Marine Microalgae: Promising Source for New Bioactive Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microalgae Cultures and Extracts Preparation
2.2. Biological Evaluation
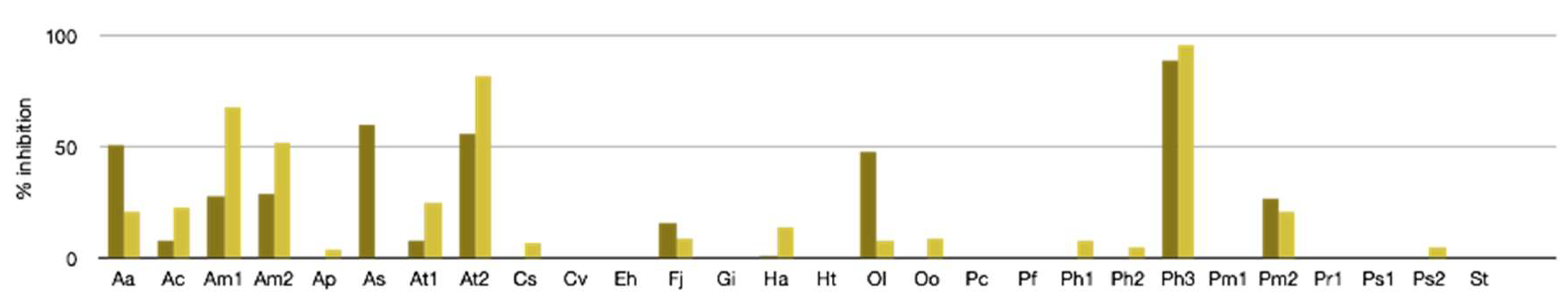
2.2.1. Antibacterial, Antifungal, and Antiviral Activity
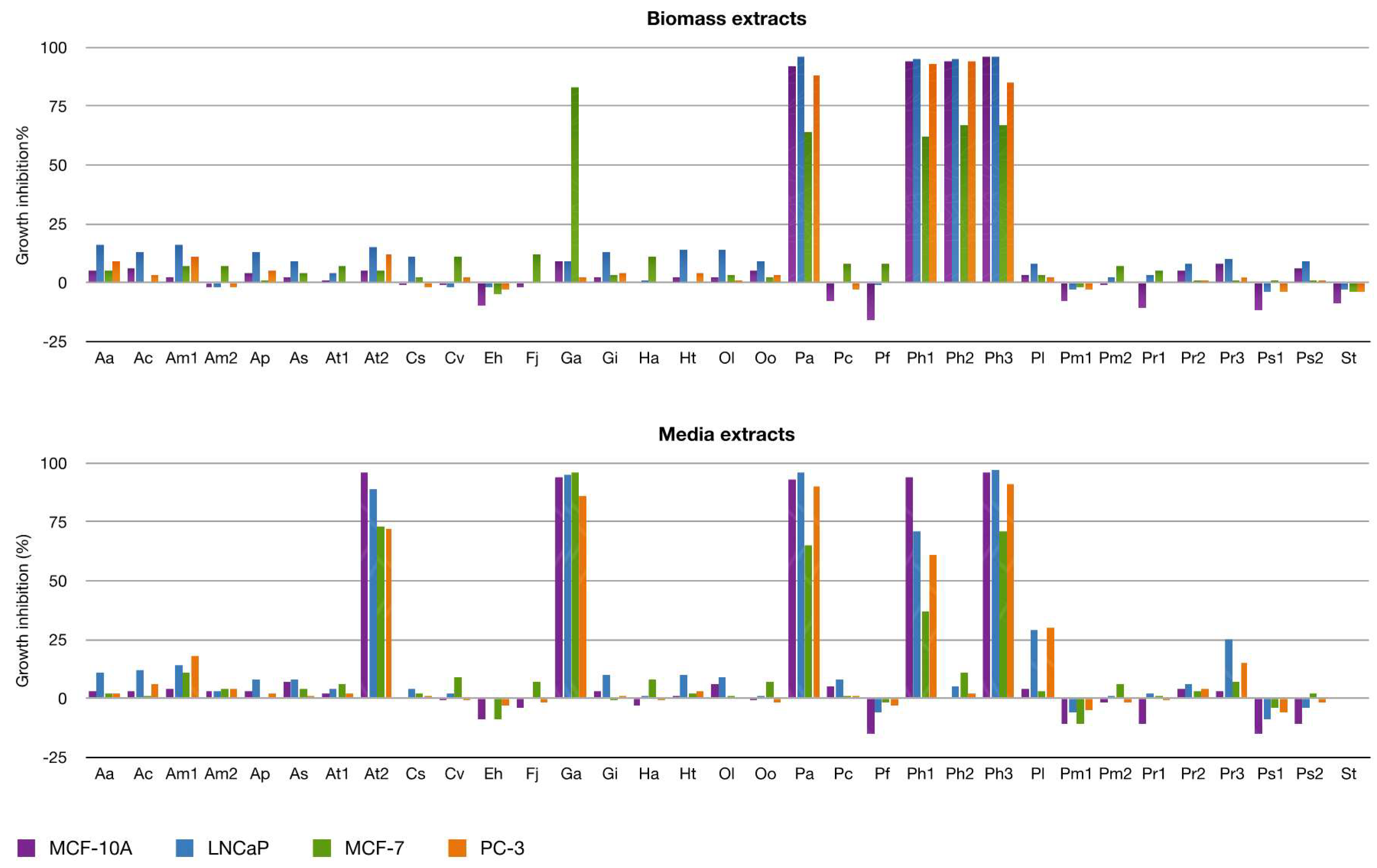
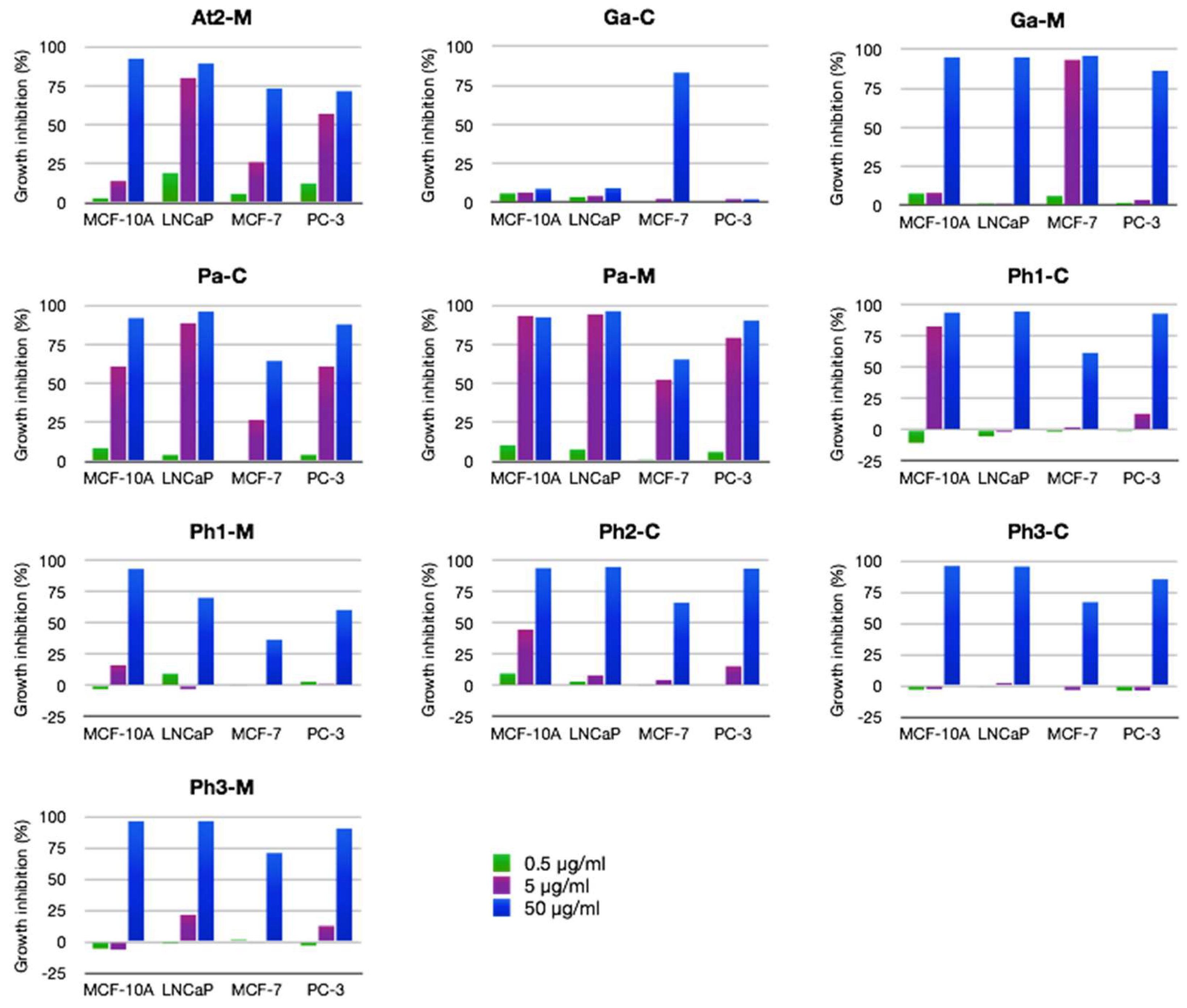
2.2.2. Antiproliferative Activity
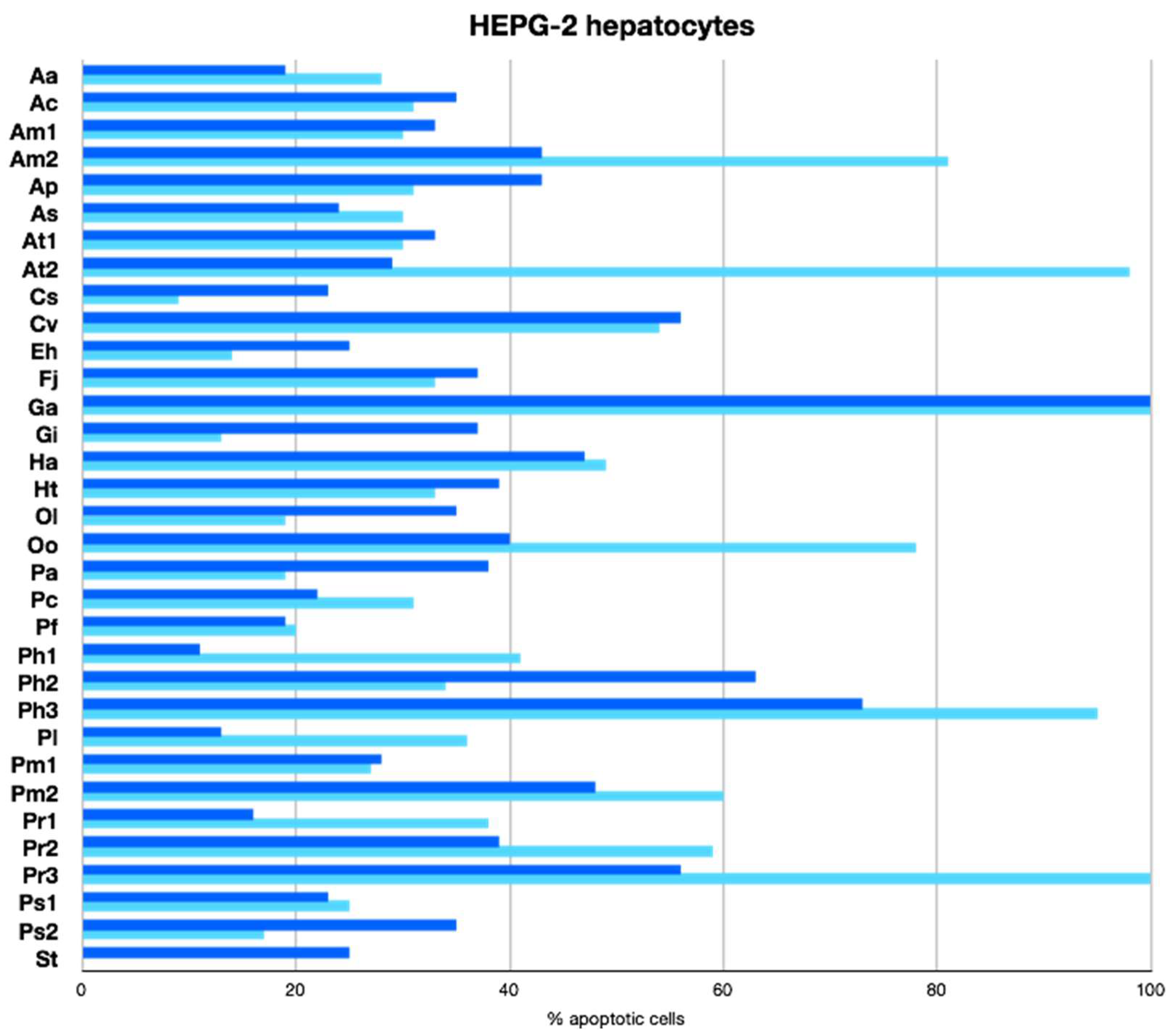
2.2.3. Apoptotic Activity
3. Materials and Methods
3.1. Microalgae Strains and Culture
3.2. Sample Preparation
3.3. Antibacterial and Antifungal Assays
3.4. Antiviral Assays
3.5. Antiproliferative Activity
3.6. Apoptotic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References and Note
- Molinski, T.F. All natural: The renaissance of natural products chemistry. J. Org. Chem. 2014, 79, 6765. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinset, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiuru, P.; D’Auria, V.M.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 1234–1246. [Google Scholar] [CrossRef]
- Bhatia, P.; Chug, A. Role of marine bioprospecting contracts in developing access and benefit sharing mechanism for marine traditional knowledge holders in the pharmaceutical industry. Global Ecol. Conserv. 2015, 3, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Bekendorff, K. Potential conservation benefits and problems associated with bioprospecting in the marine environment. In A Zoological Revolution. Using Native Fauna to Assist in Its Own Survival; Lunney, D., Dickman, C., Eds.; Royal Zoological Society of New South Wales, Mosman 2088 and Australian Museum: Lismore, Australia, 2002; pp. 90–100. [Google Scholar]
- Querellou, J.; Børresen, T.; Boyen, C.; Dobson, A.; Höfle, M.; Ianora, A.; Jaspars, M.; Kijjoa, A.; Olafsen, J.; Rigos, G.; et al. 2010 Marine Biotechnology: A New Vision and Strategy for Europe: Marine Board-ESF Postition Paper 15; European Science Foundation: Beernem, Belgium, 2010; pp. 1–91. [Google Scholar]
- Abid, F.; Zahid, M.A.; Abedin, Z.U.; Nizami, S.B.; Abid, M.J.; Kazmi, S.Z.H.; Khan, S.U.; Hasan, H.; Ali, M.; Gul, A. Omics approaches in marine biotechnology: The treasure of ocean for human betterments. In Omics Technologies and Bioengineering: Towards Improving Quality of Life; Barh, D., Acevedo, V., Eds.; Academic Press: Berlin, Germany, 2018; Volume 1, pp. 47–61. [Google Scholar]
- Sieg, R.D.; Poulson-Ellestada, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- García Camacho, F.; Gallardo Rodríguez, J.; Sánchez Mirón, A.; Cerón García, M.C.; Belarbi, E.H.; Chisti, Y.; Molina Grima, E. Biotechnological significance of toxic marine dinoflagellates. Biotech. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Prober, T.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting Marine Plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Seventh Research Framework Programme, Exploring Marine Resources for Bioactive Compounds: From Discovery to Sustainable Production and Industrial Applications, MAREX Consortium, EUFP7-KBBE-3-245137.
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Harrison, P.J.; Berges, J.A. Marine culture media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, VT, USA, 2005; pp. 21–33. [Google Scholar]
- Bravo, I.; Fernández, M.L.; Ramilo, I.; Martínez, A. Toxin composition of the toxic dinoflagellate Prorocentrum lima isolated from different locations along the Galician coast (NW Spain). Toxicon 2001, 39, 1537–1541. [Google Scholar] [CrossRef]
- Nagai, H.; Satake, M.; Yasumoto, T. Antimicrobial activities of polyether compounds of dinoflagellate origins. J. Appl. Phycol. 1990, 2, 305–308. [Google Scholar] [CrossRef]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D´Souza, L.; DÁuria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a novel MTX analog produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Turner, J.T.; Esposito, F.; Carotenuto, Y.; d’Ippolito, G.; Romano, G.; Fontana, A.; Guisande, C.; Miralto, A. Copepod egg production and hatching success is reduced by maternal diets of a non-neurotoxic strain of the dinoflagellate Alexandrium tamarense. Mar. Ecol. Prog. Ser. 2004, 280, 199–210. [Google Scholar] [CrossRef]
- Paasche, E. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia 2002, 40, 503–529. [Google Scholar] [CrossRef]
- Manning, R.S.; La Claire II, J.W. Prymnesins: Toxic metabolites of the golden alga, Prymnesium parvum Carter (Haptophyta). Mar. Drugs 2010, 8, 678–704. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Nuzzo, G.; Galasso, C.; Cassoti, R.; Fontana, A.; Romano, G.; Ianora, A. The marine dinoflagellate Alexandrium andersoni induces cell death in lung and colorectal tumor cell lines. Mar. Biotech. 2018, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Katsuo, D.; Nakayasu, S.; Salati, C.; Duan, J.; Zou, Y.; Matsuyama, Y.; Yamaguchi, K.; Oda, T. Purification and characterization of a novel high molecular weight exotoxin produced by red tide phytoplankton, Alexandrium tamarense. J. Biochem. Mol. Toxicol. 2008, 22, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Abi-Khalil, C.; Finkelstein, D.S.; Conejero, G.; Du Bois, J.; Destoumieux-Garzon, D.; Rolland, J.L. The paralytic shellfish toxin, saxitoxin, enters the cytoplasm and induces apoptosis of oyster immune cells through a caspase-dependent pathway. Aquat. Toxicol. 2017, 190, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Leung, P.T.Y.; Ip, J.C.H.; Cheng, J.; Wu, J.J.; Gu, J.R.; Lam, P.K.S. Developmental toxicity and molecular responses of marine medaka (Orzyas melastigma) embryos to ciguatoxin P-CTX-1 exposure. Aquat. Toxicol. 2017, 185, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.J.; Candenas, M.L.; Souto, M.L.; Trujillo, M.M.; Norte, M. Okadaic acid, useful tool for studying cellular processes. Curr. Med. Chem. 2012, 9, 222–262. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Gião, M.S.; Seabra, R.; Ferreira, A.C.S.; Tamagnini, P.; Moradas-Ferreira, P. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Lee, J.H.; Kwon, O.N.; Kim, S.W.; Jeon, Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 2013, 28, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.M.R.; Samarakoon, K.W.; Ko, J.Y.; Chaminda, L.H.H.; Lee, J.H.; An, S.J.; Jeon, Y.J.; Lee, J.B. Potentiality of benthic dinoflagellate cultures and screening of their bioactivities in Jeju Island, Korea. Afr. J. Biotechnol. 2014, 13, 792–805. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes and antibacterial activities. Front. Mar. Sci. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Montalvão, S.; Demirel, Z.; Devi, P.; Lombardi, V.; Hongisto, V.; Perälä, M.; Hattara, J.; Imamoglu, E.; Tilvi, S.S.; Turan, G.; et al. Large-scale bioprospecting of cyanobacteria, micro-and macroalgae from the Aegean Sea. New Biotechnol. 2016, 33, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vera, C.R.; Díaz Crespín, G.; Hernández Daranas, A.; Montalvão Looga, S.; Lillsunde, K.-E.; Tammela, P.; Perälä, M.; Hongisto, V.; Virtanen, J.; Rischer, H.; et al. Marine Microalgae: Promising Source for New Bioactive Compounds. Mar. Drugs 2018, 16, 317. https://doi.org/10.3390/md16090317
De Vera CR, Díaz Crespín G, Hernández Daranas A, Montalvão Looga S, Lillsunde K-E, Tammela P, Perälä M, Hongisto V, Virtanen J, Rischer H, et al. Marine Microalgae: Promising Source for New Bioactive Compounds. Marine Drugs. 2018; 16(9):317. https://doi.org/10.3390/md16090317
Chicago/Turabian StyleDe Vera, Caterina R., Guillermo Díaz Crespín, Antonio Hernández Daranas, Sofia Montalvão Looga, Katja-Emilia Lillsunde, Päivi Tammela, Merja Perälä, Vesa Hongisto, Johannes Virtanen, Heiko Rischer, and et al. 2018. "Marine Microalgae: Promising Source for New Bioactive Compounds" Marine Drugs 16, no. 9: 317. https://doi.org/10.3390/md16090317




