrahedron Lett. 1982, 23, 1777–1780. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Hérin, M.; Karlsson, R. Chemical studies of marine invertebrates. X. Lobophytholide, a new cembranolide diterpene from the soft coral Lobophytum cristagalli (Coelenterata, Octocorallia, Alyconacea). Tetrahedron Lett. 1974, 15, 3769–3772. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Dedeuerwaerder, H.; Karlsson, R. Chemical studies of marine invertebrates. XXXI. Crassolide, a highly oxygenated diterpene from the soft coral Lobophytum crassum (Coelenterata, Octocorallia, Alcynoacea). Bull. Soc. Chim. Belg. 1978, 87, 75–81. [Google Scholar] [CrossRef]
- Wang, S.-K.; Duh, C.-Y.; Wu, Y.-C.; Wang, Y.; Cheng, M.-C.; Soong, K.; Fang, L.-S. Studies on Formosan soft corals, II. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992, 55, 1430–1435. [Google Scholar] [CrossRef]
- Wang, L.-T.; Wang, S.-K.; Soong, K.; Duh, C.-Y. New cytotoxic cembranolides from the soft coral Lobophytum michaele. Chem. Pharm. Bull. 2007, 55, 766–770. [Google Scholar] [CrossRef]
- Pesando, D.; Graillet, C.; Braekman, J.-C.; Dubreuil, A.; Girard, J.-P.; Puiseux-Dao, S. The use of sea urchin eggs as a model to investigate the effects of diterpene isolated from a soft coral. Toxicol. In Vitro 1991, 5, 395–401. [Google Scholar] [CrossRef]
- Januar, H.I.; Zamani, N.P.; Soedharma, D.; Chasanah, E. New cytotoxic cembranoid from Indonesian soft coral Sarcophyton sp. Pharmacognosy Res. 2017, 9, 65–68. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D. Chemical studies of marine invertebrates-XIII. 2-Hydroxynepthenol, a novel cembrane diterpene from the soft coral Lithophyton viridis (Coelenterata, Octocorallia, Alconacea). Bull. Soc. Chim. Belg. 1975, 84, 767–774. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Weng, Y.-L.; Chiang, M.Y.; Dai, C.-F. Cytotoxic terpenoids from the Formosan soft coral Nephthea brassica. J. Nat. Prod. 1999, 62, 1518–1521. [Google Scholar] [CrossRef]
- Suzuki, M.; Shimada, A.; Kato, T. Cyclization of polyenes xxx1 synthesis of 2-hydroxy-and 1,2-dehydro-7,8-oxido-nephthenols and and cembrenol. Chem. Lett. 1978, 7, 759–762. [Google Scholar] [CrossRef]
- Hérin, M.; Tursch, B. Chemical studies of marine invertebrates. XXIV. Minor cembrane diterpenes from the soft coral Sinularia flexibilis (Coelenterata, Octocorallia). Bull. Soc. Chim. Belg. 1976, 85, 707–719. [Google Scholar] [CrossRef]
- Shaker, K.H.; Müller, M.; Ghani, M.A.; Dahse, H.-M.; Seifert, K. Terpenes from the soft corals Litophyton arboreum and Sarcophyton ehrenbergi. Chem. Biodiversity 2010, 7, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Januar, H.I.; Chasanah, E.; Motti, C.A.; Tapiolas, D.M.; Liptrot, C.H.; Wright, A.D. Cytotoxic cembranes from Indonesian specimens of the soft coral Nephthea sp. Mar. Drugs 2010, 8, 2142–2152. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Hérin, M.; Karlsson, H.; Losman, D. Chemical Studies of marine invertebrates-XI. Sinulariolide, a new cembranolide diterpene from the softcoral Sinularia flexibilis (Coelenterata, Octocorallia, Alyconacea). Tetrahedron 1975, 31, 129–133. [Google Scholar] [CrossRef]
- Karlsson, R. The structure and absolute configuration of sinulariolide, a cembranolide diterpene. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1977, B33, 2027–2031. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Chang, F.-R.; McPhail, A.T.; Lee, K.-H.; Wu, Y.-C. New cembranolide analogues from the Formosan soft coral Sinularia flexibilis and their cytotoxicity. Nat. Prod. Res. 2003, 17, 409–418. [Google Scholar] [CrossRef]
- Su, J.-H.; Lin, Y.-F.; Yeh, H.-C.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm. Bull. 2009, 57, 1189–1192. [Google Scholar] [CrossRef]
- Li, Y.; Su, H.-J.; Chen, Y.-H.; Wen, Z.-H.; Sheu, J.-H.; Su, J.-H. Anti-inflammatory cembranoids from the Formosan soft coral Sinularia discrepans. Arch. Pharm. Res. 2011, 34, 1263–1267. [Google Scholar] [CrossRef]
- Wang, J.; Su, P.; Gu, Q.; Li, W.D.; Guo, J.L.; Qiao, W.; Feng, D.Q.; Tang, S.A. Antifouling activity against bryozoan and barnacle by cembrane diterpenes from the soft coral Sinularia flexibilis. Int. Biodeterior. Biodegrad. 2017, 120, 97–103. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Chen, H.-Y.; Sheu, J.-H.; Chiang, M.-Y.; Wen, Z.-H.; Dai, C.-F.; Su, J.-H. Structural elucidation and structure−anti-inflammatory activity relationships of cembranoids from cultured soft corals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food. Chem. 2015, 63, 7211–7218. [Google Scholar] [CrossRef] [PubMed]
- Aceret, T.L.; Coll, J.C.; Uchio, Y.; Sammarco, P.W. Antimicrobial actvity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata, Octocorallia). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endrocrinol. 1998, 120, 121–126. [Google Scholar] [CrossRef]
- Aceret, T.L.; Sammarco, P.W.; Coll, J.C.; Uchio, Y. Discrimination between several diterpenoid compounds in feeding by Gambusia affinis. Comp. Biochem. Physiol. C Comp. Pharmacol. 2001, 128, 55–63. [Google Scholar] [CrossRef]
- Michalek, K.; Bowden, B.F. A natural algacide from soft coral Sinularia flexibilis (Coelenterata, Octocorallia, Alcyonacea). J. Chem. Ecol. 1997, 23, 259–273. [Google Scholar] [CrossRef]
- Aceret, T.L.; Brown, L.; Meiiler, J.; Coll, J.C.; Sammarco, P.W. Cardiac and vascular responses of isolated rat tissues with diterpenes from Sinularia flexibilis (Coelenterata: Octocorallia). Toxicon 1996, 34, 1165–1171. [Google Scholar] [CrossRef]
- Neoh, C.-A.; Wang, R.Y.-L.; Din, Z.-H.; Su, J.-H.; Chen, Y.-K.; Tsai, F.-J.; Weng, S.-H.; Wu, Y.-J. Induction of apoptosis by sinulariolide from soft coral through mitochondrial-related and p38MAPK pathways on human bladder carcinoma cells. Mar. Drugs 2012, 10, 2893–2911. [Google Scholar] [CrossRef]
- Li, H.-H.; Su, J.-S.; Chiu, C.-C.; Lin, J.-J.; Yang, Z.-Y.; Hwang, W.-I.; Chen, Y.-K.; Lo, Y.-H.; Wu, Y.-J. Proteomic investigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of cascapase cascade. Mar. Drugs 2013, 11, 2625–2642. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Su, J.-H.; Tsao, C.-Y.; Hung, C.-T.; Chao, H.-H.; Lin, J.-J.; Liao, M.-H.; Yang, Z.-Y.; Huang, H.H.; Tsai, F.-J.; et al. Sinulariolide induced hepatocellular carcinoma apoptosis through activation of mitochondrial-related apoptotic and PERK/eIF2α/ATF4/CHOP pathway. Molecules 2013, 18, 10146–10161. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Neoh, C.-A.; Tsao, C.-Y.; Su, J.-H.; Li, H.-H. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and P13K/Akt signaling pathways. Int. J. Mol. Sci. 2015, 16, 16469–16482. [Google Scholar] [CrossRef]
- Cheng, T.-C.; Din, Z.-H.; Su, J.-H.; Wu, Y.-J.; Liu, C.-I. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/ AKT/mTOR signalling pathway in human bladder cancer cells. Mar. Drugs 2017, 15, 238. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Wu, Y.-J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Li, Y.-R.; Huang, W.Y.; Su, J.-H.; Chan, H.-L.; Lin, S.-H.; Liu, C.-S.; Lin, S.-C.; Lin, C.-C.; Lin, C.-H. Sinulariolide suppresses LPS-induced phenotypic and functional maturation of dendritic cells. Mol. Med. Rep. 2017, 16, 6992–7000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maida, M.; Carroll, A.R.; Coll, J.C. Variability of terpene content in the soft coral Sinularia flexibilis (Coelenterata: Octocorallia), and its ecological implications. J. Chem. Ecol. 1993, 19, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Aceret, T.L.; Sammarco, P.W.; Coll, J.C. Effects of diterpenes derived from the soft coral Sinularia flexibilis on the eggs, sperm, and embryos of the scleractinian corals Montipora digitata and Acropora tenuis. Mar. Biol. 1995, 122, 317–323. [Google Scholar] [CrossRef]
- Michalek-Wagner, K.; Bowden, B.F. Effects of bleaching on secondary metabolite chemistry of alcyonacean soft corals. J. Chem. Ecol. 2000, 26, 1543–1562. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, C.-H.; Liaw, C.-C.; Chen, Y.-C.; Kuo, Y.-H.; Shen, Y.-C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Su, T.-R.; Tsai, F.-J.; Lin, J.-J.; Huang, H.H.; Chiu, C.-C.; Su, J.-H.; Yang, Y.-T.; Chen, J.Y.-F.; Wong, B.-S.; Wu, Y.-J. Induction of apoptosis by 11-dehydrosinulariolide via mitochondrial dysregulation and ER stress pathways in human melanoma cells. Mar. Drugs 2012, 10, 1883–1898. [Google Scholar] [CrossRef]
- Chen, W.-F.; Chakraborty, C.; Sung, C.-S.; Feng, C.-W.; Jean, Y.-H.; Lin, Y.-Y.; Hung, H.-C.; Huang, T.-Y.; Huang, S.-Y.; Su, T.-M.; et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 265–275. [Google Scholar] [CrossRef]
- Liu, C.-I.; Chen, C.-C.; Chen, J.-C.; Su, J.-H.; Huang, H.H.; Chen, J.Y.-F.; Wu, Y.-J. Proteomic analysis of anti-tumor effects of 11-dehydrosinulariolide on CAL-27 cells. Mar. Drugs 2011, 9, 1254–1272. [Google Scholar] [CrossRef]
- Hu, L.-C.; Yen, W.-H.; Su, J.-H.; Chiang, M.Y.-N.; Wen, Z.-H.; Chen, W.-F.; Lu, T.-J.; Chang, Y.-W.; Chen, Y.-H.; Wang, W.-H.; et al. Cembrane derivatives from the soft corals, Sinularia gaweli and Sinularia flexibilis. Mar. Drugs 2013, 11, 2154–2167. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, N.-F.; Feng, C.-W.; Cheng, S.-Y.; Hung, H.-C.; Tsui, K.-H.; Hsu, C.-H.; Sung, P.-J.; Chen, W.-F.; Wen, Z.-H. A coral-derived compound improves functional recovery after spinal cord injury through its antiapoptotic and anti-inflammatory effects. Mar. Drugs 2016, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Jean, Y.-H.; Lee, H.-P.; Chen, W.-F.; Sun, Y.-M.; Su, J.-H.; Lu, Y.; Huang, S.-Y.; Hung, H.-C.; Sung, P.-J.; et al. A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PLoS ONE 2013, e62926. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Su, J.-H.; Tsai, C.-C.; Chen, Y.-J.; Liao, M.-H.; Wu, Y.-J. 11-epi-Sinulariolide acetate reduces cell migration and invasion of human hepatocellular carcinoma by reducing the activation of ERK1/2, p38MAPK and FAK/PI3K/AKT/mTOR signaling pathways. Mar. Drugs 2014, 12, 4783–4798. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Wang, R.Y.L.; Chen, J.-C.; Chiu, C.-C.; Liao, M.-H.; Wu, Y.-J. Cytotoxicity of 11-epi-sinulariolide acetate isolated from cultured soft corals on HA22T cells through the endoplasmic reticulum stress pathway and mitochondrial dysfunction. Int. J. Mol. Sci. 2016, 17, 1787. [Google Scholar] [CrossRef] [PubMed]
- Sanduja, R.; Sanduja, S.K.; Weinheimer, A.J.; Alam, M.; Martin, G.E. Isolation of the cembranolide diterpenes dihydrosinularin and 11-epi-sinulariolide from the marine mollusk Planaxis sulcatus. J. Nat. Prod. 1986, 49, 718–719. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Irace, C.; Maffettone, C.; Bavestrello, G.; Cerrano, C. Oxygenated cembranoids of the decaryiol type from the Indonesian soft coral Lobophytum sp. Tetrahedron 2009, 65, 2898–2904. [Google Scholar] [CrossRef]
- Grote, D.; Dahse, H.-M.; Seifert, K. Furanocembranoids from the soft corals Sinularia asterolobata and Litophyton arboreum. Chem. Biodiversity. 2008, 5, 2449–2456. [Google Scholar] [CrossRef]
- Kapojos, M.M.; Lee, J.-S.; Oda, T.; Nakazawa, T.; Takahashi, O.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Wewengkang, D.S.; Tsukamoto, S.; et al. Two unprecedented cembrene-type terpenes from an Indonesian soft coral Sarcophyton sp. Tetrahedron 2010, 66, 641–645. [Google Scholar] [CrossRef]
- Fattorusso, E.; Luciano, P.; Putra, M.Y.; Tagliatela-Scafati, O.; Lanaro, A.; Pana, E.; Bavestrello, G.; Cerrano, C. Chloroscabrolides, chlorinated norcembranoids from the Indonesian soft coral Sinularia sp. Tetrahedron 2011, 67, 7983–7988. [Google Scholar] [CrossRef]
- Anta, C.; González, N.; Santafé, G.; Rodríguez, J.; Jiménez, C. New xenia diterpenoids from the Indonesian soft coral Xenia sp. J. Nat. Prod. 2002, 65, 766–768. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Tagliatela-Scafati, O.; Achmad, M.J.; Bavestrello, G.; Cerrano, C. Xenimanadins A–D, a family of xenicane diterpenoids from the Indonesian soft coral Xenia sp. Tetrahedron 2008, 64, 3141–3146. [Google Scholar] [CrossRef]
- Craig, K.S.; Williams, D.E.; Hollander, I.; Frommer, E.; Mallon, R.; Collins, K.; Wojciechowicz, D.; Tahir, A.; van Soest, R.; Andersen, R.J. Novel sesterterpenoid and norsesterterpenoid RCE-protease inhibitors isolated from the marine sponge Hippospongia sp. Tetrahedron Lett. 2002, 43, 4801–4804. [Google Scholar] [CrossRef]
- Elkhayat, E.; Edrada, R.A.; Ebel, R.; Wray, V.; van Soest, R.; Wiryowidagdo, W.; Mohamed, M.H.; Müller, W.E.G.; Proksch, P. New luffarielloide derivatives from the Indonesian sponge Acanthodendrilla sp. J. Nat. Prod. 2004, 67, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Afifi, A.H.; Kagiyama, I.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Sulawesins A–C, furanosesterterpene tetronic acids that inhibit USP7 from a Psammocinia sp. marine sponge. J. Nat. Prod. 2017, 80, 2045–20150. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R. Diacarperoxide S, new norterpene cyclic peroxide from the sponge Diacarnus megaspinorhabdosa. Nat. Prod. Commun. 2012, 7, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Al Haidari, R.A.; Mohamed, G.A. Megaspinoxide A: New norterpene cyclic peroxide from the sponge Diacarnus megaspinorhabdosa. Nat. Prod. J. 2014, 4, 38–42. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Yoshida, W.Y.; Scheuer, P.J.; Lobkovsky, E.; Clardy, J.; Kelly, M. Honulactones: New bishomoscalarane sesterterpenes from the Indonesian sponge Strepsichordaia aliena. J. Org. Chem. 2000, 65, 6837–6840. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.I.; Yoshida, W.Y.; Scheuer, P.J.; Kelly, M. Scalarane-based sesterterpenes from an Indonesian sponge Strepsichordaia aliena. J. Nat. Prod. 2000, 63, 1388–1392. [Google Scholar] [CrossRef]
- Zhang, H.; Crews, P.; Tenney, K.; Valeriote, F.A. Cytotoxic phyllactone analogs from the marine sponge Phyllospongia papyrecea. Med. Chem. (Sharjah, United Arab Emirates). 2017, 13, 295–300. [Google Scholar] [CrossRef]
- Williams, D.E.; Hollander, I.; Feldberg, L.; Frommer, E.; Mallon, R.; Tahir, A.; van Soest, R.; Andersen, R.J. Scalarane-base sesterterpenoid RCE-protease inhibitors isolated from the Indonesian Carteriospongia foliascens. J. Nat. Prod. 2009, 72, 1106–1109. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Mangindaan, R.E.P.; Namikoshi, M. Two new protein tyrosinase phosphatase 1B inhibitors, hyattellactones A and B, from the Indonesian marine sponge Hyattella sp. Bioorg. Med. Chem. Lett. 2015, 25, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.C.; Tanaka, J.; de Voogd, N.J.; Higa, T. New scalarane class sesterterpenes from an Indonesian sponge, Phyllospongia sp. J. Nat. Prod. 2002, 65, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Tahir, A.; Andersen, R.J. A new acyclic diketotriterpenoid isolated from the Indonesian marine sponge Hyrtios erectus. J. Nat. Prod. 1999, 62, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Schüßeler, T. First highly efficient asymmetric synthesis of the Hyrtios erectus diketotriterpenoid. Synthesis 2002, 2280–2288. [Google Scholar] [CrossRef]
- Fouad, M.; Edrada, R.A.; Ebel, R.; Wray, V.; Müller, W.E.G.; Lin, W.H.; Proksch, P. Cytotoxic isomalabaricane triterpenes from the marine sponge Rhabdastrella globostellata. J. Nat. Prod. 2006, 69, 211–218. [Google Scholar] [CrossRef]
- Aoki, S.; Sanagawa, M.; Watanabe, Y.; Setiawan, A.; Arai, M.; Kobayashi, M. Novel isomarabarican triterpenes, exhibiting selective anti-proliferative activity against vascular endothelial cells, from marine sponge Rhabdastrella globostellata. Bioorg. Med. Chem. 2007, 15, 4818–4828. [Google Scholar] [CrossRef]
- Constantino, V.; Sala, G.D.; Mangoni, A.; Perinu, C.; Teta, R. Blurring the boundary between bio- and geohopanoids: Plakohopanoid, a C32 biohopanoid ester from Plakortis cf. lita. Eur. J. Org. Chem. 2012, 2012, 5171–5176. [Google Scholar] [CrossRef]
- Bortolotto, M.; Braekman, J.C.; Daloze, D.; Losman, D.; Tursch, B. Chemical studies of marine invertebrates. XXIII. A novel polyhydroxylated sterol from the soft coral Lithophyton viridis (Coelenterata, Octocorallia, Alycynoacea). Steroids 1976, 28, 461–466. [Google Scholar] [CrossRef]
- Aoki, S.; Naka, Y.; Itoh, T.; Furukawa, T.; Rachmat, R.; Akiyama, S.; Kobayashi, M. Lembehsterols A and B, novel sulfated sterols inhibiting thymidine phosphorylase, from the marine sponge Petrosia strongylata. Chem. Pharm. Bull. 2002, 50, 827–830. [Google Scholar] [CrossRef]
- Bortolotto, M.; Braekman, J.C.; Daloze, D.; Losman, D.; Tursch, B. Chemical studies of marine invertebrates. XXIX. 4α-Methyl-3β, 8β-dihydroxy-5α-ergost-24(28)-en-23-one, a novel polyoxygenated sterol from the softcoral Litophyton viridis (Coelenterata, Octocroallia, Alycyonacea). Steroids 1977, 30, 159–164. [Google Scholar] [CrossRef]
- Angawi, R.F.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Fattorusso, E.; Scala, F.; Taglialatela-Scafati, O. Dehydroconicasterol and aurantoic acid, a chlorinated polyene derivative, from the Indonesian sponge Theonella swinhoei. J. Nat. Prod. 2009, 72, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Barral, M.A.; Rodríguez, J.; Jiménez, C. New cytotoxic steroids from the gorgonian Isis hippuris. Structure-activity studies. Tetrahedron 2001, 57, 3487–3497. [Google Scholar] [CrossRef]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Topsentinols, 24-isopropyl steroids from the marine sponge Topsentia sp. J. Nat. Prod. 2010, 73, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Fattorusso, E.; Taglialatela-Scafati, O.; Bavestrello, G.; Calcinai, B.; Dien, H.A.; Ligresti, A.; Marzo, V.D. Desulfohaplosamate, a new phosphate-containing steroid from Dasychalina sp., is a selective cannabinoid CB 2 receptor ligand. Steroids 2011, 76, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Jurek, J.; Scheuer, P.J.; Kelly-Borges, M. Two steroidal alkaloids from a sponge, Corticium sp. J. Nat. Prod. 1994, 57, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, M.; Braekman, J.C.; Daloze, D.; Tursch, B. Chemical studies of marine invertebrates. XVIII. Four novel polyhydroxylated steroids from Sinularia dissecta. Bull. Soc. Chim. Belg. 1976, 85, 27–34. [Google Scholar] [CrossRef]
- Ushiyama, S.; Umaoka, H.; Kato, H.; Suwa, Y.; Morioka, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the ubc13-uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012, 75, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Putra, M.Y.; Bavestrello, G.; Cerrano, C.; Renga, B.; D’Amore, C.; Fiorucci, S.; Fattorusso, E.; Taglialatela-Scafati, O. Polyhydroxylated sterols from the Indonesian soft coral Sinularia sp. and their effect on farnesoid X-activated receptor. Steroids 2012, 77, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.A.; Christie, E.M.; Jaspars, M.; van Ofwegen, L.P. A bioactive secosterol with an unusual A- and B-ring oxygenation pattern isolated from an Indonesian soft coral Lobophytum sp. J. Nat. Prod. 1998, 61, 538–541. [Google Scholar] [CrossRef]
- Anta, C.; González, N.; Rodríguez, J.; Jiménez, C. A new secosterol from the Indonesian octocoral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, J.-S.; Nakazawa, T.; Ukai, K.; Mangindaan, R.E.P.; Wewengkang, D.; Rotinsulu, H.; Kobayashi, H.; Tsukamoto, S.; Namikoshi, M. (25S)-Cholesten-26-oic acid derivatives from an Indonesian soft coral Minabea sp. Steroids 2009, 74, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, W.A.; Cabral, C.; Kelly, M.; Scheuer, P.J. A novel D-ring unsaturated A-nor sterol from the Indonesian sponge, Axinella carteri Dendy. Nat. Prod. Res. 2008, 22, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Flyer, A.N.; Si, C.; Myers, A.G. Synthesis of cortistatins A, J, K, and L. Nat. Chem. 2010, 2, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, I.; Tursch, B.M.; Djerassi, C. Terpenoids. LXVIII. 23ξ-acetoxy-17-deoxy-7,8-dihydroholothurinogenin, a new triterpenoid sapogenin from a sea cucumber. J. Org. Chem. 1973, 38, 209–214. [Google Scholar] [CrossRef]
- Dai, H.-F.; Edrada, R.A.; Ebel, R.; Nimtz, M.; Wray, V.; Proksch, P. Norlanostane triterpenoidal saponins from the marine sponge Melophlus sarassinorum. J. Nat. Prod. 2005, 68, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeon, J.-e.; Lee, Y.-J.; Lee, H.-S.; Sim, C.J.; Oh, K.-B. Nortriterpene gylcosides of the sarasinoside class from the sponge Lipastrotethya sp. J. Nat. Prod. 2012, 75, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Maarisit, W.; Yamazaki, H.; Kanno, S.-I.; Tomizawa, A.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Ukai, K.; Kapojos, M.M.; Namikoshi, M. A tetramic acid derivative with protein tyrosinase phospahatase 1B inhibitory activity and a new nortriterpene glycoside from the Indonesian marine sponge Petrosia sp. Bioorg. Med. Chem. Lett. 2017, 27, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Piña, I.C.; Sanders, M.L.; Crews, P. Puupehenone congeners from an Indo-Pacific Hyrtios sponge. J. Nat. Prod. 2003, 66, 2–6. [Google Scholar] [CrossRef]
- Wahab, H.A.; Pham, N.B.; Muhammad, T.S.T.; Hooper, J.N.A.; Quinn, R.J. Merosesquiterpene congeners from the Australian sponge Hyrtios digitatus as potential drug leads for atherosclerosis disease. Mar. Drugs 2017, 15, 6. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nakayama, W.; Takahashi, O.; Kirikoshi, R.; Izumikawa, Y.; Iwasaki, K.; Toraiwa, K.; Ukai, K.; Rotinsulu, H.; Wewengkang, D.S.; et al. Verruculides A and B, two new protein tyrosine phosphatase 1B inhibitors from an Indonesian ascidian-derived Penicillium verruculosum. Bioorg. Med. Chem. Lett. 2015, 25, 3087–3090. [Google Scholar] [CrossRef]
- Xu, J.; Takasaki, A.; Kobayashi, H.; Oda, T.; Yamada, J.; Mangindaan, R.E.P.; Ukai, K.; Nagai, H.; Namikoshi, M. Four new macrocyclic trichothecenes from two strains of marine-derived fungi of the genus Myrothecium. J. Antibiot. 2006, 59, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ebel, R.; Rusman, Y.; Brauers, G.; Proksch, P.; Frank, W.; Wray, V. Novel oxygenated meroterpenoids from the sponge-derived fungus Penicillium citreonigrum. Planta Med. 2006, 72, 961–1089. [Google Scholar] [CrossRef]
- Rusman, Y. Isolation of New Secondary Metabolites from Sponge-Associated and Plant-Derived Endophytic Fungi. Ph.D. Thesis, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany, 2006. [Google Scholar]
- Issa, H.H.; Tanaka, J.; Rachmat, R.; Higa, T. Floresolides, new metacyclophane hydroquinone lactones froman ascidian, Aplidium sp. Tetrahedron Lett. 2003, 44, 1243–1245. [Google Scholar] [CrossRef]
- Nicolau, K.C.; Xu, H. Total synthesis of floresolide B and Δ6,7-Z-floresolide B. Chem. Commun. 2006, 6, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; de Voogd, N.; Kalscheuer, R.; Müller, W.E.G.; Chaidir; Proksch, P. Cytotoxic drimane meroterpenoids from the Indonesian marine sponge Dactylospongia elegans. Phytochem. Lett. 2017, 22, 154–158. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Rachmat, R.; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem. Pharm. Bull. 2004, 52, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; de Voogd, N.J.; Muller, W.E.G.; Wray, V.; Lin, W.; Feger, D.; Kubbutat, M.; Aly, A.H.; Proksch. Cytotoxic and protein kinase inhibiting nakijiquinones and nakijiquinols from the sponge Dactylospongia metachromia. J. Nat. Prod. 2014, 77, 218–226. [Google Scholar] [CrossRef]
- Suna, H.; Arai, M.; Tsubotani, Y.; Hayashi, A.; Setiawan, A.; Kobayashi, M. Dysideamine, a new sesquiterpene aminoquinone, protects hippocampal neuronal cells against iodoacetic acid-induced cell death. Bioorg. Med. Chem. 2009, 17, 3968–3972. [Google Scholar] [CrossRef]
- Williams, D.E.; Telliez, J.-B.; Liu, J.; Tahir, A.; van Soest, R.; Andersen, R.J. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the Indonesian marine sponge Acanthodendrilla sp. J. Nat. Prod. 2004, 67, 2127–2129. [Google Scholar] [CrossRef]
- Basabe, P.; Martín, M.; Bodero, O.; Blanco, A.; Marcos, I.S.; Díez, D.; Urones, J.G. Synthesis of (+)-makassaric acid, a protein kinase MK2 inhibitor. Tetrahedron 2010, 66, 6008–6012. [Google Scholar] [CrossRef]
- Trianto, A.; Hermawan, I.; de Voogd, N.J.; Tanaka, J. Halioxepine, a new meroditerpene from an Indonesian sponge Haliclona sp. Chem. Pharm. Bull. 2011, 59, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Harrison, B. New triterpene-ketides (merotriterpenes), haliclotriols A and B, from an Indo-Pacific Haliclona sp. sponge. Tetrahedron 2000, 56, 9039–9046. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Kelly, M.; Kara, U.A.K.; Ang, K.K.H.; Katsuyama, I.; Dunbar, D.C.; Khan, A.A.; Hamann, M.T. New manzamine alkaloids with potent activity against infectious diseases. J. Am. Chem. Soc. 2001, 123, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; El Sayed, K.; Rao, K.V.; Lim, C.W.; Hu, J.-F.; Kelly, M.; Franzblau, S.G.; Zhang, F.; Peraud, O.; Hill, R.T.; et al. 12,34-Oxamanzamines, novel biocatalytic and natural products from manzamine producing Indo-Pacific sponges. Tetrahedron 2002, 58, 7397–7402. [Google Scholar] [CrossRef]
- Ang, K.K.H.; Holmes, M.J.; Higa, T.; Hamann, M.T.; Kara, U.A.K. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob. Agents Chemother. 2000, 44, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R.; et al. Marine natural products as prototype agrochemical agents. J. Agric. Food. Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef]
- Rao, K.V.; Donia, M.S.; Peng, J.; Garcia-Palomero, E.; Alonso, D.; Martinez, A.; Medina, M.; Franzblau, S.G.; Tekwani, B.L.; Khan, S.I.; et al. Manzamine B and E and ircinal A related alkaloids from an Indonesian Acanthostrongylophora sponge and their activity againstinfectious, tropical parasitic, and Alzheimer’s diseases. J. Nat. Prod. 2006, 69, 1034–1040. [Google Scholar] [CrossRef]
- Kim, C.-K.; Riswanto, R.; Won, T.-H.; Kim, H.; Elya, B.; Sim, C.J.; Oh, D.-C.; Oh, K.-B.; Shin, J. Manzamine alkaloids from an Acanthostrongylophora sp. sponge. J. Nat. Prod. 2017, 80, 1575–1583. [Google Scholar] [CrossRef]
- Rao, K.V.; Santarsiero, B.D.; Mesecar, A.D.; Schinazi, R.F.; Tekwani, B.L.; Hamann, M.T. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J. Nat. Prod. 2003, 66, 823–828. [Google Scholar] [CrossRef]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.S.; Hamann, M.T. New manzamine alkaloids from an Indo-Pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory disease. J. Med. Chem. 2004, 47, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.E.; Fromentin, Y.; Zou, Y.; Hamann, M.T. Acantholactone, a new manzamine related alkaloid with an unprecedented δ-lactone and ε-lactam ring system. Tetrahedron Lett. 2012, 53, 6329–6331. [Google Scholar] [CrossRef] [PubMed]
- El-desoky, A.; Kato, H.; Eguchi, K.; Kawabata, T.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Takeya, M.; Yokosawa, H.; et al. Acantholactam and pre-neo-kauluamine, manzamine-related alkaloids from the Indonesia Acanthostrongylophora ingens. J. Nat. Prod. 2014, 77, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Furusato, A.; Kato, H.; Nehira, T.; Eguchi, K.; Kawabata, T.; Fujiawara, Y.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Takeya, M.; et al. Acanthomanzamines A−E with new manzamine frameworks from the marine sponge Acanthostrongylophora ingens. Org. Lett. 2014, 16, 3888–3891. [Google Scholar] [CrossRef] [PubMed]
- AlTarabeen, M.; Daletos, G.; Ebrahim, W.; Müller, W.G.; Hartmann, R.; Lin, W.; Proksch, P. Ircinal E, a new manzamine derivative from the Indonesian marine sponge Acanthostrongylophora ingens. Nat. Prod. Commun. 2015, 10, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.I.; Ichiba, T.; Isobe, M.; Kelly-Borges, M.; Scheuer, P.J. Kauluamine: An unprecedented manzamine dimer from an Indonesian marine sponge, Prianos sp. J. Am. Chem. Soc. 1995, 117, 10743–10744. [Google Scholar] [CrossRef]
- Jaspars, M.; Pasupathy, V.; Crews, P. A tetracyclic diamine alkaloid, halicyclamine A, from the marine sponge Haliclona sp. J. Org. Chem. 1994, 59, 3253–3255. [Google Scholar] [CrossRef]
- Matsunaga, S.; Miyata, Y.; van Soest, R.W.M.; Fusetani, N. Tetrahydrohalicylamine A and 22-hydrocylamine A, new cytotoxic bis-piperidine alkaloids from a marine sponge Amphimedon sp. J. Nat. Prod. 2004, 67, 1758–1760. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Sobou, M.; Vilchéze, C.; Baughn, A.; Hashizume, H.; Prusakorn, P.; Ishida, S.; Matsumoto, M.; Jacobs, W.R., Jr.; Kobayashi, M. Halicylcamine A, a marine spongean alkaloid as a lead for anti-tubercolosis agent. Bioorg. Med. Chem. 2008, 16, 6732–6736. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Liu, L.; Fujimoto, T.; Setiawan, A.; Kobayashi, M. DedA protein relates to action-mechanism of halicylamine A, a marine spongean macrocyclic alkaloid, as an anti-dorman mycobacterial substance. Mar. Drugs 2011, 9, 984–993. [Google Scholar] [CrossRef]
- Arai, M.; Ishida, S.; Setiawan, A.; Kobayashi, M. Haliclonacyclamines, tetracyclic alkylpiperidine alkaloids, as anti-dormant Mycobacterial substances from a marine sponge of Haliclona sp. Chem. Pharm. Bull. 2009, 57, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; Katavic, P.L.; Lambert, L.K.; Hayes, P.Y.; Banwell, M.G.; Munro, M.H.G.; Bernhardt, P.V.; Garson, M.J. Structure and absolute configuration of 3-alkylpiperidine alkaloids from an Indonesian sponge of the genus Halichondria. Tetrahedron 2010, 66, 2752–2760. [Google Scholar] [CrossRef]
- Dewi, A.S.; Hadi, T.A.; Fajarningsih, N.D.; Blanchfield, J.T.; Bernhardt, P.V.; Garson, M.J. Acanthocyclamine A from the Indonesian marine sponge Acanthostrongylophora ingens. Aust. J. Chem. 2014, 67, 1205–1210. [Google Scholar] [CrossRef]
- Esposito, G.; Bourguet-Kondracki, M.-L.; Mai, L.H.; Longeon, A.; Teta, R.; Meijer, L.; van Soest, R.; Mangoni, A.; Constantino, V. Chloromethylhalicyclamine B, a marine-derived protein kinase CK1δ/ε Inhibitor. J. Nat. Prod. 2016, 79, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.I.; Goetz, G.; Mau, C.M.S.; Yoshida, W.Y.; Scheuer, P.J.; Williamson, R.T.; Kelly, M. ‘Upenamide: An unprecedented macrocyclic alkaloid from the Indonesian sponge Echinochalina sp. J. Org. Chem. 2000, 65, 8465–8469. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct imine acylation: Synthesis of the proposed structures of upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Achmad, M.J.; Bavestrello, G.; Cerrano, C. Lobozoanthamine, a new zoanthamine-type alkaloid from the Indonesian soft coral Lobophytum sp. Tetrahedron Lett. 2008, 49, 2189–2192. [Google Scholar] [CrossRef]
- Issa, H.H.; Tanaka, J.; Rachmat, R.; Setiawan, A.; Trianto, A.; Higa, T. Polycitorols A and B, new tricyclic alkaloids from an ascidian. Mar. Drugs 2005, 3, 78–83. [Google Scholar] [CrossRef]
- Lin, W.H.; Fu, H.Z.; Li, J.; Proksch, P. Novel chromone derivatives from marine fungus Aspergillus versicolor isolated from the sponge Xestospongia exigua. Chin. Chem. Lett. 2001, 12, 235–238. [Google Scholar]
- Lin, W.; Brauers, G.; Ebel, R.; Wray, V.; Berg, A.; Sudarsono; Proksch, P. Novel chromone derivatives from the fungus Aspergillus versicolor isolated from the marine sponge Xestospongia exigua. J. Nat. Prod. 2003, 66, 57–61. [Google Scholar] [CrossRef]
- Sperry, S.; Crews, P. A novel alkaloid from the Indo-Pacific sponge Clathria basilana. Tetrahedron Lett. 1996, 37, 2389–2390. [Google Scholar] [CrossRef]
- Arai, M.; Kamiya, K.; Shin, D.; Matsumoto, H.; Hisa, T.; Setiawan, A.; Kotoku, N.; Kobayashi, M. N-Methylniphatyne A, a new 3-alkylpyridine alkaloid as an inhibitor of the cancer cells adapted to nutrient starvation, from an Indonesian marine sponge of Xestospongia sp. Chem. Pharm. Bull. 2016, 64, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Loveridge, S.T.; Tenney, K.; Crews, P. A new 3-alkylpyridine alkaloid from the marine sponge Haliclona sp. and its cytotoxic activity. Nat. Prod. Res. 2016, 30, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Maarisit, W.; Abdjul, D.; Yamazaki, H.; Kato, H.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Kapojos, M.M.; Ukai, K.; Namikoshi, M. Anti-mycobacterial alkaloids, cyclic 3-alkyl pyridinium dimers, fron the Indonesian marine sponge Haliclona sp. Bioorg. Med. Chem. Lett. 2017, 27, 3503–3506. [Google Scholar] [CrossRef] [PubMed]
- Shaker, K.H.; Göhl, M.; Müller, T.; Seifert, K. Indole alkaloids from the sea anemone Heteractis aurora and homarine from Octopus syanea. Chem. Biodiversity. 2015, 12, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Wewengkang, D.; Nishikawa, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Namikoshi, M. Two new tryptamine derivatives, leptoclinidamide and (–)-leptoclinidamine B, from an Indonesian ascidian Leptoclinides dubius. Mar. Drugs 2012, 10, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Salmoun, M.; Devijer, C.; Daloze, D.; Braekman, J.-C.; van Soest, R.W.M. 5-Hydroxytryptamine-derived alkaloids from two marine sponges of the genus Hyrtios. J. Nat. Prod. 2002, 65, 1173–1176. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Wan, S.B.; Ren, S.M.; Jiang, T. First total synthesis of marine alkaloid hyrtiosulwesine. Chin. Chem. Lett. 2010, 21, 1307–1309. [Google Scholar] [CrossRef]
- Sauleau, P.; Martin, M.-T.; Dau, M.-E.T.H.; Youssef, D.T.A.; Bourguet-Kondracki, M.-L. Hyrtiazepine, an azepino-indole-type alkaloid from the red sea marine sponge Hyrtios erectus. J. Nat. Prod. 2006, 69, 1676–1679. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Zayed, M.F.; Sayed, H.M. Ingenines A and B, two new alkaloids from the sponge Acanthostrongylophora ingens. Drug Res. 2015, 65, 361–365. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenines C and D, new cytotoxic pyrimidine-β-carboline alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Phytochem. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenine E, a new cytotoxic β-carboline alkaloid from the Indonesian sponge Acanthostrongylophora ingens. J. Asian. Nat. Prod. Res. 2017, 19, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Ebel, R.A.; Ebel, R.; Proksch, P. Achantomine A, a new pyrimidine-β-carboline from the sponge Acanthostrongylophora ingens. Nat. Prod. Commun. 2008, 3, 175–178. [Google Scholar]
- Sakai, E.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Variabines A and B: New β-carboline alkaloids from the marine sponge Luffariella variabilis. J. Nat. Med. 2014, 68, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Jompa, J.; Tahir, A.; Ireland, C.M. Trypargine alkaloids from a previously undescribed Eudistoma Ascidian. J. Nat. Prod. 1999, 62, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Yamanokuchi, R.; Imada, K.; Miyazaki, M.; Kato, H.; Watanabe, T.; Fujimuro, M.; Saeki, Y.; Yoshinaga, S.; Terasawa, H.; Iwasaki, N.; et al. Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg. Med. Chem. 2012, 20, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Segraves, N.L.; Lopez, S.; Johnson, T.A.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz; Valeriote, F.A.; Crews, P. Structures and cytotoxicities of fascaplysin and related alkaloids from two marine phyla—Fascaplysinopsis sponges and Didemnum tunicates. Tetrahedron Lett. 2003, 44, 3471–3475. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Baranova, O.V.; Balaneva, N.N.; Fedorov, S.N.; Radchenko, O.S.; Dubovitskii, S.V. The first syntheses of 3-bromofascaplysin-marine alkaloids from Fascaplysinopsis reticulata and Didemnum sp. by application of a simple and effective approach to the pyrido [1,2-a:3,4-b’]diindole system. Tetrahedron Lett. 2007, 48, 7998–8000. [Google Scholar] [CrossRef]
- Kuzmich, A.S.; Fedorov, S.N.; Shastina, V.V.; Shubina, L.K.; Radchenko, O.S.; Balaneva, N.N.; Zhidkov, M.; Park, J.-I.; Kwak, J.Y.; Stonik, V.A. The anticancer activity of 3- and 10-bromofascaplysins is mediated by caspase -8, -9, -3-dependent apoptosis. Bioorg. Med. Chem. 2010, 18, 3834–3840. [Google Scholar] [CrossRef]
- Segraves, N.L.; Robinson, S.J.; Garcia, D.; Said, S.A.; Fu, X.; Schmitz, F.J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Comparison of fascaplysin and related alkaloids: A study of structures, cytotoxicities, and sources. J. Nat. Prod. 2004, 67, 783–792. [Google Scholar] [CrossRef]
- Bry, D.; Banaigs, B.; Long, C.; Bontemps, N. New pyridoacridine alkaloids from the purple morphof the ascidian Cystodytes dellechiajei. Tetrahedron Lett. 2011, 52, 3041–3044. [Google Scholar] [CrossRef]
- Thale, Z.; Johnson, T.; Tenney, K.; Wenzel, P.J.; Lobkovsky, E.; Clardy, J.; Media, J.; Pietraszkiewics, H.; Valeriote, F.A.; Crews, P. Structures and cytotoxic properties of sponge-derived bisannulated acridines. J. Org. Chem. 2002, 67, 9384–9391. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Wei, Ho.; Matsui, K.; Rachmat, R.; Kobayashi, M. Pyridoacridine alkaloids inducing neuronal differentiation in a neuroblastoma cell line, from marine sponge Biemna fortis. Bioorg. Med. Chem. 2003, 11, 1969–1973. [Google Scholar] [CrossRef]
- Moran, D.A.P.; Takada, K.; Ise, Y.; Bontemps, N.; Davis, R.A.; Furihata, K.; Okada, S.; Matsunaga, S. Two cell differentiation inducing pyridoacridines from a marine sponge Biemna sp. and their chemical conversions. Tetrahedron 2015, 71, 5013–5018. [Google Scholar] [CrossRef]
- Barnes, E.C.; Said, N.A.B.M.; Williams, E.D.; Hooper, J.N.A.; Davis, R.A. Ecionines A and B, two new cytotoxic pyridoacridine alkaloids from the Australian marine sponge, Ecionemia geodides. Tetrahedron 2010, 66, 283–287. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Elkhayat, E.S.; Fouad, M.; Proksch, P. Sagitol C, a new cytotoxic pyridoacridine alkaloid from the sponge Oceanapia sp. Bull. Fac. Pharm. (Cairo Univ.) 2013, 51, 229–232. [Google Scholar] [CrossRef]
- Smith, C.J.; Venables, D.A.; Hopmann, C.; Salomon, C.E.; Jompa, J.; Tahir, A.; Faulkner, D.J.; Ireland, C.M. Plakinidine D, a new pyrroloacridine alkaloid from two ascidians of the genus Didemnum. J. Nat. Prod. 1997, 60, 1048–1050. [Google Scholar] [CrossRef]
- Calcul, L.; Longeon, A.; Al Mourabit, A.; Guyot, M.; Bourguet-Kondracki, M.-L. Novel alkaloids of the aaptamine class from an Indonesian marine sponge of the genus Xestospongia. Tetrahedron 2003, 59, 6539–6544. [Google Scholar] [CrossRef]
- Pham, C.-D.; Hartmann, R.; Müller, W.E.G.; de Voogd, N.; Lai, D.; Proksch, P. Aaptamine derivatives from the Indonesian sponge Aaptos suberitoides. J. Nat. Prod. 2013, 76, 103–106. [Google Scholar] [CrossRef]
- Arai, M.; Han, C.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Aaptamines, marine spongean alkaloids, as anti-dormant mycobacterial substances. J. Nat. Med. 2014, 68, 372–376. [Google Scholar] [CrossRef]
- Herlt, A.; Mander, L.; Rombang, W.; Rumampuk, R.; Soemitro, S.; Steglich, W.; Tarigan, P.; von Nussbaum, F. Alkaloids from marine organisms. Part 8: Isolation of bisdemethylaaptamine and bisdemethylaaptamine-9-O-sulfate from an Indonesian Aaptos sp. marine sponge. Tetrahedron 2004, 60, 6101–6104. [Google Scholar] [CrossRef]
- Larghi, E.L.; Bohn, M.L.; Kaufman, T.S. Aaptamine and related products. Their isolation, chemical syntheses, and biological activity. Tetrahedron 2009, 65, 4257–4282. [Google Scholar] [CrossRef]
- Kudo, Y.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Aaptoline A, a new quinoline alklaoid from the marine sponge Aaptos suberitoides. Heterocycles 2014, 88, 591–594. [Google Scholar] [CrossRef]
- Park, S.K.; Jurek, J.; Carney, J.R.; Scheuer, P.J. Two more bastadins, 16 and 17, from an Indonesian sponge Ianthella basta. J. Nat. Prod. 1994, 57, 407–410. [Google Scholar] [CrossRef]
- Couladourus, E.A.; Pitsinos, E.N.; Moutsos, V.I.; Sarakinos, G. A general method for the synthesis of bastarenes and isobastarenes: First total synthesis of bastadins 5, 10, 12, 16, 20, and 21. Chem. Eur. J. 2005, 11, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Wauthoz, N.; Lefranc, F.; Niemann, H.; Amighi, K.; Kiss, R.; Proksch, P. Cyclic versus hemi-bastadins. Pleiotropic anti-cancer effects: From apoptosis to anti-angiogenic and anti-migratory effects. Molecules 2013, 18, 3543–3561. [Google Scholar] [CrossRef]
- Ortlepp, S.; Sjögren, M.; Dahlström, M.; Weber, H.; Ebel, R.; Edrada, R.; Thoms, C.; Schupp, P.; Bohlin, L.; Proksch, P. Antifouling activity of bromotyrosine-derived sponge metabolites and synthetic analogues. Mar. Biotechnol. 2007, 9, 776–785. [Google Scholar] [CrossRef]
- Reddy, A.V.; Ravinder, K.; Narasimhulu, M.; Sridevi, A.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New anticancer bastadin alkaloids from the sponge Dendrilla cactos. Bioorg. Med. Chem. 2006, 14, 4452–4457. [Google Scholar] [CrossRef]
- Niemann, H.; Lin, W.; Müller, W.E.G.; Kubbutat, M.; Lai, D.; Proksch, P. Trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar] [CrossRef]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef]
- Dai, J.; Parrish, S.M.; Yoshida, W.Y.; Yip, M.L.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504. [Google Scholar] [CrossRef] [PubMed]
- McCauley, E.P.; Lam, H.; Lorig-Roach, N.; Luu, J.; Lloyd, C.; Tenney, K.; Pietraszkiewicz, H.; Diaz, C.; Valeriote, F.; Auerbuch, V.; et al. Investigation of the physical and bioactive properties of bromo-and iodo-containing sponge-derived compounds possessing an oxyphenylethanime core. J. Nat. Prod. 2017, 80, 3225–3266. [Google Scholar] [CrossRef] [PubMed]
- Supriyono, A.; Schwarz, B.; Wray, V.; Witte, L.; Müller, W.E.G.; van Soest, R.; Sumaryono, W.; Proksch, P. Bioactive alkaloids from the tropical marine sponge Axinella carteri. Z. Naturforsch. 1995, 50c, 669–674. [Google Scholar] [CrossRef]
- Hamed, A.N.E.; Schmitz, R.; Bergermann, A.; Totzke, F.; Kubbutat, M.; Müller, W.E.G.; Youssef, D.T.A.; Bishr, M.M.; Kamel, M.S.; Edrada-Ebel, R.A.; et al. Bioactive pyrrole alkaloids isolated from the Red sea: Marine sponge Stylissa carteri. Z. Naturforsch. C J. Biosci. 2018, 73, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Proksch, P.; Wray, V.; Steube, K.l.; Bringmann, G.; van Soest, R.W.M.; Sudarsono; Ferdinandus, E.; Pattisina, L.A.; Wiryowidagdo, S.; et al. New alkaloids from the Indopacific sponge Stylissa carteri. J. Nat. Prod. 1999, 62, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.; Debbab, A.; Wray, V.; Müller, W.E.G.; Proksch, P. New bioactive alkaloids from the marine sponge Stylissa sp. Tetrahedron 2012, 68, 10176–10179. [Google Scholar] [CrossRef]
- Abjul, D.B.; Yamazaki, H.; Kanno, S.; Tomizama, A.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Ukai, K.; Kapojos, M.M.; Namikoshi, M. An anti-mycobacterial bisfunctionalized spingolipid and new bromopyrrole alkaloid from the Indonesian marine sponge Agelas sp. J. Nat. Med. 2017, 71, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Carlile, G.W.; Keyzers, R.A.; Teske, K.A.; Robert, R.; Williams, D.E.; Linington, R.G.; Gray, C.A.; Centko, R.M.; Yan, L.; Anjos, S.; et al. Correction of F508del-CFTR trafficking by the sponge alkaloid latonduine is modulated by interaction with PARP. Chem. Biol. 2012, 19, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Linh, M.H.; Longeon, A.; de Voogd, N.J.; Durieu, E.; Meijer, L.; Bourguet-Kondracki, M.-L.; Singab, A.N.B.; Müller, W.E.G.; Proksch, P. Dispacamide E and other bioactive bromopyrrole alkaloids from two Indonesian marine sponges of the genus Stylissa. Nat. Prod. Res. 2015, 29, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Edrada-Ebel, R.; de Voogd, N.J.; Wray, V.; Proksch, P. Dibromopyrrole alkaloids from the marine sponge Acanthostylotella sp. Nat. Prod. Commun. 2009, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, Y.; Kato, H.; Rotinsulu, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Spironaamidine, a new spiroquinone-containing alkaloid from the marine sponge Leucetta microraphis. Tetrahedron Lett. 2011, 52, 5342–5344. [Google Scholar] [CrossRef]
- Hassan, W.; Edrada, R.A.; Ebel, R.; Wray, V.; Berg, A.; van Soest, R.; Wiryowidagdo, S.; Proksch, P. New imidazole alkaloids from the Indonesian sponge Leucetta chagosensis. J. Nat. Prod. 2004, 67, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Koswatta, P.B.; Lovely, C.J. Concise total synthesis of naamine G and naamidine H. Chem. Commun. 2010, 46, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Koswatta, P.B.; Jones, J.D.; Yousufuddin, M.; Lovely, C.J. Total syntheses of kealiinines A–C. Org. Lett. 2012, 14, 6210–6213. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.B.; Gligorich, K.M.; Welm, B.E.; Looper, R.E. Synthesis of the reported structures for kealiinines B and C. Org. Lett. 2012, 14, 4734–4737. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.P.; van Soest, R.W.M.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, cytotoxic imidazole alkaloids from the Indonesian marine sponge Leucetta chaogosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Oda, T.; Fujita, A.; Mangindaan, R.E.P.; Nakazawa, T.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Three new sulfur-containing alkaloids, polycarpaurines A, B, and C, from an Indonesian ascidian Polycarpa aurata. Tetrahedron 2007, 63, 409–412. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Z.; Li, G.; Liu, Y.; Xie, Y.; Wang, Q. First discovery of polycarpine, polycarpaurines A and C, and their derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food. Chem. 2016, 64, 4264–4272. [Google Scholar] [CrossRef]
- Liu, H.; Fujiawara, T.; Nishikawa, T.; Mishima, Y.; Nagai, H.; Shida, T.; Tachibana, K.; Kobayashi, H.; Mangindaan, R.E.P.; Namikoshi, M. Lissoclibadins 1–3, three new polysulfur alkaloids, from the ascidian Lissoclinum cf. badium. Tetrahedron 2005, 61, 8611–8615. [Google Scholar] [CrossRef]
- Oda, T.; Kamoshita, K.; Maruyama, S.; Masuda, K.; Nishimoto, M.; Xu, J.; Ukai, K.; Mangindaan, R.E.P.; Namikoshi, M. Cytotoxicity of lissoclibadins and lissoclinotoxins, isolated from a tropical ascidian Lissoclinum cf. badium, against human soild-tumor-derived cell lines. Bio. Pharm. Bull. 2007, 30, 385–387. [Google Scholar] [CrossRef]
- Tatsuta, T.; Hosono, M.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Namikoshi, M.; Yamazaki, H. Lissoclibadin 1, a polysulfur aromatic alkaloid from the Indonesian ascidian Lissoclinum cf. badium, induces caspase-dependent apoptosis in human colon cancer cells and suppresses tumor growth in nude mice. J. Nat. Prod. 2017, 80, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Fujiawara, T.; Liu, H.; Ukai, K.; Mangindaan, R.E.P.; Mochizuki, M.; Namikoshi, M. Effect of lissoclibadins and lissoclinotoxins, isolated from a tropical ascidian Lissoclinum cf. badium, on IL-8 production in a PMA-stimulated promyelocytic leukemia cell line. Mar. Drugs 2006, 4, 15–21. [Google Scholar] [CrossRef]
- Nakazawa, T.; Xu, J.; Nishikawa, T.; Oda, T.; Fujita, A.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Kobayashi, H.; Namikoshi, M. Lissoclibadins 4–7, polysulfur aromatic alkaloids from the indonesian Ascidian Lissoclinum cf. badium. J. Nat. Prod. 2007, 70, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Takahashi, O.; Oda, T.; Nakazawa, T.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Wewengkang, D.S.; Kobayashi, H.; Tsukamoto, S.; et al. Lissoclibadins 8–14, polysulfur dopamine-derived alkaloids from the colonial ascidian Lissoclinum cf. badium. Tetrahedron 2009, 65, 9598–9603. [Google Scholar] [CrossRef]
- González, N.; Rodríguez, J.; Jiménez, C. Didemniserinolipids A–C, unprecedented serinolipids from the tunicate Didemnum sp. J. Org. Chem. 1999, 64, 5705–5707. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, H.; Dixon, D.J.; Luscombe, C.K.; Hettsedt, S.; Ley, S.V. Synthesis, structure revision, and absolute configuration of (+)-didemniserinolipid B, a serinol marine natural product from a tunicate Didemnum sp. Org. Lett. 2002, 4, 3223–3226. [Google Scholar] [CrossRef]
- Ren, J.; Tong, R. Asymmetric total synthesis of (+)-didemniserinolipid B via Achmatowicz rearrangement/ bicycloketalization. J. Org. Chem. 2014, 79, 6987–6995. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kato, H.; Nishikawa, T.; Iwasaki, N.; Suwa, Y.; Rotinsulu, H.; Losung, F.; Maarisit, W.; Mangindaan, R.E.P.; Morioka, H.; et al. Siladenoserinols A–L: New sulfonated serinol derivatives from a tunicate as inhibitors of p53-Hdm2 interaction. Org. Lett. 2013, 15, 322–325. [Google Scholar] [CrossRef]
- Yoshida, M.; Saito, K.; Kato, H.; Tsukamoto, S.; Doi, T. Total synthesis and biological evaluation of siladenoserinol A and its analogue. Angew. Chem. Int. Ed. 2018, 57, 5147–5150. [Google Scholar] [CrossRef]
- Carney, J.R.; Scheuer, P.J.; Kelly-Borges, M. Makaluvamine G, a cytotoxic pigment from an Indonesian sponge Histodermella sp. Tetrahedron 1993, 49, 8483–8486. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Makarieva, T.; Utkina, N.; Santalova, E.; Kryukova, E.; Methfessel, C.; Tsetlin, V.; Stonik, V.; Kasheverov, I. Marine natural products acting on the acetylcholine-protein and nicotinic receptors: From computer modelling to binding studies and electrophysiology. Mar. Drugs 2014, 12, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, M.M.; Chaikina, E.L.; Utkina, N.K. Alkaloids from marine sponges as stimulators of initial stages of development of agricultural plants. Nat. Prod. Commun. 2014, 9, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; El-Khayat, E.S.; Gouda, Y.G.; Proksch, P. Strepsiamides A–C, new ceramides from the marine sponge Strepsichordaia lendenfeldi. Nat. Prod. Commun. 2008, 3, 205–209. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Fouad, M.A.; El-Khayat, E.S.; Proksch, P. Iotrochotamides I and II: New ceramides from the Indonesian sponge Iotrochota purpurea. Nat. Prod. Res. 2009, 23, 86–92. [Google Scholar] [CrossRef]
- Aoki, S.; Higuchi, K.; Ye, Y.; Satari, R.; Kobayashi, M. Melophlins A and B, novel tetramic acids reversing the phenotype of ras-transformed cells, from the marine sponge, Melophlus sarassinorum. Tetrahedron 2000, 56, 1833–1836. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, B.-G.; Wiryowidagdo, S.; Wray, V.; van Soest, R.; Steube, K.G.; Guan, H.-S.; Proksch, P.; Ebel, R. Melophlins C–O, thirteen novel tetramic acids from the marine sponge Melophlus sarassinorum. J. Nat. Prod. 2003, 66, 51–56. [Google Scholar] [CrossRef]
- Ichiba, T.; Nakao, Y.; Scheuer, P.J. Kumusine, a chloroadenine riboside from a sponge, Theonella sp. Tetrahedron Lett. 1995, 36, 3977–3980. [Google Scholar] [CrossRef]
- Searle, P.A.; Molinski, T.F. Trachycladines A and B: 2’-C-Methyl-5’-deoxyribofuranosyl nucleosides from the marine sponge Trachycladus laevispirulifer. J. Org. Chem. 1995, 60, 4296–4298. [Google Scholar] [CrossRef]
- Setyowati, E.P.; Jenie, U.A.; Sudarsono; Kardono, L.B.S.; Rahmat, R. Identification of cytotoxic constituent of Indonesian sponge Kaliapsis sp. Pak. J. Biol. Sci 2008, 11, 2560–2566. [Google Scholar] [CrossRef]
- Liang, Z.; Sulzmaier, F.J.; Yoshida, W.Y.; Kelly, M.; Ramos, J.W. Neopetrocyclamines A and B, polycyclic diamine alkaloids from the sponge Neopetrosia cf. exigua. J. Nat. Prod. 2015, 78, 543–547. [Google Scholar] [CrossRef]
- Rodríguez, J.; Jimenez, C.; Blanco, M.; Tarazona, G.; Fernandez, R.; Cuevas, C. Lanesoic acid: A cytotoxic zwitterion from Theonella sp. Org. Lett. 2016, 18, 5832–5835. [Google Scholar] [CrossRef] [PubMed]
- van Wagoner, R.M.; Jompa, J.; Tahir, A.; Ireland, C.M. A novel modified pterin from a Eudistoma species Ascidian. J. Nat. Prod. 2001, 64, 1100–1101. [Google Scholar] [CrossRef]
- Mokhlesi, A.; Hartmann, R.; Kurtán, T.; Weber, H.; Lin, W.; Chaidir, C.; Müller, W.E.G.; Daletos, G.; Proksch, P. New 2-methoxy acetylenic acids and pyrazole alkaloids from the marine sponge Cinachyrella sp. Mar. Drugs 2017, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Trianto, A.; de Voogd, N.J.; Tanaka, J. Two new compounds from an Indonesian sponge Dysidea sp. J. Asian. Nat. Prod. Res. 2014, 16, 163–168. [Google Scholar] [CrossRef]
- Mokhlesi, A.; Stuhldreier, F.; Wex, K.W.; Berscheid, A.; Hartmannn, R.; Rehberg, N.; Sureechatchaiyan, P.; Chaidir, C.; Kassack, M.U.; Kalscheuer, R.; et al. Cyclic cysteine-bridged peptides from the marine sponge Clathria basilana induce apoptosis in tumor cells and depolarize the bacterial cystoplasmic membrane. J. Nat. Prod. 2017, 80, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Ardá, A.; Rodríguez, J.; Nieto, R.M.; Bassarello, C.; Gomez-Paloma, L.; Bifulco, G.; Jiménez, C. NMR J-based analysis of nitrogen-containing moieties and application to dysithiazolamide, a new polychlorinated dipeptide from Dysidea sp. Tetrahedron 2005, 61, 10093–10098. [Google Scholar] [CrossRef]
- Ardá, A.; Soengas, R.G.; Nieto, M.I.; Jiménez, C.; Rodríguez, J. Total synthesis of (–)-dysithiazolamide. Org. Lett. 2008, 10, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Sadar, M.D.; Williams, D.E.; Mawji, N.R.; Patrick, B.O.; Wikanta, T.; Chasanah, E.; Irianto, H.E.; van Soest, R.; Andersen, R.J. Sintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cells. Org. Lett. 2008, 10, 4947–4950. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zakarian, A. Concise total synthesis of sintokamides A, B, and E by a unified, protecting- group-free strategy. Angew. Chem. Int. Ed. 2010, 49, 9702–9705. [Google Scholar] [CrossRef]
- Banuelos, C.A.; Travakoli, I.; Tien, A.H.; Caley, D.P.; Mawji, N.R.; Li, Z.; Wang, J.; Yang, Y.C.; Imamura, Y.; Yan, L.; et al. Sintokamide A is a novel antagonist of adrogen receptor that uniquely binds activation function-1 in its amino-terminal domain. J. Biol. Chem. 2016, 291, 22231–22242. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Wang, Z.; Kwong, S.; Xu, Z.; Ye, T. Total synthesis of sintokamide C. Org. Lett. 2010, 12, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Weeson, K.J.; Hamann, M.T. Keenamide A, a bioactive cyclic peptide from the marine mollusk Pleurobranchus forskalii. J. Nat. Prod. 1996, 59, 629–631. [Google Scholar] [CrossRef]
- Donia, M.S.; Wang, B.; Dunbar, D.C.; Desai, P.V.; Patny, A.; Avery, M.; Hamann, M.T. Mollamides B and C, cyclic hexapeptides from the Indonesian tunicate Didemnum molle. J. Nat. Prod. 2008, 71, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Mau, C.M.S.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borger, M. Waiakeamide, a cyclic hexapeptide from the sponge Ircinia dendroides. J. Org. Chem. 1996, 61, 6302–6304. [Google Scholar] [CrossRef] [PubMed]
- Urda, C.; Fernández, R.; Rodríguez, J.; Pérez, M.; Jiménez, C.; Cuevas, C. Bistramides M and N, oxazole-thiazole containing cyclic hexapeptides isolated from Lissoclinum bistratum interaction of zinc (II) with bistramide K. Mar. Drugs 2017, 15, 209. [Google Scholar] [CrossRef]
- Bonnington, L.S.; Tanaka, J.; Higa, T.; Kimura, J.; Yoshimura, Y.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J. Cupolamide A: A cytotoxic cylcic heptapeptide from two samples of the sponge Theonella cupola. J. Org. Chem. 1997, 62, 7765–7767. [Google Scholar] [CrossRef]
- Tan, L.T.; Williamson, R.T.; Gerwick, W.H.; Watts, K.S.; McGough, K.; Jacobs, R. Cis,cis-and trans, trans-ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodicyton spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000, 65, 419–425. [Google Scholar] [CrossRef]
- Yokokawa, F.; Sameshima, H.; Shiori, T. Total synthesis of cis,cis-ceratospongamide, a bioactive thiazole-containing cyclic peptide from marine origin. Synlett 2001, SI, 968–988. [Google Scholar] [CrossRef]
- Deng, S.; Taunton, J. Kinetic control of proline amide rotamers: Total synthesis of trans, trans- and cis, cis- ceratospongamide. J. Am. Chem. Soc. 2002, 124, 916–917. [Google Scholar] [CrossRef]
- Kutsumura, N.; Sata, N.U.; Nishiyama, S. Synthetic studies on ceratospongamides, cyclic heptapeptides containing thiazole and oxazoline units: Total synthesis of cis, cis-ceratospongamide. Bull. Chem. Soc. Jpn. 2002, 75, 847–850. [Google Scholar] [CrossRef]
- Yokokawa, F.; Sameshima, H.; In, Y.; Minoura, K.; Ishida, T.; Shiori, T. Total synthesis and conformational studies of ceratospongamide, a bioactive cyclic heptapeptide from marine origin. Tetrahedron 2002, 58, 8127–8143. [Google Scholar] [CrossRef]
- Morris, L.A.; van den Bosch, J.J.K.; Versluis, K.; Thompson, G.S.; Jaspars, M. Structure determination and MSn analysis of two new lissoclinamides isolated from the Indo-Pacific ascidian Lissoclinum patella: NOE restrained molecular dynamics confirms the absolute stereochemistry derived by degradative methods. Tetrahedron 2000, 56, 8345–8353. [Google Scholar] [CrossRef]
- Morris, L.A.; Milne, B.F.; Jaspars, M.; Kettenes-van den Bosch, J.J.; Versluis, K.; Heck, A.J.R.; Kelly, S.M.; Price, N.C. Metal binding of Lissoclinum patella metabolites. Part 2: Lissoclinamides 9 and 10. Tetrahedron 2001, 57, 3199–3207. [Google Scholar] [CrossRef]
- Afifi, A.H.; El-Desoky, A.; Kato, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Carteritins A and B, cyclic heptapeptides from the marine sponge Stylissa carteri. Tetrahedron Lett. 2016, 57, 1285–1288. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, M.; Fujita, M.; Setiawan, A.; Kobayashi, M. Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorg. Med. Chem. Lett. 2012, 22, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.C.; Ohtani, I.I.; Tanaka, J.; Higa, T.; Satari, R. Barangamide A, a new cyclic peptide from the Indonesian sponge Theonella swinhoei. Tetrahedron Lett. 1999, 40, 5373–5376. [Google Scholar] [CrossRef]
- Roy, M.C.; Ohtani, I.I.; Ichiba, T.; Tanaka, J.; Satari, R.; Higa, T. New cyclic peptides from the Indonesian Sponge Theonella swinhoei. Tetrahedron 2000, 56, 9079–9092. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Edrada-Ebel, R.A.; Mohamed, G.A.; Youssef, D.T.A.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. ARKIVOC 2008, xii, 164–171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoscheke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the marine sponge Callyspongia aerizusa: cyclic peptides with antitubercular activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef]
- Zhang, S.; de Leon Rodriguez, L.M.; Leung, I.K.H.; Cook, G.M.; Harris, P.W.R.; Brimble, M.A. Total synthesis and conformational study of callyaerin A: Anti tubercular cylic peptide bearing a rare rigidifying (Z)-2,3-diaminoacrylamide moiety. Angew. Chem. Int. Ed. 2018, 130, 3693–3697. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Cartner, L.K.; Shigematsu, N.; Pannel, L.K.; Boyd, M.R. Microspinosamide, a new HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J. Nat. Prod. 2001, 64, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Cao, L.; Matsui, K.; Rachmat, R.; Akiyama, S.; Kobayashi, M. Kendarimide A, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of Haliclona sp. Tetrahedron 2004, 60, 7053–7059. [Google Scholar] [CrossRef]
- Kotoku, N.; Cao, L.; Aoki, S.; Kobayashi, M. Absolute stereo-structure of kendarimide A, a novel MDR modulator, from a marine sponge. Heterocylces 2005, 65, 563–578. [Google Scholar] [CrossRef]
- Clark, D.P.; Carrol, J.; Naylor, S.; Crews, P. An antifungal cyclodepsipeptide, cyclolithistide A, from the sponge Theonella swinhoei. J. Org. Chem. 1998, 63, 8757–8764. [Google Scholar] [CrossRef]
- Tajima, H.; Wakimoto, T.; Takada, K.; Ise, Y.; Abe, I. Revised structure of cylclolithistide A, a cyclic depsipeptide from the marine sponge Discodermia japonica. J. Nat. Prod. 2014, 77, 154–158. [Google Scholar] [CrossRef]
- Sinisi, A.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Zampella, A.; D’Amore, C.; Renga, B.; Fiorucci, S.; Taglialatela-Scafati, O. New tridecapeptides of the theonellapeptolide family from the Indonesian sponge Theonella swinhoei. Beilstein J. Org. Chem. 2013, 9, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A–C and theopapuamides B–D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef]
- Ebada, S.S.; Wray, V.; de Voogd, N.J.; Deng, Z.; Lin, W.; Proksch, P. Two new jaspamide derivatives from the marine sponge Jaspis splendens. Mar. Drugs 2009, 7, 435–444. [Google Scholar] [CrossRef]
- Urda, C.; Fenández, R.; Rodríguez, J.; Pérez, M.; Jiménez, C.; Cuevas, C. Daedophamide, a cytotoxic cyclodepsipeptide from a Daedalopelta sp. collected in Indonesia. J. Nat. Prod. 2017, 80, 3054–3059. [Google Scholar] [CrossRef]
- Fernández, R.; Martín, M.J.; Rodríguez-Acebes, R.; Reyes, F.; Franscesh, A.; Cuevas, C. Diazonamides C–E, new cytotoxic metabolites from the ascidian Diazona sp. Tetrahedron Lett. 2008, 49, 2283–2285. [Google Scholar] [CrossRef]
- Juagdan, E.G.; Kalidindi, R.S.; Scheuer, P.J.; Kelly-Borges, M. Elenic acid, an inhibitor of topoisomerase II, from a sponge, Plakinastrella sp. Tetrahedron Lett. 1995, 36, 2905–2908. [Google Scholar] [CrossRef]
- Takahanashi, S.; Takagi, M.; Takikawa, H.; Mori, K. Synthesis of elenic acid, an inhibitor of topoisomerase II from the sponge Plakinastrella sp. J. Chem. Soc. Perkin Trans 1 1998, 1603–1606. [Google Scholar] [CrossRef]
- Hoye, R.C.; Baigorria, A.S.; Danielson, M.E.; Pragman, A.A.; Rajapakse, H.A. Synthesis of elenic acid, an inhibitor of topoisomerase II. J. Org. Chem. 1999, 64, 2450–2453. [Google Scholar] [CrossRef]
- Smith, C.J.; Abbanat, D.; Bernan, V.S.; Maiese, W.M.; Greenstein, M.; Jompa, J.; Tahir, A.; Ireland, C.M. Novel polyketide metabolites from a species of marine fungi. J. Nat. Prod. 2000, 63, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.; Rao, N.N.; Sujitha, P.; Kumar, C.G. Protecting group-free syntheses of (4S,5S,11R)- and (4S,5S,11S)-iso-cladospolide B and their biological evaluation. Synthesis 2012, 44, 1663–1666. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, H.; Wu, C.; Zhu, T.; Che, Q.; Gu, Q.; Guo, P.; Li, D. Lipid-lowering polyketides from a soft coral-derived fungus Cladosporium sp. TZP29. Bioorg. Med. Chem. Lett. 2015, 25, 3606–3609. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Tanaka, K.; Satari, R.; Kobayashi, M. Lembehyne A, a novel neuritogenic polyacetylene, from a marine sponge of Haliclona sp. Tetrahedron 2000, 56, 9945–9948. [Google Scholar] [CrossRef]
- Murakami, N.; Nakajima, T.; Kobayashi, M. Total synthesis of lembehyne A, a neuritogenic spongean polyacetylene. Tetrahedron Lett. 2001, 42, 1941–1943. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Takata, T.; Kobayashi, M. In situ photoaffinity labeling of the target protein for a neuronal differentiation inducer. FEBS Lett. 2003, 544, 223–227. [Google Scholar] [CrossRef]
- Aoki, S.; Matsui, K.; Takata, T.; Wei, H.; Kobayashi, M. Lembehyne A, a spongean polyacetylene, induces neuronal differentiation in neuroblastoma cell. Biochem. Biophys. Res. Commun. 2001, 289, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Matsui, K.; Wei, H.; Murakami, N.; Kobayashi, M. Structure-activity relationship of neuritogenic spongean acetylene alcohols, lembehynes. Tetrahedron 2002, 58, 5417–5422. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; D’yakonov, V.A.; Makarov, A.A.; Andreev, E.N.; Yunusbaeva, M.M.; Dzhemilev, U.M. The first total synthesis of the marine acetylenic alcohol, lembehyne B-a selective inducer of early apoptosis in leukemia cancer cells. Org. Biomol. Chem. 2017, 15, 470–476. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makrov, A.A.; Dzemileva, L.U.; Andreev, E.N.; Dzhemilev, U.M. The first total synthesis of lembehyne B. Mendeleev Commun. 2017, 27, 122–124. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Andreev, E.N.; Dzhemilev, U.M. Short and efficient synthetic route to lembehyne B possesing neuritogenic activity. Russ. J. Org. Chem. 2016, 52, 1844–1846. [Google Scholar] [CrossRef]
- Balansa, W.; Trianto, A.; de Voogd, N.J.; Tanaka, J. A new cytotoxic polyacetylenic alcohol from a sponge Callyspongia sp. Nat. Prod. Commun. 2017, 12, 1909–1911. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Devijver, C.; Dubut, D.; van Soest, R.W.M. A new C-20 polyacetylene from the sponge Callyspongia pseudoreticulata. J. Nat. Prod. 2003, 871–872. [Google Scholar] [CrossRef]
- Aratake, S.; Trianto, A.; Hanif, N.; de Voogd, N.J.; Tanaka, J. A new polyunsaturated brominated fatty acid from a Haliclona sponge. Mar. Drugs 2009, 7, 523–527. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D. Cytotoxic metabolites from an Indonesian Sponge Lendenfeldia sp. J. Nat. Prod. 2007, 70, 1824–1826. [Google Scholar] [CrossRef]
- Ichiba, T.; Scheuer, P.J.; Kelly-Borges, M. Two cytotoxic 3,6-epidioxy fatty acids from an Indonesian sponge, Plakortis sp. Tetrahedron 1995, 51, 12195–12202. [Google Scholar] [CrossRef]
- Pettit, G.R.; Nogawa, T.; Knight, J.C.; Doubek, D.L.; Hooper, J.N.A. Antineoplastic agents. 535. Isolation and structure of plakorstatins 1 and 2 from the Indo-Pacific sponge Plakortis nigra. J. Nat. Prod. 2004, 67, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, C.; Persico, M.; Calcinai, B.; Cerrano, C.; Parapini, S.; Taramelli, D.; Novellino, E.; Romano, A.; Scala, F.; Fattorusso, E.; et al. Manadoperoxides A–D from the Indonesian sponge Plakortis cfr. simplex. Further insights on the structure-activity relationships of simple 1,2-dioxane antimalarials. J. Nat. Prod. 2010, 73, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Fattorusso, E.; Scala, F.; Teta, R.; Calcinai, B.; Bavestrello, G.; Dien, H.A.; Kaiser, M.; Tasdemir, D.; Taglialatela-Scafati, O. Manadoperoxides, a new class of potent antitrypanosomal agents of marine origin. Org. Biomol. Chem. 2012, 10, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Abrell, L.M.; Borgeson, B.; Crews, P. A new polyketide secocurvularin from the salt water culture of a sponge derived fungus. Tetrahedon Lett. 1996, 37, 8983–8984. [Google Scholar] [CrossRef]
- Yoo, H.-D.; Ketchum, S.O.; France, D.; Bair, K.; Gerwick, W. Vidalenolone, a novel phenolic metabolite from the tropical red alga Vidalia sp. J. Nat. Prod. 2002, 65, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Sheng-Ding, Z.; Hua-Jie, Z. Absolute configuration determination for natural products (1)-absolute configuration determination for ketone, lactone, and alcohol by comparing computed optical rotations and 13C NMR with the experimental results. Chem. J. Chin. Univ. 2011, 32, 2568–2573. [Google Scholar]
- Shiono, Y.; Miyazaki, N.; Murayama, T.; Koseki, T.; Katja, H.D.G.; Supratman, U.; Nakata, J.; Kakihara, Y.; Saeki, M.; Yoshida, J.; et al. GSK-3β inhibitory activities of novel dichroloresorcinol derivatives from Cosmospora vilior isolated from a mangrove plant. Phytochem. Lett. 2016, 18, 122–127. [Google Scholar] [CrossRef]
- Jadulco, R.; Proksch, P.; Wray, V.; Sudarsono; Berg, A.; Gräfe, U. New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J. Nat. Prod. 2001, 64, 527–530. [Google Scholar] [CrossRef]
- Tarman, K.; Lindequist, U.; Wende, K.; Porzel, A.; Arnold, N.; Wessjohann, L.A. Isolation of a new natural product and cytotoxic and antimicrobial activities of extracts from fungi of Indonesian marine habitats. Mar. Drugs 2011, 9, 294–306. [Google Scholar] [CrossRef]
- Handayani, D.; Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Soest, R.W.; Kunzmann, A.; Soedarsono. Four new bioactive polybrominated diphenyl ethers of the sponge Dysidea herbacea from West Sumatra, Indonesia. J. Nat. Prod. 1997, 60, 1313–1316. [Google Scholar] [CrossRef]
- Hanif, N.; Tanaka, J.; Setiawan, A.; Trianto, A.; de Voogd, N.J.; Murni, A.; Tanaka, C.; Higa, T. Polybrominated diphenyl ethers from the Indonesian sponge Lamellodysidea herbacea. J. Nat. Prod. 2007, 70, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Calcul, L.; Chow, R.; Oliver, A.G.; Tenney, K.; White, K.N.; Wood, A.W.; Fiorilla, C.; Crews, P. NMR strategy for unravelling structures of bioactive sponge-derived oxy-polyhalogenated diphenyl ethers. J. Nat. Prod. 2009, 72, 443–449. [Google Scholar] [CrossRef]
- Sumilat, D.A.; Yamazaki, H.; Endo, K.; Rotinsulu, H.; Wewengkang, D.S.; Ukai, K.; Namikoshi, M. A new biphenyl ether derivative produced by Indonesian ascidian-derived Penicilium albobiverticillium. J. Nat. Med. 2017, 71, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Y.; Miao, C.; Liu, P.; Hong, K.; Zhu, W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef]
- Kuramochi, K.; Saito, F.; Nakazaki, A.; Takeuchi, T.; Tsubaki, K.; Sugawara, F.; Kobayashi, S. Synthesis of pseudodeflectusin and ustusorane C: Structural revision of aspergiones A and B. Biosci. Biotechnol. Biochem. 2010, 74, 1635–1640. [Google Scholar] [CrossRef]
- Saito, F.; Kuramochi, K.; Nakazaki, A.; Mizushina, Y.; Sugawara, F.; Kobayashi, S. Synthesis and absolute configuration of (+)-pseudodeflectusin: Structural revision of aspergione B. Eur. J. Org. Chem. 2006, 4796–4799. [Google Scholar] [CrossRef]
- Kuramochi, K.; Tsubaki, K. Synthesis and structural characterization of natural benzofuranoids. J. Nat. Prod. 2015, 78, 1056–1066. [Google Scholar] [CrossRef]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef]
- Xu, J.; Nakazawa, T.; Ukai, K.; Kobayashi, H.; Mangindaan, R.E.P.; Wewengkang, D.S.; Rotinsulu, H.; Namikoshi, M. Tetrahydrobostrycin and 1-deoxytetrahydrobostrycin, two new hexahydroanthrone derivatives, from a marine-derived fungus Aspergillus sp. J. Antibiot. 2008, 61, 415–419. [Google Scholar] [CrossRef]
- Shiono, Y.; Sasaki, T.; Shibuya, F.; Yasuda, Y.; Koseki, T.; Supratman, U. Isolation of a phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from mangrove, Rhizhopora mucronata. Nat. Prod. Commun. 2013, 8, 1735–1737. [Google Scholar] [CrossRef]
- Rönsberg, D.; Debbab, A.; Mándi, A.; Vasylyeva, V.; Böhler, P.; Stork, B.; Engelke, L.; Hamacher, A.; Sawadogo, R.; Diedrich, M.; et al. Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla. J. Org. Chem. 2013, 78, 12409–12425. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kinoshita, M.; Aoki, S.; Kobayashi, M. Komodoquinone A, a novel neuritogenic anthracycline, from marine Streptomyces sp. KS3. J. Nat. Prod. 2003, 66, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kinoshita, M.; Wei, H.; Kobayashi, M. Stereostrcuture of komodoquinone A, a neuritogenic anthracyline, from marine Streptomyces sp. KS3. Chem. Pharm. Bull. 2003, 51, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorg. Med. Chem. Lett. 2014, 24, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Wray, V.; Berg, A.; Gräfe, U.; Sudarsono; Brauers, G.; Proksch, P. Novel spiciferone derivatives from the fungus Drechslera hawaiiensis isolated from the marine sponge Callyspongia aerizusa. Z. Naturforsch. 2000, 55c, 218–221. [Google Scholar] [CrossRef]
- Spery, S.; Samuels, G.J.; Crews, P. Vertinoid polyketides from the salt culture of the fungus Tricoderma longibrachiatum separated from a Haliclona sponge. J. Org. Chem. 1998, 63, 10011–10014. [Google Scholar] [CrossRef]
- Smith, C.J.; Jompa, J.; Tahir, A.; Buchanan, M.V.; Ireland, C.M. Cadiolides A and B, new metabolites from an ascidian of the genus Botryllus. J. Org. Chem. 1998, 63, 4147–4150. [Google Scholar] [CrossRef]
- Franck, X.; Vaz Araujo, M.E.; Jullian, J.C.; Hocquemiller, R.; Figadère, B. Synthesis and structure determination of iso-cladospolide B. Tetrahedron Lett. 2001, 42, 2801–2803. [Google Scholar] [CrossRef]
- Trost, B.M.; Aponick, A. Palladium-catalyzed asymmetric allylic alkylation of meso- and dl-1,2-divinylethylene carbonate. J. Am. Chem. Soc. 2006, 128, 3931–3933. [Google Scholar] [CrossRef]
- Choi, P.J.; Sperry, J.; Brimble, M.A. Heteroatom-directed reverse Wacker oxidations. Synthesis of the reported structure of (–)-herbaric acid. J. Org. Chem. 2010, 75, 7388–7392. [Google Scholar] [CrossRef]
- Costantino, V.; Sala, G.D.; Saurav, K.; Teta, R.; Bar-Shalom, R.; Mangoni, A.; Steindler, L. Plakofuranolactone as a quorum quenching agent from the Indonesian sponge Plakortis cf. lita. Mar. Drugs 2017, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Shibuya, F.; Koseki, T.; Harizon; Supratman, U.; Uesugi, S.; Kimura, K. A new α-pyrone metabolite from a mangrove plant endophytic fungus Fusarium sp. J. Asian. Nat. Prod. Res. 2014, 17, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Tarman, K.; Palm, G.J.; Porzel, A.; Merzweiler, K.; Arnold, N.; Wessjohann, L.A.; Unterseher, M.; Lindequist, U. Helicascolide C, a new lactone from an Indonesian marine algicolous strain of Daldinia eschscholzii (Xylariaceae, Ascomycota). Phytochem. Lett. 2012, 5, 83–86. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, A.B.; Shankar, K.S. Concise total synthesis of helicascolides A, B, and C. Synthesis 2013, 45, 1034–1038. [Google Scholar] [CrossRef]
- Haydal, A.M.; Berthold, D.; Spreider, P.A.; Breit, B. Stereodivergent and protecting-group-free synthesis of the helicascolide family: A rhodium-catalyzed atom-economical lactonization strategy. Angew. Chem. Int. Ed. 2016, 55, 5765–5769. [Google Scholar] [CrossRef]
- Sirirath, S.; Tanaka, J.; Ohtani, I.I.; Ichiba, T.; Rachmat, R.; Ueda, K.; Osada, H.; Higa, T. Bitungolides A–F, new polyketides from the Indonesian sponge Theonella cf. swinhoei. J. Nat. Prod. 2002, 65, 1820–1823. [Google Scholar] [CrossRef]
- Reddy, K.M.; Shashidar, J.; Ghosh, S. A concise approach for the synthesis of bitungolides: Total syntheses of (–)-bitungolides B & E. Org. Biomol. Chem. 2014, 12, 4002–4012. [Google Scholar] [CrossRef] [PubMed]
- Shashidar, J.; Reddy, K.M.; Ghosh, S. The first total synthesis of (–)-bitungolide E. Tetrahedron Lett. 2011, 52, 3106–3109. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, S.U.; Shashidhar, J. Total synthesis of (–)-bitungolide F and determination of its absolute stereochemistry. J. Org. Chem. 2008, 73, 1582–1585. [Google Scholar] [CrossRef]
- Edrada, R.A.; Heubes, M.; Brauers, G.; Wray, V.; Berg, A.; Gräfe, U.; Wohlfarth, M.; Mühlbacher, J.; Schaumann, K.; Sudarsono; et al. Online analysis of xestodecalactones A–C, novel bioactive metabolites from the fungus Penicillium cf. montanense and their subsequent isolation from the sponge Xestospongia exigua. J. Nat. Prod. 2002, 65, 1598–1604. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Michel, M.; Heubes, M. Stereochemical assignment of the fungal metabolite xestodecalactone A by total synthesis. Tetrahedron Lett. 2004, 45, 2829–2831. [Google Scholar] [CrossRef]
- Yoshino, T.; Ng, F.; Danishefsky, S.J. A total synthesis of xestodecalactone A and proof of its absolute stereochemistry: Interesting observations on dienophilic control with 1,3-disubtituted nonequivalent allenes. J. Am. Chem. Soc. 2006, 128, 14185–14191. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, J.; Quan, W.; Sun, Y.; She, X.; Pan, X. The first asymmetric total syntheses and determination of absolute configurations of xestodecalactones B and C. J. Org. Chem. 2007, 72, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Thrimurtulu, N.; Gayathri, K.U.; Reddy, B.V.S.; Prasad, A.R. The stereoselective total synthesis of xestodecalactone C and epi-sporostatin via the prins cylisation. Tetrahedron Lett. 2008, 49, 6617–6620. [Google Scholar] [CrossRef]
- Rajesh, K.; Suresh, V.; Selvan, J.J.P.; Rao, C.B.; Venkateswarlu, Y. Stereoselective total synthesis of xestodecalactone C. Helv. Chim. Acta. 2009, 92, 1866–1872. [Google Scholar] [CrossRef]
- Yadav, J.S.; Rao, Y.G.; Ravindar, K.; Reddy, B.V.S.; Narsaiah, A.V. Total synthesis of xestodecalactone C from L-malic acid. Synthesis 2009, 3157–3161. [Google Scholar] [CrossRef]
- Gesner, S.; Cohen, N.; Ilan, M.; Yarden, O.; Carmeli, S. Pandangolide 1a, a metabolite of the sponge-associated fungus Cladosporium sp., and the absolute stereochemistry of pandangolide 1 and iso-cladospolide B. J. Nat. Prod. 2005, 68, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.-D.; Hartmann, R.; Böhler, P.; Störk, B.; Wesselborg, S.; Lin, W.; Lai, D.; Proksch, P. Callyspongiolide, a cytotoxic macrolide from the marine sponge Callyspongia sp. Org. Lett. 2014, 16, 266–269. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kassekert, L.A.; Bungard, J.D. Enantioselective total synthesis and structural assignment of callyspongiolide. Org. Biomol. Chem. 2016, 14, 11357–11370. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Gao, B.; Xu, Z.; Ye, T. Total synthesis and stereochemical assignment of callyspongiolide. J. Am. Chem. Soc. 2016, 138, 6948–6951. [Google Scholar] [CrossRef]
- Williamson, R.T.; Boulanger, A.; Vulpanovici, A.; Roberts, M.A.; Gerwick, W.H. Structure and absolute stereochemistry of phormidolide, a new toxic metabolite from the marine cyanobacterium Phormidium sp. J. Org. Chem. 2002, 67, 7927–7936. [Google Scholar] [CrossRef] [PubMed]
- Trianto, A.; Hermawan, I.; Suzuka, T.; Tanaka, J. Two new cytotoxic candidaspongiolides from an Indonesian sponge. ISRN Pharm. 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.G.; Herb, R.; Moore, R.E.; Scheuer, P.J.; Paul, V.J. Laulimalides: New potent cytotoxic macrolides from a marine sponge and a nudibranch predator. J. Org. Chem. 1988, 53, 3644–3646. [Google Scholar] [CrossRef]
- Quiñoà, E.; Kakou, Y.; Crews, P. Fijianolide, polyketide heterocycles from a marine sponge. J. Org. Chem. 1988, 53, 3642–3644. [Google Scholar] [CrossRef]
- Jefford, C.W.; Bernardinelli, G.; Tanaka, J.; Higa, T. Structures and absolute configurations of the marine toxins, latrunculin A and laulimalide. Tetrahedron Lett. 1996, 37, 159–162. [Google Scholar] [CrossRef]
- Paterson, I.; Menche, D.; Britton, R.; Håkanson, A.E.; Silva-Martínez, M.A. Conformational studies and solution structure of laulimalide and simplified analogues using NMR spectroscopy and molecular modelling. Tetrahedron Lett. 2005, 46, 3677–3682. [Google Scholar] [CrossRef]
- Thepchatri, P.; Cicero, D.O.; Monteagudo, E.; Ghosh, A.K.; Cornett, B.; Weeks, E.R.; Snyder, J.P. Conformations of laulimalide in DMSO-d6. J. Am. Chem. Soc. 2005, 127, 12838–12846. [Google Scholar] [CrossRef]
- Prota, A.E.; Bargsten, K.; Northcote, P.T.; Marsh, M.; Altmann, K.-H.; Miller, J.H.; Díaz, J.F.; Steinmatez, M.O. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew. Chem. Int. Ed. 2014, 53, 1621–1625. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T.; Bernardinelli, G.; Jefford, C.W. New cytotoxic macrolides from the sponge Fasciospongia rimosa. Chem. Lett. 1996, 25, 255–256. [Google Scholar] [CrossRef]
- Moobery, S.L.; Tien, G.; Hernandez, A.H.; Plubrukarn, A.; Davidson, B.S. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999, 59, 653–660. [Google Scholar]
- Ghosh, A.K.; Wang, Y. Total synthesis of (–)-laulimalide. J. Am. Chem. Soc. 2000, 122, 11027–11028. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hoegenauer, E.K.; Enev, V.S.; Hanbauer, M.; Kaehlig, H.; Öhler, E.; Mulzer, J. Total synthesis of the microtubule stabilizing antitumor agent laulimalide and some nonnatural analogues: The power of Sharpless’ asymmetric epoxidation. J. Org. Chem. 2003, 68, 3026–3042. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.M., Jr.; Fang, F.G.; Johannes, C.W.; Pesant, M.; Tremblay, M.R.; Zhao, H.; Akasaka, K.; Li, X.-Y.; Liu, J.; Littlefield, B.A. Synthesis and biological evaluation of (–)-laulimalide analogues. Bioorg. Med. Chem. Lett. 2004, 14, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Gollner, A.; Altmann, K.-H.; Gertsch, J.; Mulzer, J. The laulimalide family: Total synthesis and biological evaluation of neolaulimalide, isolaulimalide, laulimalide and a nonatural analogue. Chem. Eur. J. 2009, 15, 5979–5997. [Google Scholar] [CrossRef] [PubMed]
- Pryor, D.E.; O’Brate, A.; Bilcer, G.; Diaz, J.F.; Wang, Y.; Kabaki, M.; Jung, M.K.; Andreu, J.M.; Ghosh, A.K.; Giannakakou, P.; et al. The microtubule stabilizing agent laulimalide does not bind in the taxoid site, kill cells resistant to paclitaxel and epothilones and may not require its epoxide moeity for activity. Biochemistry 2002, 41, 9109–9115. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, S.L.; Randall-Hlubek, D.A.; Leal, R.M.; Hedge, S.G.; Hubbard, R.D.; Zhang, L.; Wender, P.A. Microtubule-stabilizing agents based on designed laulimalide analogues. Proc. Natl. Acad. Sci. USA 2004, 101, 8803–8808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollner, A.; Mulzer, J. Total synthesis of neolaulimalide and isolaulimalide. Org. Lett. 2008, 10, 4701–4704. [Google Scholar] [CrossRef] [PubMed]
- Sinisi, A.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Zampella, A.; D’Amore, C.; Renga, B.; Fiorucci, S.; Taglialatela-Scafati, O. Isoswinholide B and swinholide K, potently cytotoxic dimeric macrolides from Theonella swinhoei. Bioorg. Med. Chem. Lett. 2013, 21, 5332–5338. [Google Scholar] [CrossRef]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Xestosaprols from the Indonesian Marine Sponge Xestospongia sp. J. Nat. Prod. 2010, 73, 1188–1191. [Google Scholar] [CrossRef]
- Millán-Aguiñaga, N.; Soria-Mercado, I.E.; Williams, P. Xestosaprols D and E from the Indonesian marine sponge Xestospongia sp. Tetrahedron Lett. 2010, 51, 751–753. [Google Scholar] [CrossRef]
- He, F.; Mai, L.H.; Longeon, A.; Copp, B.R.; Loaëc, N.; Bescond, A.; Meijer, L.; Bourguet-Kondracki, M.-L. Novel adociaquinone derivatives from the Indonesian sponge Xestospongia sp. Mar. Drugs 2015, 13, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Tanokashira, N.; Kukita, S.; Kato, H.; Nehira, T.; Angkouw, E.D.; Mangindaan, R.E.P.; de Voogd, N.J.; Tsukamoto, S. Petroquinones: Trimeric xestoquinone derivatives isolated from the marine sponge Petrosia alfiani. Tetrahedron 2016, 72, 5530–5540. [Google Scholar] [CrossRef]
- Zhu, Y.; Yoshida, W.Y.; Kelly-Borges, M.; Scheuer, P.J. Noelaquinone, a new hexacyclic triazine quinone from an Indonesian Xestospongia sp. Heterocylces 1998, 49, 355–360. [Google Scholar] [CrossRef]
- Cao, S.; Foster, C.; Brisson, M.; Lazo, J.S.; Kinston, D.G.I. Halenaquinone and xestoquinone derivatives, inhibitors of Cdc25B phosphatase from a Xestospongia sp. Bioorg. Med. Chem. 2005, 13, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Ishida, R.; Matsumoto, H.; Arai, M.; Toda, K.; Setiawan, A.; Muraoka, O.; Kobayashi, M. Biakamides A–D, unique polyketides from a marine sponge, act as selective growth inhibitors of tumor cells adapted to nutrient starvation. J. Org. Chem. 2017, 82, 1705–1718. [Google Scholar] [CrossRef]
- Riccio, R.; Kinnel, R.B.; Bifulco, G.; Scheuer, P.J. Kakelokelose, a sulfated mannose polysaccharide with anti-HIV activity from the Pacific tunicate Didemnum molle. Tetrahedron Lett. 1996, 37, 1979–1982. [Google Scholar] [CrossRef]




















































© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, N.; Murni, A.; Tanaka, C.; Tanaka, J. Marine Natural Products from Indonesian Waters. Mar. Drugs 2019, 17, 364. https://doi.org/10.3390/md17060364
Hanif N, Murni A, Tanaka C, Tanaka J. Marine Natural Products from Indonesian Waters. Marine Drugs. 2019; 17(6):364. https://doi.org/10.3390/md17060364
Chicago/Turabian StyleHanif, Novriyandi, Anggia Murni, Chiaki Tanaka, and Junichi Tanaka. 2019. "Marine Natural Products from Indonesian Waters" Marine Drugs 17, no. 6: 364. https://doi.org/10.3390/md17060364