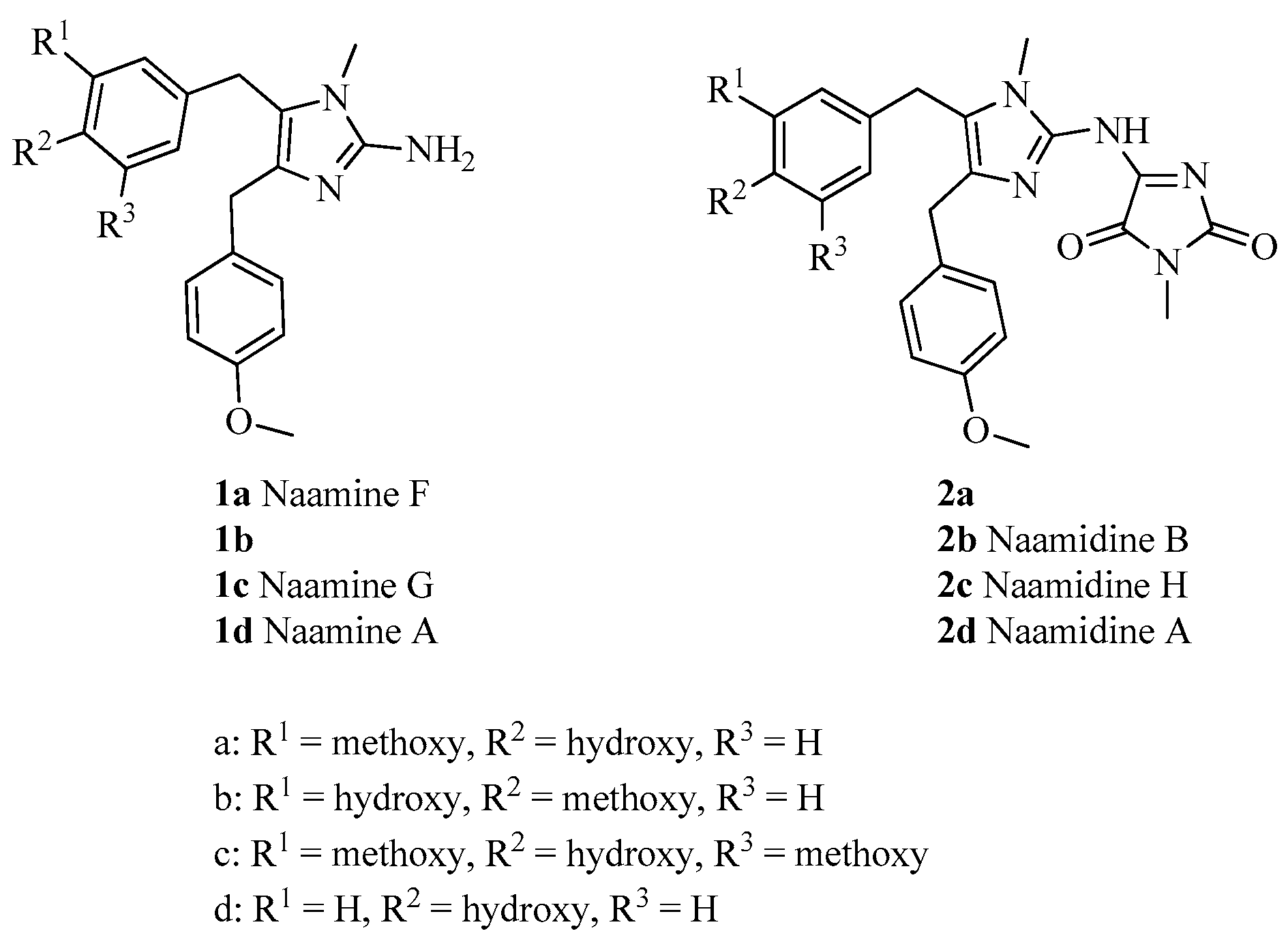
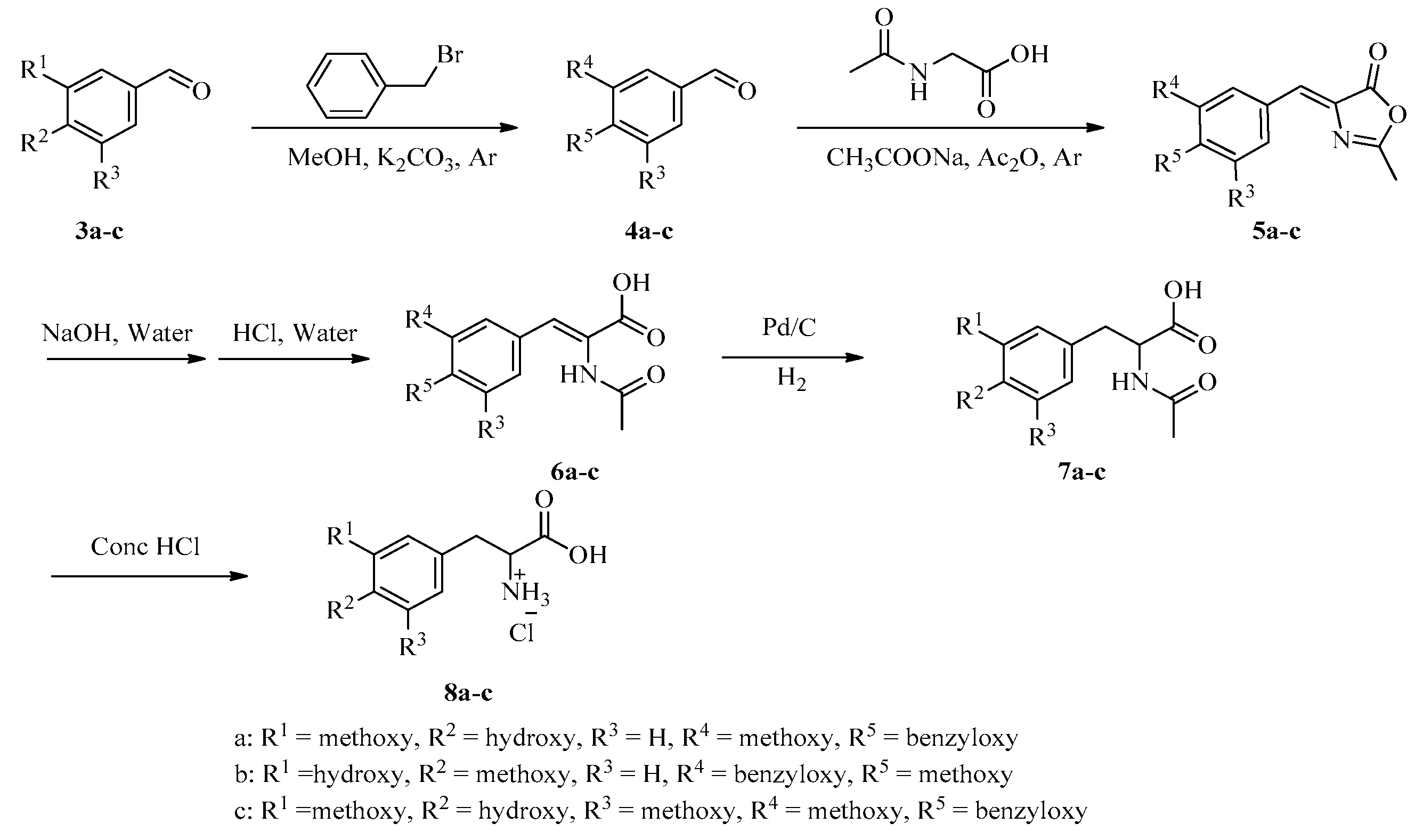
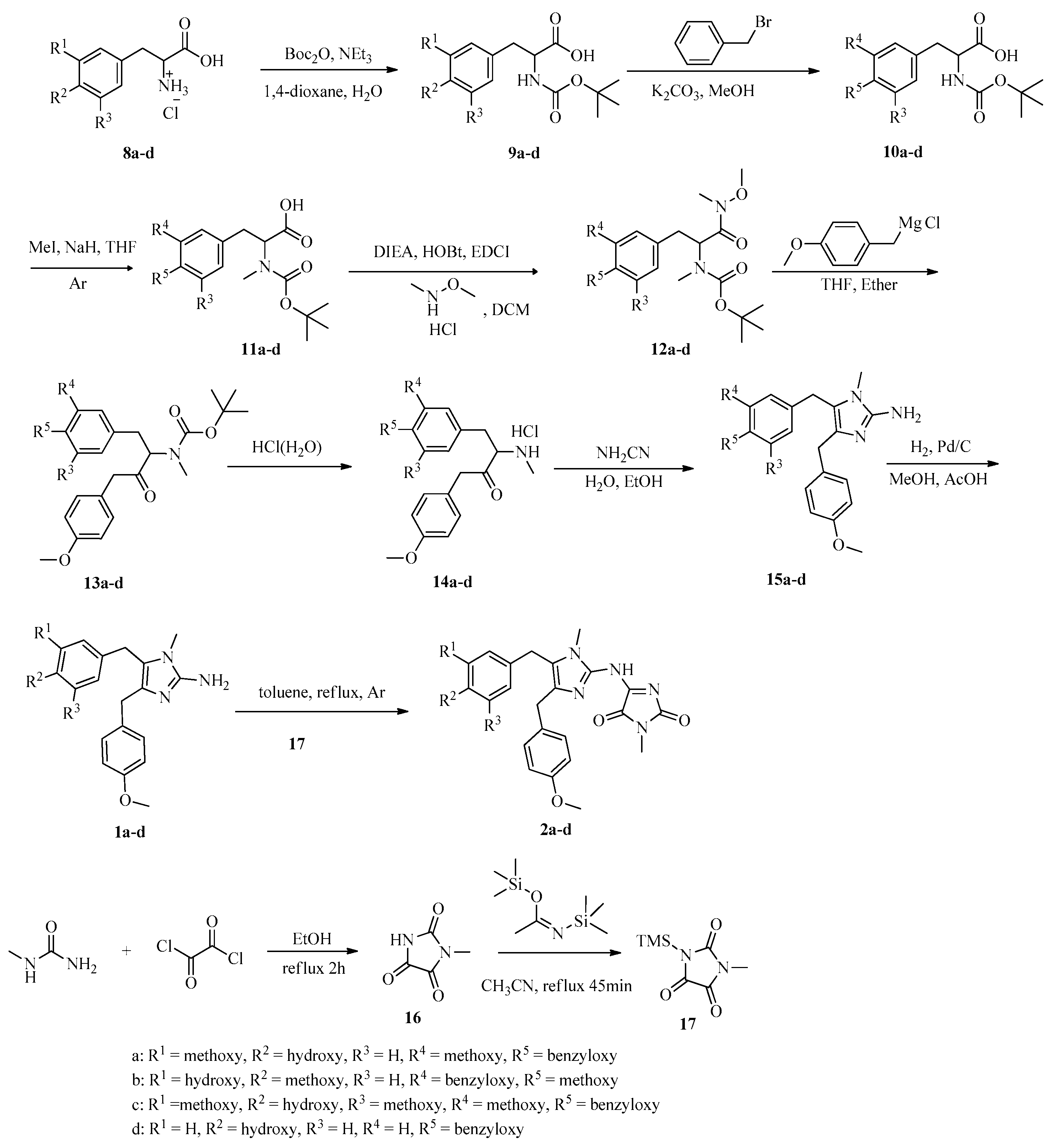
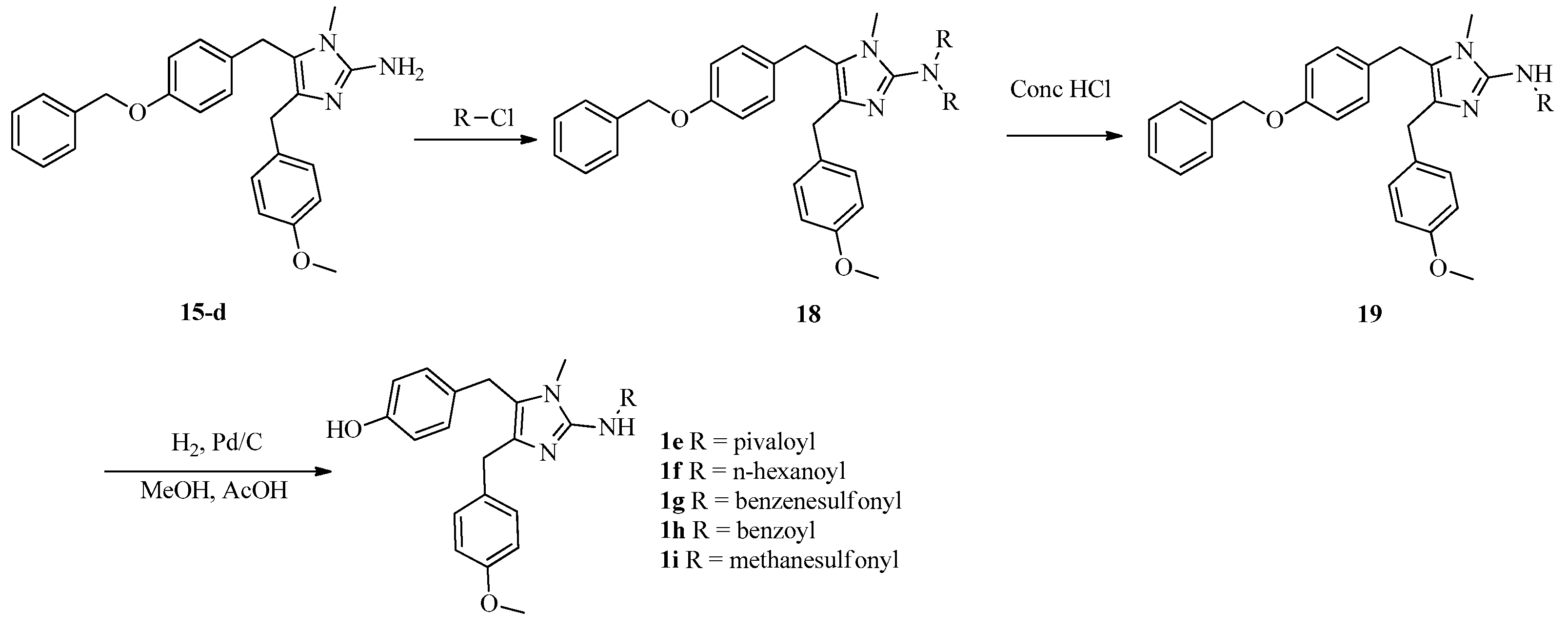
3.1. General Experimental Procedures
The melting points were determined on an X-4 binocular microscope (Beijing Tech Instruments Co., Beijing, China). NMR spectra were obtained by using Bruker AV 400 spectrometer (Bruker Co., Fallanden, Switzerland). Chemical shifts (δ) were given in parts per million (ppm) and measured downfield from internal tetramethylsilane. High-resolution mass spectra were obtained with an FT-ICR MS spectrometer (Ionspec, 7.0 T, Kuala Lumpur, Malaysia). All reagents were of analytical reagent grade or chemically pure and purified prior to use when necessary.
General procedure for the preparation of benzaldehydes 4. The mixture of benzaldehydes 3 (0.06 mol), K2CO3 (11.0 g, 0.07 mol), benzyl bromide (9.4 g, 0.07 mol) and methanol 250 mL was stirred and refluxed for 4 h under argon. Then the reaction mixture was filtered and evaporated under vacuum. The residue was dissolved in CH2Cl2 (200 mL) and washed with H2O (100 mL), brine (100 mL), and dried with MgSO4 anhydrous. The solution was filtered, evaporated under vacuum. Then acetone (10 mL) was added. The mixture was kept at 0 °C for 4 h, filtered to give benzaldehydes 4 as a white powder.
For 4-(benzyloxy)-3-methoxybenzaldehyde (4a): Yield 85%; m.p.: 67–68 °C; 1H NMR (400 MHz, CDCl3) δ 9.83 (s, 1H, COH), 7.45–7.31 (m, 7H, Ar-H), 6.99 (d, J = 8.2 Hz, 1H, Ar-H), 5.25 (s, 2H, O-CH2), 3.95 (s, 3H, O-CH3).
For 3-(benzyloxy)-4-methoxybenzaldehyde (4b): Yield 95%; m.p.: 63–65 °C; 1H NMR (400 MHz, CDCl3) δ 9.82 (s, 1H, COH), 7.47–7.30 (m, 7H, Ar-H), 6.99 (d, J = 8.1 Hz, 1H, Ar-H), 5.19 (s, 2H, O-CH2), 3.96 (s, 3H, O-CH3).
For 4-(benzyloxy)-3,5-dimethoxybenzaldehyde (4c): Yield 88%; m.p.: 63–64 °C; 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H, COH), 7.47 (d, J = 7.1 Hz, 2H, Ar-H), 7.32 (dd, J = 11.2, 7.0 Hz, 3H, Ar-H), 7.11 (s, 2H, Ar-H), 5.13 (s, 2H, O-CH2), 3.90 (s, 6H, O-CH3).
General procedure for the preparation of 5. The mixture of benzaldehydes 4 (0.02 mol), acetic anhydride (10 mL), N-acetyl-glycine (0.02 mol) and sodium acetate (0.02 mol) was stirred at 115 °C for 3.5 h under argon. Then, the reaction mixture was cooled to room temperature, and ethanol (100 mL) was added. After ultrasonic oscillation, the mixture was filtered to give 5 as a yellow powder.
For (Z)-4-(4-(benzyloxy)-3-methoxybenzylidene)-2-methyloxazol-5(4H)-one (5a): Yield 44%; m.p.: 157–159 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 1.8 Hz, 1H, Ar-H), 7.48–7.29 (m, 6H, Ar-H), 7.07 (s, 1H, CH), 6.91 (d, J = 8.4 Hz, 1H, Ar-H), 5.23 (s, 2H, O-CH2), 3.96 (s, 3H, O-CH3), 2.39 (s, 3H, C-CH3).
For (Z)-4-(3-(benzyloxy)-4-methoxybenzylidene)-2-methyloxazol-5(4H)-one (5b): Yield 28%; m.p.: 108–110 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (s, 1H, CH), 7.52–7.28 (m, 6H, Ar-H), 7.03 (s, 1H, Ar-H), 6.92 (d, J = 8.4 Hz, 1H, Ar-H), 5.22 (s, 2H, O-CH2), 3.94 (s, 3H, O-CH3), 2.39 (s, 3H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 168.1, 164.8, 152.6, 148.2, 136.7, 131.6, 130.5, 128.6, 128.0, 127.6, 126.3, 116.4, 111.3, 71.0, 56.0, 15.6; HRMS (ESI) calcd. for C19H18NO4+ [M + H]+ 324.1230, found 324.1232.
For (Z)-4-(4-(benzyloxy)-3,5-dimethoxybenzylidene)-2-methyloxazol-5(4H)-one (5c): Yield 52%; m.p.: 102–104 °C; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 7.5 Hz, 2H, Ar-H), 7.41–7.28 (m, 5H, Ar-H), 7.04 (s, 1H, CH), 5.10 (s, 2H, O-CH2), 3.88 (s, 6H, O-CH3), 2.40 (s, 3H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 167.9, 165.6, 153.6, 139.9, 137.4, 131.6, 131.5, 128.7, 128.5, 128.2, 128.0, 109.6, 75.1, 56.2, 15.8; HRMS (ESI) calcd. for C20H20NO5+ [M + H]+ 354.1336, found 354.1338.
General procedure for the preparation of acids 6. The mixture of 5 (13.10 mmol), NaOH (1.6 g, 39.20 mmol) and H2O (100 mL) was stirred and refluxed for 1 h. Then, the reaction mixture was cooled to room temperature and acidified to pH 5–6 with dilute hydrochloric acid. The mixture was filtered to give acids 6 as a white powder.
For (Z)-2-acetamido-3-(4-(benzyloxy)-3-methoxyphenyl)acrylic acid (6a): Yield 93%; m.p.: 200–202 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.52 (s, 1H, COOH), 9.41 (s, 1H, NH), 7.45–7.35 (m, 5H, Ar-H), 7.33 (s, 1H, Ar-H), 7.21 (s, 1H, CH), 7.18 (d, J = 8.4 Hz, 1H, Ar-H), 7.07 (d, J = 8.4 Hz, 1H, Ar-H), 5.13 (s, 2H, O-CH2), 3.77 (s, 3H, O-CH3), 1.99 (s, 3H, C-CH3).
For (Z)-2-acetamido-3-(3-(benzyloxy)-4-methoxyphenyl)acrylic acid (6b): Yield 61%; m.p.: 209–211 °C; 1H NMR (400 MHz, DMSO) δ 12.53 (s, 1H, COOH), 9.46 (s, 1H, NH), 7.55–7.30 (m, 6H, Ar-H), 7.24 (d, J = 8.2 Hz, 1H, Ar-H), 7.19 (s, 1H, CH), 7.02 (d, J = 8.2 Hz, 1H, Ar-H), 5.10 (s, 2H, O-CH2), 3.80 (s, 3H, O-CH3), 1.97 (s, 3H, CO-CH3).
For (Z)-2-acetamido-3-(4-(benzyloxy)-3,5-dimethoxyphenyl)acrylic acid (6c): Yield 53%; m.p.: 155–157 °C; 1H NMR (400 MHz, CD3OD) δ 7.46 (s, 1H, CH), 7.44 (d, J = 6.7 Hz, 2H, Ar-H), 7.36–7.24 (m, 3H, Ar-H), 6.93 (s, 2H, Ar-H), 4.99 (s, 2H, O-CH2), 3.82 (s, 6H, O-CH3), 2.11 (s, 3H, CO-CH3); 13C NMR (100 MHz, CD3OD) δ 173.2, 168.3, 154.7, 139.2, 139.0, 136.0, 130.7, 129.6, 129.2, 129.1, 126.2, 108.5, 76.1, 56.6, 22.6; HRMS (ESI) calcd. for C20H22NO6+ [M + H]+ 372.1442, found 372.1444.
General procedure for the preparation of acids 7. The mixture of acids 6 (3.28 mmol) and Pd/C (10 wt%) (0.15 g) in ethanol (100 mL) was bubbled with hydrogen and stirred at room temperature for 12 h. Then, the mixture was filtered and concentrated to give acids 7 as a slight yellow powder.
For 2-acetamido-3-(4-(benzyloxy)-3-methoxyphenyl)propanoic acid (7a): Yield 98%; m.p.: 73–75 °C; 1H NMR (400 MHz, CD3OD) δ 6.79 (d, J = 1.8 Hz, 1H, Ar-H), 6.70 (d, J = 8.0 Hz, 1H, Ar-H), 6.64 (dd, J = 8.0, 1.8 Hz, 1H, Ar-H), 4.61 (dd, J = 8.9, 5.1 Hz, 1H, CH), 3.82 (s, 3H, O-CH3), 3.10 (dd, J = 14.0, 5.1 Hz, 1H, CH2), 2.85 (dd, J = 14.0, 8.9 Hz, 1H, CH2), 1.91 (s, 3H, C-CH3).
For 2-acetamido-3-(3-(benzyloxy)-4-methoxyphenyl)propanoic acid (7b): Yield 95%; m.p.: 147–150 °C; 1H NMR (400 MHz, CD3OD) δ 6.82 (d, J = 8.0 Hz, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.65 (d, J = 8.0 Hz, 1H, Ar-H), 4.57 (dd, J = 8.7, 5.0 Hz, 1H, CH), 3.81 (s, 3H, O-CH3), 3.06 (dd, J = 13.9, 5.0 Hz, 1H, CH2), 2.82 (dd, J = 13.9, 8.7 Hz, 1H, CH2), 1.91 (s, 3H, CO-CH3); 13C NMR (100 MHz, CD3OD) δ 174.9, 173.2, 148.0, 147.4, 131.2, 121.5, 117.2, 112.7, 56.4, 55.4, 37.8, 22.4; HRMS (ESI) calcd. for C12H16NO5+ [M + H]+ 254.1023, found 254.1019.
For 2-acetamido-3-(4-(benzyloxy)-3,5-dimethoxyphenyl)propanoic acid (7c): Yield 96%; m.p.: 148–151 °C; 1H NMR (400 MHz, CD3OD) δ 6.54 (s, 2H, Ar-H), 4.68 (dd, J = 8.8, 5.0 Hz, 1H, CH), 3.86 (s, 6H, O-CH3), 3.16 (dd, J = 13.9, 5.0 Hz, 1H, CH2), 2.90 (dd, J = 13.9, 8.8 Hz, 1H, CH2), 1.97 (s, 3H, CO-CH3); 13C NMR (100 MHz, CD3OD) δ 175.0, 173.2, 149.2, 135.4, 129.0, 107.4, 56.8, 55.4, 38.6, 22.4; HRMS (ESI) calcd. for C13H18NO6+ [M + H]+ 284.1129, found 284.1133.
General procedure for the preparation of amino acids 8. The mixture of acids 7 (19.78 mmol) in 4 N HCl solution (500 mL) was refluxed for 24 h and concentrated. Then, methanol (50 mL) was added. The mixture was kept at 0 °C for 4 h, filtered to give amino acids 8, which were used directly for the next step.
General procedure for the preparation of amino acids 9. The mixture of acids 8 (4.03 mmol), (Boc)2O (4.44 mmol), Et3N (12.1 mmol), H2O (20 mL) and 1,4-dioxane (20 mL) was stirred at room temperature for 18 h. Then, the mixture was concentrated and dissolved in H2O (100 mL), acidified to pH 5–6 with dilute hydrochloric acid, and extracted with ethyl acetate (100 mL × 3). The combined organic layer was washed with brine (100 mL), dried with MgSO4 anhydrous. The solution was filtered, evaporated under vacuum. Methanol (10 mL) was added. The mixture was kept at 0 °C for 4 h, filtered to give amino acids 9, which were used directly for the next step.
General procedure for the preparation of Boc amino acids 10. The mixture of amino acids 9 (1.89 mmol), K2CO3 (4.55 mmol), benzyl bromide (2.27 mmol) and methanol (50 mL) was refluxed for 4 h. Then, the mixture was concentrated and dissolved in H2O (50 mL), acidified to pH 5–6 with dilute hydrochloric acid, and extracted with ethyl acetate (50 mL × 3). The combined organic layer was washed with brine (100 mL), dried with MgSO4 anhydrous. The solution was filtered, evaporated under vacuum to give Boc amino acids 10.
For 3-(4-(benzyloxy)-3-methoxyphenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid (10a): Slight yellow powder; Yield for three steps 68%; m.p.: 128–130 °C; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.51–7.27 (m, 5H, Ar-H), 6.81 (d, J = 8.1 Hz, 1H, Ar-H), 6.72 (s, 1H, Ar-H), 6.65 (d, J = 7.8 Hz, 1H, Ar-H), 6.02 and 4.93 (d, J = 7.3 Hz, 1H, NH), 5.12 (s, 2H, O-CH2), 4.55 and 4.34 (s, 1H, CH), 3.85 (s, 3H, O-CH3), 3.13–2.85 (m, 2H, CH2), 1.42 and 1.32 (two s, 9H, C-CH3).
For 3-(3-(benzyloxy)-4-methoxyphenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid (10b): White powder; Yield for three steps 53%; m.p.: 143–145 °C; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.46–7.28 (m, 5H, Ar-H), 6.81 (d, J = 8.6 Hz, 1H, Ar-H), 6.75–6.68 (m, 2H, Ar-H), 5.84 and 4.89 (two d, 1H, NH), 5.11 (s, 2H, O-CH2), 4.59–4.46 and 4.35–4.25 (two m, 1H, CHCH2), 3.85 (s, 3H, O-CH3), 3.12–2.76 (m, 2H, CHCH2), 1.42 and 1.35 (two s, 9H, C-CH3).
For 3-(4-(benzyloxy)-3,5-dimethoxyphenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid (10c): Brown powder; Yield for three steps 23%; m.p.: 123–125 °C; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.47 (d, J = 7.1 Hz, 2H, Ar-H), 7.36–7.27 (m, 3H, Ar-H), 6.38 (s, 2H, Ar-H), 4.98 (s, 2H, O-CH2), 4.94 (d, J = 6.9 Hz, 1H, NH), 4.59 (s, 1H, CH), 3.79 (s, 6H, O-CH3), 3.18–3.08 (m, 1H, CH2), 3.05–2.96 (m, 1H, CH2), 1.43 and 1.35 (two s, 9H, C-CH3).
For 3-(4-(benzyloxy)phenyl)-2-((tert-butoxycarbonyl)amino)propanoic acid (10d): White powder; Yield for three steps 93%; m.p.: 110–111 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.28 (m, 5H, Ar-H), 7.10 (d, J = 8.3 Hz, 2H, Ar-H), 6.91 (d, J = 8.4 Hz, 2H, Ar-H), 6.11 and 4.92 (two d, J = 6.6 Hz, 1H, NH), 5.03 (s, 2H, O-CH2), 4.57 and 4.36 (two d, J = 5.4 Hz, 1H, CHCH2), 3.06 (m, 2H, CHCH2), 1.37 (d, J = 38.4 Hz, 9H, CCH3).
General procedure for the preparation of acids 11. To the solution of acids 10 (10.02 mmol) in THF (30 mL) was added 70% NaH (30.06 mmol) and stirred for 30 min at 0 °C. Then CH3I (20.04 mmol) was added. The reaction mixture was stirred for 24 h at room temperature, and quenched with H2O (20 mL), and extracted with ethyl acetate (100 mL × 3). The combined organic layer was washed with Na2S2O3 solution (100 mL), NaHCO3 solution (100 mL), brine (100 mL) and dried with MgSO4 anhydrous. The solution was filtered, evaporated under vacuum to give acids 11.
For 3-(4-(benzyloxy)-3-methoxyphenyl)-2-((tert-butoxycarbonyl)(methyl)amino)propanoic acid (11a): Yellow oil; Yield 88%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.47–7.27 (m, 5H, Ar-H), 6.83–6.62 (m, 3H, Ar-H), 5.12 (s, 2H, O-CH2), 4.73 and 4.52 (two d, J = 5.9 Hz, 1H, CH), 3.87 (s, 3H, O-CH3), 3.26–3.19 (m, 1H, CH2), 3.12–2.92 (m, 1H, CH2), 2.74 and 2.37 (two s, 3H, N-CH3), 1.41 and 1.33 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 176.3, 156.3, 155.1, 149.6, 146.9, 137.2, 130.6, 130.3, 128.5, 127.8, 127.3, 121.1, 121.0, 114.2, 112.7, 112.5, 80.7, 80.6, 71.1, 61.8, 60.3, 56.0, 34.8, 34.3, 32.8, 28.3, 28.2; HRMS (ESI) calcd. for C23H29NNaO6+ [M + Na]+ 438.1887, found 438.1880.
For 3-(3-(benzyloxy)-4-methoxyphenyl)-2-((tert-butoxycarbonyl)(methyl)amino)propanoic acid (11b): Brown oil; Yield 76%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.44–7.27 (m, 5H, Ar-H), 6.83–6.67 (m, 3H, Ar-H), 5.11 (s, 2H, O-CH2), 4.72–4.66 and 4.45–4.35 (two m, 1H, CHCH2), 3.84 (s, 3H, O-CH3), 3.25–3.10 (m, 1H, CHCH2), 3.04–2.84 (m, 1H, CHCH2), 2.64 and 2.57 (two s, 3H, N-CH3), 1.39 and 1.33 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 176.4, 156.4, 155.0, 148.6, 148.5, 148.0, 137.2, 137.1, 130.0, 129.6, 127.9, 127.4, 127.4, 121.8, 121.7, 115.1, 114.8, 112.1, 111.9, 80.7, 77.31, 71.2, 71.0, 61.8, 60.6, 60.5, 56.1, 34.8, 34.2, 33.0, 14.2; HRMS (ESI) calcd. for C23H29NNaO6+ [M + Na]+ 438.1887, found 438.1883.
For 3-(4-(benzyloxy)-3,5-dimethoxyphenyl)-2-((tert-butoxycarbonyl)(methyl)amino)propanoic acid (11c): Brown oil; Yield 79%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.47 (d, J = 7.0 Hz, 2H, Ar-H), 7.36–7.27 (m, 3H, Ar-H), 6.42 and 6.37 (two s, 2H, Ar-H), 4.98 (s, 2H, O-CH2), 4.80–4.70 and 4.55–4.45 (two m, 1H, CH), 3.80 (s, 6H, O-CH3), 3.33–3.17 (m, 1H, CH2), 3.15–2.95 (m, 1H, CH2), 2.73 and 2.67 (two s, 3H, N-CH3), 1.43 and 1.36 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 176.0, 156.2, 154.9, 153.5, 153.4, 137.8, 137.7, 135.7, 135.5, 133.3, 132.9, 130.2, 128.5, 128.1, 127.8, 106.0, 105.8, 80.7, 75.0, 61.8, 60.3, 56.1, 35.5, 34.9, 33.1, 33.0, 28.3, 28.2; HRMS (ESI) calcd. for C24H31NNaO7+ [M + Na]+ 468.1993, found 468.1989.
For 3-(4-(benzyloxy)phenyl)-2-((tert-butoxycarbonyl)(methyl)amino)propanoic acid (11d): White powder; m.p.: 126–127 °C Yield 79%; 1H NMR (400 MHz, CDCl3, exists as a 1:1 mixture of two rotamers) δ 7.47–7.29 (m, 5H, Ar-H), 7.14–7.08 (m, 2H, Ar-H), 6.91 (d, J = 8.0 Hz, 2H, Ar-H), 5.04 (s, 2H, O-CH2), 4.71–4.66 and 4.60–4.53 (two m, 1H, CHCH2), 3.33–3.17 (m, 1H, CHCH2), 3.16–2.92 (m, 1H, CHCH2), 2.75 and 2.67 (two s, 3H, N-CH3), 1.41 and 1.35 (two s, 9H, C-CH3).
General procedure for the preparation of carbamates 12. The mixture of acids 11 (0.75 mol), N,N-diisopropylethylamine (232.87 mmol), 1-hydroxybenzotriazole (82.63 mmol), 1-ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride (EDC·HCl) (82.63 mmol) and N,O-dimethylhydroxylamine hydrochloride (82.63 mmol) in dichloromethane (200 mL) was stirred at room temperature for 12 h. Then, the reaction mixture was acidified to pH 5–6 with dilute hydrochloric acid and filtered. The filtrate was extracted with dichloromethane (100 mL × 3). The combined organic layer was washed with brine (100 mL) and dried with MgSO4 anhydrous. The solution was filtered and evaporated under vacuum. The residue was purified by column chromatography on silica gel to give carbamates 12.
For tert-butyl (3-(4-(benzyloxy)-3-methoxyphenyl)-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)(methyl)carbamate (12a): Yellow oil; Yield 73%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.45–7.28 (m, 5H, Ar-H), 6.83–6.61 (m, 3H, Ar-H), 5.52 and 5.09 (two s, 1H, CH), 5.12 (s, 2H, O-CH2), 3.87 (s, 3H, O-CH3), 3.62 and 3.59 (two s, 3H, O-CH3), 3.18 and 3.15 (two s, 3H, N-CH3), 3.15–2.86 (m, 2H, CH2), 2.84 (s, 3H, N-CH3), 1.37 and 1.24 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 155.6, 154.9, 149.5, 149.4, 146.8, 146.7, 137.3, 131.2, 130.6, 128.5, 127.7, 127.2, 121.3, 114.2, 114.1, 113.1, 112.9, 79.7, 71.1, 61.3, 60.4, 57.3, 55.9, 54.4, 34.5, 32.3, 30.2, 29.9, 28.3, 28.1, 21.0, 14.2; HRMS (ESI) calcd. for C25H35N2O6+ [M + H]+ 459.2490, found 459.2498.
For tert-butyl (3-(3-(benzyloxy)-4-methoxyphenyl)-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)(methyl)carbamate (12b): Yellow oil; Yield 80%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.50–7.28 (m, 5H, Ar-H), 6.87–6.70 (m, 3H, Ar-H), 5.49 and 5.06 (two s, 1H, CHCH2), 5.12 (s, 2H, O-CH2), 3.85 (s, 3H, O-CH3), 3.62 and 3.57 (two s, 3H, O-CH3), 3.17 and 3.14 (two s, 3H, N-CH3), 3.11–2.81 (m, 2H, CHCH2), 2.79 and 2.78 (two s, 3H, N-CH3), 1.38 and 1.28 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 155.7, 155.0, 148.5, 148.3, 148.2, 148.0, 137.3, 137.2, 130.6, 129.9, 128.6, 127.8, 127.4, 127.3, 122.1, 122.0, 115.4, 115.1, 112.1, 111.9, 79.8, 79.7, 77.3, 71.1, 71.0, 61.6, 61.3, 57.4, 56.2, 56.1, 54.5, 34.5, 32.4, 32.1, 30.2, 30.0, 28.3, 28.2; HRMS (ESI) calcd. for C25H35N2O6+ [M + H]+ 459.2490, found 459.2490.
For tert-butyl (3-(4-(benzyloxy)-3,5-dimethoxyphenyl)-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)(methyl)carbamate (12c): Brown oil; Yield 67%; 1H NMR (400 MHz, CDCl3) δ 7.53–7.45 (m, 2H, Ar-H), 7.38–7.28 (m, 3H, Ar-H), 6.46 and 6.37 (two s, 2H, Ar-H), 5.57 and 5.12 (two s, 1H, CH), 4.96 (s, 2H, O-CH2), 3.80 (s, 6H, O-CH3), 3.63 and 3.60 (two s, 3H, O-CH3), 3.20 and 3.16 (two s, 3H, N-CH3), 3.14–3.07 (m, 1H, CH2), 2.96–2.88 (m, 1H, CH2), 2.85 and 2.84 (two s, 3H, N-CH3), 1.39 and 1.27 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 155.6, 154.9, 153.4, 153.2, 137.9, 137.8, 135.6, 135.4, 134.0, 133.1, 128.5, 128.1, 128.1, 127.8, 127.7, 106.3, 106.1, 79.8, 75.0, 75.0, 61.5, 61.3, 60.8, 60.4, 57.4, 56.1, 56.0, 53.9, 35.3, 32.3, 32.0, 30.2, 30.0, 28.3, 28.15, 21.0, 14.2; HRMS (ESI) calcd. for C26H37N2O7+ [M + H]+ 489.2595, found 489.2603.
For tert-butyl (3-(4-(benzyloxy)phenyl)-1-(methoxy(methyl)amino)-1-oxopropan-2-yl)(methyl)carbamate (12d): Yellow oil; Yield 85%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.43–7.30 (m, 5H, Ar-H), 7.16 and 7.08 (two d, J = 8.2 Hz, 2H, Ar-H), 6.93–6.85 (m, 2H, Ar-H), 5.50 and 5.12 (two s, 1H, CHCH2), 5.03 (s, 2H, O-CH2), 3.63 and 3.60 (two s, 3H, O-CH3), 3.19 and 3.16 (two s, 3H, N-CH3), 3.10–2.89 (m, 2H, CHCH2), 2.84 (s, 3H, N-CH3), 1.36 and 1.25 (two s, 9H, CCH3).
General procedure for the preparation of carbamates 13. To the stirring mixture of magnesium ribbon (22.50 mmol) in THF (20 mL) was added dropwise the solution of 4- methoxy benzyl chloride (13.58 mmol) in THF (10 mL) and absolute ether (5 mL) at 0 °C under argon atmosphere. The mixture was stirred at room temperature for 1 h. Then, to the reaction mixture was added the solution of 12 (6.79 mmol) in THF (50 mL) at 0 °C. The mixture was stirred at room temperature for 3 h. Then, saturated ammonium chloride solution (30 mL) and H2O (20 mL) were added. The reaction solution was extracted with absolute ether (50 mL × 3). The combined organic layer was washed with brine (100 mL) and dried with MgSO4 anhydrous. The solution was filtered and evaporated under vacuum. The residue was purified by column chromatography on silica gel to give carbamates 13.
For tert-butyl (1-(4-(benzyloxy)-3-methoxyphenyl)-4-(4-methoxyphenyl)-3-oxobutan-2-yl)(methyl)carbamate (13a): Yellow oil; Yield 85%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.45–7.28 (m, 5H, Ar-H), 7.12–7.04 (m, 2H, Ar-H), 6.88–6.80 (m, 2H, Ar-H), 6.77–6.74 (m, 1H, Ar-H), 6.68–6.52 (m, 2H, Ar-H), 5.11 (s, 2H, O-CH2), 4.78–4.70 and 4.30–4.20 (two m, 1H, CH), 3.83 (s, 3H, O-CH3), 3.79 and 3.78 (two s, 3H, O-CH3), 3.74–3.61 (m, 2H, C-CH2), 3.19–3.01 (m, 1H, CH2), 2.85–2.76 (m, 1H, CH2), 2.58 and 2.51 (two s, 3H, N-CH3), 1.43 and 1.34 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 206.0, 205.9, 158.7, 158.6, 155.8, 154.9, 149.6, 149.5, 146.8, 146.6, 137.3, 137.2, 131.2, 130.8, 130.5, 130.5, 128.5, 127.8, 127.3, 127.3, 125.8, 125.7, 121.2, 121.1, 114.3, 114.2, 114.1, 114.0, 112.8, 112.7, 80.8, 80.3, 71.1, 66.8, 65.9, 64.4, 56.0, 55.9, 55.3, 55.2, 46.2, 45.7, 33.4, 32.9, 32.3, 28.4, 28.2; HRMS (ESI) calcd. for C31H37NNaO6+ [M + Na]+ 542.2513, found 542.2515.
For tert-butyl (1-(3-(benzyloxy)-4-methoxyphenyl)-4-(4-methoxyphenyl)-3-oxobutan-2-yl)(methyl)carbamate (13b): Yellow oil; Yield 91%; 1H NMR (400 MHz, CDCl3, exists as a complex mixture of two rotamers at room temperature) δ 7.48–7.27 (m, 5H, Ar-H), 7.07 (dd, J = 8.5, 3.1 Hz, 2H, Ar-H), 6.91–6.73 (m, 3H, Ar-H), 6.71–6.58 (m, 2H, Ar-H), 5.15–5.01 (m, 2H, O-CH2), 4.70–4.60 and 4.19–4.09 (two m, 1H, CHCH2), 3.84 (s, 3H, O-CH3), 3.79–3.76 (m, 3H, O-CH3), 3.73–3.58 (m, 2H, CO-CH2), 3.14–3.00 (m, 1H, CHCH2), 2.83–2.70 (m, 1H, CHCH2), 2.48 and 2.43 (two s, 3H, N-CH3), 1.44 and 1.36 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 205.9, 158.7, 154.8, 148.5, 148.1, 137.1, 130.5, 130.5, 130.1, 128.6, 127.8, 127.4, 127.3, 125.8, 125.7, 121.9, 115.2, 115.0, 114.2, 114.0, 112.0, 111.9, 80.9, 80.3, 71.0, 70.9, 67.0, 64.5, 56.1, 55.3, 46.2, 45.7, 33.4, 32.8, 32.3, 28.4, 28.3; HRMS (ESI) calcd. for C31H37NNaO6+ [M + Na]+ 542.2513, found 542.2517.
For tert-butyl (1-(4-(benzyloxy)-3,5-dimethoxyphenyl)-4-(4-methoxyphenyl)-3-oxobutan-2-yl)(methyl)carbamate (13c): Yellow oil; Yield 85%; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.4 Hz, 2H, Ar-H), 7.35–7.26 (m, 3H, Ar-H), 7.16–7.05 (m, 2H, Ar-H), 6.92–6.81 (m, 2H, Ar-H), 6.33 and 6.26 (two s, 2H, Ar-H), 4.96 (s, 2H, O-CH2), 4.76–4.70 and 4.25–4.15 (two m, 1H, CH), 3.79 and 3.78 (two s, 3H, O-CH3), 3.76 (s, 6H, O-CH3), 3.74–3.61 (m, 2H, CO-CH2), 3.17–3.05 (m, 1H, CHCH2), 2.89–2.72 (m, 1H, CHCH2), 2.56 and 2.51 (two s, 3H, N-CH3), 1.46 and 1.37 (two s, 9H, C-CH3); 13C NMR (100 MHz, CDCl3) δ 205.9, 205.8, 158.8, 158.6, 155.8, 154.8, 153.6, 153.4, 138.0, 137.8, 135.5, 135.4, 134.0, 133.5, 130.6, 130.5, 128.6, 128.6, 128.2, 127.9, 127.8, 125.8, 125.7, 114.2, 114.1, 113.9, 113.6, 106.1, 106.1, 80.9, 80.4, 75.0, 67.0, 64.9, 64.3, 56.2, 56.1, 56.0, 55.3, 55.3, 55.2, 46.2, 45.7, 34.2, 33.7, 28.4, 28.3; HRMS (ESI) calcd. for C32H39NNaO7+ [M + Na]+ 572.2619, found 572.2614.
For tert-butyl (1-(4-(benzyloxy)phenyl)-4-(4-methoxyphenyl)-3-oxobutan-2-yl)(methyl)carbamate (13d): Light liquid; Yield 92%; 1H NMR (400 MHz, CDCl3, exists as a 1:1 mixture of two rotamers) δ 7.45–7.27 (m, 5H, Ar-H), 7.10–6.98 (m, 4H, Ar-H), 6.89–6.79 (m, 4H, Ar-H), 5.02 (s, 2H, O-CH2), 4.76–4.70 and 4.35–4.25 (two m, 1H, CH), 3.78 and 3.79 (two s, 3H, O-CH3), 3.73–3.61 (m, 2H, CH2CO), 3.15–3.07 (m, 1H, CHCH2), 2.87–2.75 (m, 1H, CHCH2), 2.58 and 2.52 (two s, 3H, N-CH3), 1.43 and 1.34 (two s, 9H, CCH3).
General procedure for the preparation of amines 15. To the solution of absolute ether (11.4 mL) and ethanol (8.7 mL) was added dropwise acetyl chloride (7.9 mL) at -30 °C, and stirred at room temperature for 10 min. Then, carbamates 13 (1.43 mmol) was added. The reaction solution was stirred at room temperature for 1 h and concentrated. Then, absolute ether (20 mL) was added. The mixture was filtrated to give hydrochlorides 14, which was used for the next step directly. The mixture of hydrochlorides 14 and NH2CN (1.32 mmol) in H2O (30 mL) was stirred at 90 °C for 1 h, cooled to room temperature, filtrated to give amines 15.
For 5-(4-(benzyloxy)-3-methoxybenzyl)-4-(4-methoxybenzyl)-1-methyl-1H-imidazol-2-amine (15a): Brown powder; Yield 73%; m.p.: 95 °C (dec.); 1H NMR (400 MHz, CDCl3) δ 7.44–7.29 (m, 5H, Ar-H), 7.18 (d, J = 8.4 Hz, 2H, Ar-H), 6.79–6.76 (m, 3H, Ar-H), 6.55–6.48 (m, 2H, Ar-H), 5.64 (s, 2H, NH2), 5.12 (s, 2H, O-CH2), 3.80 (s, 2H, CH2), 3.77 (s, 2H, CH2), 3.75 (s, 3H, O-CH3), 3.71 (s, 3H, O-CH3), 3.08 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 157.8, 149.9, 147.0, 146.8, 137.2, 132.9, 132.1, 131.8, 129.4, 128.5, 127.8, 127.3, 120.8, 119.8, 114.1, 113.8, 111.6, 71.1, 55.9, 55.2, 32.1, 29.2, 29.1; HRMS (ESI) calcd. for C27H30N3O3+ [M + H]+ 444.2282, found 444.2289.
For 5-(3-(benzyloxy)-4-methoxybenzyl)-4-(4-methoxybenzyl)-1-methyl-1H-imidazol-2-amine (15b): Brown powder; Yield 88%; m.p.: 56–58 °C; 1H NMR (400 MHz, CDCl3) δ 7.35–7.24 (m, 5H, Ar-H), 7.19 (d, J = 8.4 Hz, 2H, Ar-H), 6.85–6.75 (m, 3H, Ar-H), 6.64 (d, J = 7.8 Hz, 1H, Ar-H), 6.40 (s, 1H, Ar-H), 5.11 (brs, 2H, NH2), 4.93 (s, 2H, O-CH2), 3.86 (s, 3H, O-CH3), 3.74 (s, 2H, CH2), 3.73 (s, 2H, CH2), 3.71 (s, 3H, O-CH3), 2.87 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 158.0, 148.3, 148.1, 146.9, 137.1, 132.8, 131.6, 130. 9, 129.6, 129.6, 128.6, 127.8, 127.4, 120.8, 120.4, 113.9, 111.9, 70.6, 56.1, 55.2, 32.0, 29.1, 28.9; HRMS (ESI) calcd. for C27H30N3O3+ [M + H]+ 444.2282, found 444.2291.
For 5-(4-(benzyloxy)-3,5-dimethoxybenzyl)-4-(4-methoxybenzyl)-1-methyl-1H-imidazol-2-amine (15c): Brown powder; Yield 77%; m.p.: 90–93 °C; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.0 Hz, 2H, Ar-H), 7.36–7.27 (m, 3H, Ar-H), 7.19 (d, J = 8.3 Hz, 2H, Ar-H), 6.78 (d, J = 8.3 Hz, 2H, Ar-H), 6.22 (s, 2H, Ar-H), 4.96 (s, 2H, O-CH2), 4.69 (brs, 2H, NH2), 3.81 (s, 2H, CH2), 3.76 (s, 2H, CH2), 3.75 (s, 3H, O-CH3), 3.67 (s, 6H, O-CH3), 3.07 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 158.0, 153.7, 147.0, 137.8, 135.5, 134.2, 132.5, 129.5, 128.5, 128.1, 127.8, 120.6, 113.9, 104.9, 75.0, 56.1, 55.3, 31.9, 29.8, 29.3; HRMS (ESI) calcd. for C28H32N3O4+ [M + H]+ 474.2387, found 474.2389.
For 5-(4-(benzyloxy)benzyl)-4-(4-methoxybenzyl)-1-methyl-1H-imidazol-2-amine (15d): Brown powder; Yield 70%; m.p.: 120–123 °C; 1H NMR (400 MHz, CDCl3) δ 7.43–7.30 (m, 5H, Ar-H), 7.16 (d, J = 8.3 Hz, 2H, Ar-H), 6.98 (d, J = 8.3 Hz, 2H, Ar-H), 6.87 (d, J = 8.4 Hz, 2H, Ar-H), 6.79 (d, J = 8.4 Hz, 2H, Ar-H), 5.03 (s, 2H, O-CH2), 3.85 (s, 2H, NH2), 3.81 (s, 2H, CH2), 3.75 (s, 3H, O-CH3), 3.74 (s, 2H, CH2), 3.07 (s, 3H, N-CH3); HRMS (ESI) calcd. for C26H28N3O2+ [M + H]+ 414.2176, found 414.2179.
General procedure for the preparation of naamines 1a–d. The mixture of amines 15 (10.45 mmol), Pd/C (10 wt%) (0.56 g), methanol (400 mL) and acetic acid (4 mL) was bubbled with hydrogen and stirred at room temperature for 24 h. Then, the mixture was filtered and concentrated. Then acetone (15 mL) was added, and filtered to give naamines 1.
For naamine F (1a): Brick red powder; Yield 95%; m.p.: 163–165 °C; 1H NMR (400 MHz, CD3OD) δ 7.14 (d, J = 8.5 Hz, 2H, Ar-H), 6.77 (d, J = 8.5 Hz, 2H, Ar-H), 6.66 (d, J = 8.0 Hz, 1H, Ar-H), 6.53 (dd, J = 8.0, 1.4 Hz, 1H, Ar-H), 6.45 (d, J = 1.4 Hz, 1H, Ar-H), 3.80 (s, 2H, CH2), 3.73 (s, 3H, O-CH3), 3.72 (s, 2H, CH2), 3.58 (s, 3H, O-CH3), 3.09 (s, 3H, N-CH3); 13C NMR (100 MHz, CD3OD) δ 159.4, 149.7, 149.2, 146.1, 134.7, 132.7, 131.8, 130.5, 122.6, 121.6, 116.1, 114.7, 112.6, 56.2, 55.7, 32.8, 29.7, 29.5; HRMS (ESI) calcd. for C20H24N3O3+ [M + H]+ 354.1812, found 354.1818.
For 1b: Brown powder; Yield 96%; m.p.: 80–83 °C; 1H NMR (400 MHz, CD3OD) δ 7.13 (d, J = 8.5 Hz, 2H, Ar-H), 6.88–6.83 (m, 3H, Ar-H), 6.61–6.58 (m, 2H, Ar-H), 3.88 (s, 2H, CH2), 3.84 (s, 2H, CH2), 3.82 (s, 3H, O-CH3), 3.75 (s, 3H, O-CH3), 3.20 (s, 3H, N-CH3); 13C NMR (100 MHz, CD3OD) δ 160.2, 148.2, 148.1, 147.8, 130.8, 130.8, 130.6, 124.0, 123.8, 120.3, 116.1, 115.3, 113.1, 56.5, 55.8, 30.1, 29.8, 28.5; HRMS (ESI) calcd. for C20H24N3O3+ [M + H]+ 354.1812, found 354.1818.
For naamine G (1c): Brick red powder; Yield 97%; m.p.: 172–174 °C; 1H NMR (400 MHz, CD3OD) δ 7.05 (d, J = 8.3 Hz, 2H, Ar-H), 6.67 (d, J = 8.3 Hz, 2H, Ar-H), 6.16 (s, 2H, Ar-H), 3.71 (s, 2H, CH2), 3.62 (s, 5H, CH2 and O-CH3), 3.54 (s, 6H, O-CH3), 3.01 (s, 3H, N-CH3); 13C NMR (100 MHz, CD3OD) δ 159.4, 149.7, 149.3, 134.9, 134.7, 132.8, 131.1, 130.5, 122.4, 114.7, 106.2, 56.6, 55.7, 32.8, 30.1, 29.5; HRMS (ESI) calcd. for C21H26N3O4+ [M + H]+ 384.1918, found 384.1919.
For naamine A (1d): Grey powder; Yield 87%; m.p.: 202–205 °C; 1H NMR (400 MHz, CD3OD) δ 7.09 (d, J = 8.6 Hz, 2H, Ar-H), 6.85 (d, J = 8.4 Hz, 2H, Ar-H), 6.77 (d, J = 8.6 Hz, 2H, Ar-H), 6.65 (d, J = 8.4 Hz, 2H, Ar-H), 3.78 (s, 2H, CH2), 3.73 (s, 3H, O-CH3), 3.71 (s, 2H, CH2), 3.09 (s, 3H, N-CH3); 13C NMR (100 MHz, CD3OD) δ 160.0, 157.5, 148.4, 131.5, 130.5, 130.2, 129.0, 125.2, 123.8, 116.6, 115.1, 55.7, 30.2, 29.8, 28.5; HRMS (ESI) calcd. for C19H22N3O2+ [M + H]+ 324.1707, found 324.1707.
Synthesis of 1-methylimidazolidine-2,4,5-trione (16). The mixture of N-monomethylurea (2.96 g, 0.04 mol), (COCl)2 (3.3 mL, 0.04 mol) in absolute ether (100 mL) was refluxed under argon for 2 h and concentrated. Then, dichloromethane (10 mL) was added, filtered to give 16 (2.87 g, 59%) as a white powder. m.p.: 146–149 °C, 1H NMR (400 MHz, DMSO-d6) δ 11.99 (s, 1H, NH), 2.92 (s, 3H, N-CH3).
General procedure for the preparation of naamidines 2a–d. The solution of 16 (1.28 g, 10 mmol) and N,O-bis(trimethylsilyl)acetamide (2.52 g, 12.4 mmol) in acetonitrile was refluxed under argon for 45 min and concentrated to give 17. The mixture of naamines 1 (2 mmol) and 17 in toluene (16 mL) was refluxed for 18 h. The mixture was diluted with ethyl acetate (180 mL), washed with dilute hydrochloric acid (100 mL), H2O (100 mL), brine (100 mL), dried with MgSO4 anhydrous, and evaporated under vacuum. The residue was purified by column chromatography on silica gel to give naamidines 2.
For 2a: Yellow powder; Yield 54%; m.p.: 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 8.6 Hz, 2H, Ar-H), 6.84–6.77 (m, 3H, Ar-H), 6.54 (dd, J = 8.1, 1.8 Hz, 1H, Ar-H), 6.34 (d, J = 1.8 Hz, 1H, Ar-H), 3.90 (s, 4H, CH2), 3.77 (s, 3H, O-CH3), 3.65 (s, 3H, O-CH3), 3.49 (s, 3H, N-CH3), 3.18 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 162.2, 158.2, 155.4, 146.8, 146.4, 144.6, 144.5, 135.8, 131.6, 129.3, 128.8, 126.9, 120.7, 114.5, 114.0, 110.2, 55.8, 55.3, 32.2, 30.0, 29.2, 24.7; HRMS (ESI) calcd. for C24H26N5O5+ [M + H]+ 464.1928, found 464.1932.
For naamidine B (2b): Yellow powder; Yield 37%; m.p.: 157–159 °C; 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 8.5 Hz, 2H, Ar-H), 6.82 (d, J = 8.5 Hz, 2H, Ar-H), 6.72 (d, J = 8.1 Hz, 1H, Ar-H), 6.58 (s, 1H, Ar-H), 6.46 (d, J = 8.1 Hz, 1H, Ar-H), 3.89 (s, 2H, CH2), 3.86 (s, 5H, CH2 and O-CH3), 3.78 (s, 3H, O-CH3), 3.49 (s, 3H, N-CH3), 3.17 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 162.4, 158.2, 155.8, 146.4, 145.9, 145.4, 145.2, 135.5, 131.4, 130.2, 129.3, 126.7, 119.2, 114.2, 114.0, 110.8, 56.0, 55.3, 32.2, 29.9, 28.8, 24.7; HRMS (ESI) calcd. for C24H26N5O5+ [M + H]+ 464.1928, found 464.1934.
For naamidine H (2c): Brown powder; Yield 81%; m.p.: 144–146 °C; 1H NMR (400 MHz, CDCl3) δ 7.15 (d, J = 7.9 Hz, 2H, Ar-H), 6.80 (d, J = 7.9 Hz, 2H, Ar-H), 6.15 (s, 2H, Ar-H), 3.91 (s, 2H, CH2), 3.89 (s, 2H, CH2), 3.76 (s, 3H, O-CH3), 3.70 (s, 6H, O-CH3), 3.50 (s, 3H, N-CH3), 3.18 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 162.1, 158.2, 155.2, 147.2, 146.5, 144.2, 136.2, 133.5, 131.7, 129.3, 128.1, 126.7, 114.0, 104.6, 56.2, 55.3, 32.2, 30.0, 29.7, 24.7; HRMS (ESI) calcd. for C25H28N5O6+ [M + H]+ 494.2034, found 494.2030.
For naamidine A (2d): Yellow powder; Yield 85%; m.p.: 186–190 °C; 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 8.5 Hz, 2H, Ar-H), 6.85–6.79 (m, 4H, Ar-H), 6.74 (d, J = 8.4 Hz, 2H, Ar-H), 3.88 (s, 2H, CH2), 3.87 (s, 2H, CH2), 3.77 (s, 3H, O-CH3), 3.37 (s, 3H, N-CH3), 3.17 (s, 3H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 163.5, 158.3, 157.7, 155.2, 148.5, 146.4, 133.7, 130.9, 129.3, 129.0, 128.1, 126.8, 115.8, 114.1, 55.3, 31.7, 29.7, 28.6, 24.8; HRMS (ESI) calcd. for C23H24N5O4+ [M + H]+ 434.1823, found 434.1826.
General procedure for the preparation of naamines 1e–i. To the solution of 15d (4.84 mmol) and Et3N (9.68 mmol) in dichloromethane (120 mL) was added dropwise the solution of corresponding acyl chlorides (9.68 mmol) in dichloromethane (10 mL), and stirred at room temperature for 20 min. Then, con. HCl solution (50 mL) was added and stirred for further 20 min. The layers were separated. The organic layer was washed with saturated NaHCO3 (100 mL), dried over MgSO4 anhydrous, and evaporated to give 19. The solution of 19 and Pd/C (10 wt%) (0.3 g) in methanol (100 mL) was bubbled H2 and stirred at room temperature for 24 h. The mixture was filtered and concentrated to give naamines 1e–i.
For 1e: White powder; Yield 92% for three steps; m.p.: 161–163 °C; 1H NMR (400 MHz, CDCl3) δ 13.41 (s, 1H), 11.03 (s, 1H), 7.13 (d, J = 8.1 Hz, 2H, Ar-H), 6.85–6.77 (m, 6H, Ar-H), 3.90 (s, 2H, CH2), 3.86 (s, 2H, CH2), 3.76 (s, 3H, O-CH3), 3.38 (s, 3H, N-CH3), 1.37 (s, 9H, CCH3); 13C NMR (100 MHz, DMSO-d6) δ 178.2, 158.1, 156.2, 136.3, 129.7, 129.5, 129.0, 127.4, 127.0, 126.3, 115.5, 114.0, 55.1, 31.5, 28.0, 26.8, 26.6; HRMS (ESI) calcd. for C24H30N3O3+ [M + H]+ 408.2282, found 408.2282.
For 1f: White powder; Yield 87% for three steps; m.p.: 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.09 (d, J = 8.5 Hz, 2H, Ar-H), 6.85–6.77 (m, 4H, Ar-H), 6.63 (d, J = 8.3 Hz, 2H, Ar-H), 3.85 (s, 2H, CH2), 3.79 (s, 2H, CH2), 3.76 (s, 3H, O-CH3), 3.13 (s, 3H, N-CH3), 2.39 (t, J = 7.5 Hz, 2H, COCH2), 1.68–1.59 (m, 2H, CH2), 1.30–1.25 (m, 4H, CH2 CH2), 0.84 (t, J = 6.6 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 158.3, 156.0, 130.5, 129.4, 128.7, 127.7, 123.1, 115.9, 114.1, 55.2, 37.7, 31.5, 30.8, 30.0, 28.2, 25.6, 22.5, 14.0; HRMS (ESI) calcd. for C25H32N3O3+ [M + H]+ 422.2438, found 422.2440.
For 1g: White powder; Yield 68% for three steps; m.p.: 172–174 °C; 1H NMR (400 MHz, CDCl3) δ 9.57 (s, 1H, NH), 7.84 (d, J = 7.3 Hz, 2H, Ar-H), 7.48–7.35 (m, 3H, Ar-H), 7.03 (d, J = 8.6 Hz, 2H, Ar-H), 6.91 (d, J = 8.5 Hz, 2H, Ar-H), 6.85 (d, J = 8.6 Hz, 2H, Ar-H), 6.78 (d, J = 8.6 Hz, 2H, Ar-H), 3.81 (s, 3H, O-CH3), 3.76 (s, 4H, CH2), 3.07 (s, 3H, N-CH3); 13C NMR (100 MHz, DMSO-d6) δ 157.7, 156.0, 145.2, 144.8, 131.3, 130.9, 129.1, 128.9, 128.5, 127.3, 125.5, 121.5, 121.4, 115.3, 113.7, 55.0, 28.7, 27.8, 27.0; HRMS (ESI) calcd. for C25H26N3O4S+ [M + H]+ 464.1639, found 464.1632.
For 1h: White powder; Yield 57% for three steps; m.p.: 207–209 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.16 (s, 1H), 9.28 (s, 1H), 8.09 (s, 2H, Ar-H), 7.43 (s, 3H, Ar-H), 7.20 (d, J = 7.6 Hz, 2H, Ar-H), 6.91 (d, J = 7.8 Hz, 2H, Ar-H), 6.85 (d, J = 7.7 Hz, 2H, Ar-H), 6.68 (d, J = 7.3 Hz, 2H, Ar-H), 3.93 (s, 4H, CH2), 3.71 (s, 3H, O-CH3), 3.25 (s, 3H, N-CH3); 13C NMR (100 MHz, DMSO-d6) δ 157.7, 155.9, 131.4, 130.6, 129.4, 129.0, 128.4, 128.2, 127.8, 127.6, 115.4, 113.8, 55.0, 28.9, 27.2; HRMS (ESI) calcd. for C26H26N3O3+ [M + H]+ 428.1969, found 428.1971.
For 1i: White powder; Yield 59% for three steps; m.p.: 153–154 °C; 1H NMR (400 MHz, CDCl3) δ 10.47 (s, 1H), 7.08 (d, J = 8.0 Hz, 2H, Ar-H), 6.91 (d, J = 7.9 Hz, 2H, Ar-H), 6.84 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 7.9 Hz, 2H, Ar-H), 3.80 (s, 4H, CH2), 3.79 (s, 3H, O-CH3), 3.15 (s, 3H, N-CH3), 3.02 (s, 3H, S-CH3); 13C NMR (100 MHz, CDCl3) δ 158.7, 155.4, 145.5, 129.3, 128.9, 128.8, 127.5, 121.7, 121.2, 116.0, 114.4, 100.0, 55.4, 42.4, 29.3, 28.0; HRMS (ESI) calcd. for C20H24N3O4S+ [M + H]+ 402.1482, found 402.1481.
General procedure for the preparation of naamines 1j and k. The solution of naamine A (1d, 3.00 mmol), benzaldehyde (12.36 mmol) and acetic acid (0.5 mL) in ethanol (120 mL) was refluxed for 12 h, evaporated part of ethanol, filtered to give 20. The mixture of 20 and NaBH4 (6.5 mmol) in ethanol (100 mL) was stirred at 65 °C for 2 h, quenched with H2O (10 mL), and then concentrated. The residue was purified by column chromatography on silica gel to give naamines 1j and 1k.
For 1j: White powder; Yield 16%; m.p.: 226 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.18 (s, 1H, OH), 7.37 (d, J = 7.6 Hz, 2H, Ar-H), 7.33–7.19 (m, 3H, Ar-H), 7.12 (d, J = 8.2 Hz, 2H, Ar-H), 6.84 (d, J = 7.9 Hz, 2H, Ar-H), 6.77 (d, J = 8.2 Hz, 2H, Ar-H), 6.62 (d, J = 7.9 Hz, 2H, Ar-H), 5.88 (t, J = 5.8 Hz, 1H, NHCH2), 4.32 (d, J = 5.8 Hz, 2H, NHCH2), 3.71 (s, 2H, CH2), 3.69 (s, 3H, O-CH3), 3.63 (s, 2H, CH2), 3.03 (s, 3H, N-CH3); 13C NMR (101 MHz, DMSO-d6) δ 157.1, 155.5, 148.8, 140.7, 133.8, 131.5, 129.7, 129.3, 128.8, 128.0, 127.7, 126.5, 120.6, 115.1, 113.3, 54.9, 46.5, 31.9, 28.6, 27.8; HRMS (ESI) calcd. for C26H28N3O2+ [M + H]+ 414.2176, found 414.2167.
For 1k: White powder; Yield 16%; m.p.: 226–228 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.20 (s, 1H), 7.12 (d, J = 7.9 Hz, 2H, Ar-H), 6.83 (d, J = 7.8 Hz, 2H, Ar-H), 6.77 (d, J = 7.9 Hz, 2H, Ar-H), 6.61 (d, J = 7.8 Hz, 2H, Ar-H), 5.02 (t, J = 6.1 Hz, 1H, NH), 3.69 (s, 5H, O-CH3 and CH2), 3.62 (s, 2H, CH2), 3.02 (s, 3H, N-CH3), 2.96 (d, J = 6.1 Hz, 2H, NHCH2), 0.88 (s, 9H, CCH3); 13C NMR (100 MHz, DMSO-d6) δ 157.1, 155.4, 149.5, 133.8, 131.2, 129.8, 129.2, 128.8, 120.4, 115.0, 113.3, 54.9, 54.3, 31.8, 31.8, 28.6, 27.8, 27.4; HRMS (ESI) calcd. for C24H32N3O2+ [M + H]+ 394.2489, found 394.2483.
Synthesis of 4,5-bis(4-methoxybenzyl)-1,3-dimethyl-1H-imidazol-2(3H)-one (1l). To the solution of 1d (0.50 g, 1.54 mmol) in THF (100 mL) was added 70% NaH (12.36 mmol) and methyliodide (12.36 mmol) successively at 0 °C under argon. Then the mixture was stirred at 70 °C for 24 h, quenched with H2O (10 mL), concentrated, acidified to pH 4–5 with dilute hydrochloric acid and extracted with dichloromethane. The combined organic layer was washed successively with saturated aqueous NaHCO3 solution (100 mL), H2O (100 mL), and brine (100 mL), then dried over MgSO4 anhydrous, filtered and concentrated. The residue was purified by column chromatography on silica gel to give 1l (0.37 g, 68%) as a white powder. m.p.: 157–159 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.08 (d, J = 8.6 Hz, 4H, Ar-H), 6.86 (d, J = 8.6 Hz, 4H, Ar-H), 3.84 (s, 4H, CH2), 3.72 (s, 6H, O-CH3), 2.89 (s, 6H, N-CH3); 13C NMR (100 MHz, CDCl3) δ 158.4, 153.8, 129.9, 128.8, 117.5, 114.1, 55.3, 28.3, 27.8; HRMS (ESI) calcd. for C21H25N2O3+ [M + H]+ 353.1860, found 353.1879.
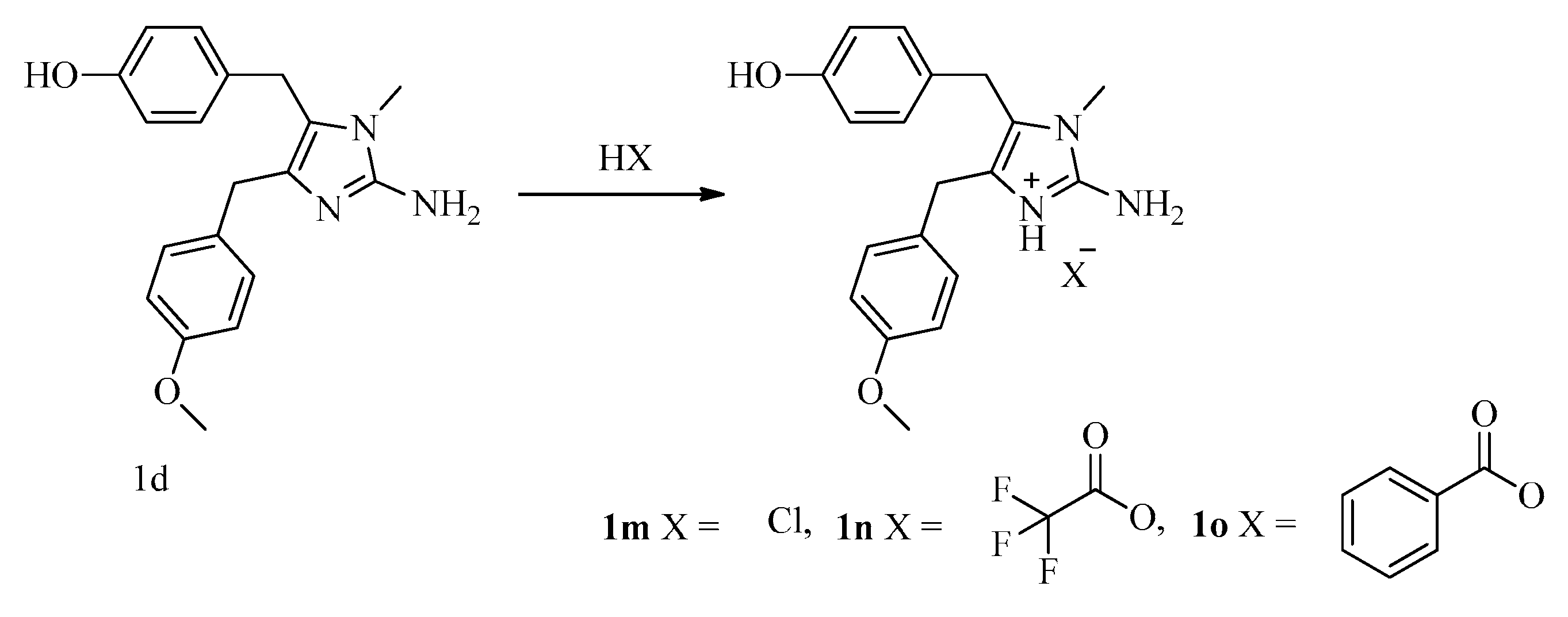
General procedure for the preparation of naamines 1m–o. The solution of 1d (0.35 g, 1.08 mmol) and corresponding acids (2.16 mmol) in methanol (100 mL) was stirred at 50 °C for 2 h and concentrated. Then, acetone (5 mL) and petroleum ether (5 mL) were added, filtered to give 1m–o.
For 1m: White powder; Yield 98%; m.p.: 45–47 °C; 1H NMR (400 MHz, DMSO) δ 12.14 (s, 1H, OH), 9.37 (s, 1H, NH), 7.47 (s, 2H, NH2), 7.15 (d, J = 8.7 Hz, 2H, Ar-H), 6.91 (d, J = 8.5 Hz, 2H, Ar-H), 6.86 (d, J = 8.7 Hz, 2H, Ar-H), 6.69 (d, J = 8.5 Hz, 2H, Ar-H), 3.86 (s, 2H, CH2), 3.81 (s, 2H, CH2), 3.72 (s, 3H, O-CH3), 3.15 (s, 3H, N-CH3); 13C NMR (101 MHz, DMSO) δ 157.9, 156.1, 146.1, 130.2, 129.4, 128.9, 127.1, 122.2, 121.7, 115.4, 113.9, 55.1, 29.5, 27.9, 26.6.
For 1n: White powder; Yield 98%; m.p.: 123–125 °C; 1H NMR (400 MHz, DMSO) δ 12.21 (s, 1H, OH), 9.36 (s, 1H, NH), 7.47 (s, 2H, NH2), 7.14 (d, J = 8.5 Hz, 2H, Ar-H), 6.92 (d, J = 8.3 Hz, 2H, Ar-H), 6.86 (d, J = 8.5 Hz, 2H, Ar-H), 6.68 (d, J = 8.3 Hz, 2H, Ar-H), 3.87 (s, 2H, CH2), 3.81 (s, 2H, CH2), 3.72 (s, 3H, O-CH3), 3.15 (s, 3H, N-CH3); 13C NMR (100 MHz, DMSO) δ 158.8 (q, J = 32.7 Hz), 158.0, 156.1, 146.2, 130.1, 129.3, 129.0, 127.1, 122.2, 121.7, 118.2, 115.4, 113.9, 55.0, 29.3, 28.0, 26.6.
For 1o: White powder; Yield 58%; m.p.: 172–174 °C; 1H NMR (400 MHz, DMSO) δ 7.94 (d, J = 7.4 Hz, 2H, Ar-H), 7.54–7.40 (m, 3H, Ar-H), 7.20 (d, J = 8.3 Hz, 2H, Ar-H), 6.95–6.84 (m, 4H, Ar-H and NH2), 6.80 (d, J = 8.3 Hz, 2H, Ar-H), 6.66 (d, J = 8.2 Hz, 2H, Ar-H), 3.81 (s, 2H, CH2), 3.70 (s, 2H, CH2), 3.69 (s, 3H, O-CH3), 3.06 (s, 3H, N-CH3); 13C NMR (100 MHz, DMSO) δ 170.5, 158.1, 156.3, 148.3, 132.0, 131.4, 130.0, 129.6, 129.4, 128.7, 128.4, 121.1, 115.8, 114.1, 55.5, 30.0, 29.4, 27.5.
Synthesis of naamidine-metal complex 2e. The mixture of 2d (0.50 g, 1.15 mmol), ZnSO4·7H2O (69 mmol) in H2O (200 mL) and dichloromethane (100 mL) was stirred at room temperature for 3 h, filtered to give 2e. Yellow powder; Yield 38%; m.p.: 185–187 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.30 (s, 2H, OH), 6.95 (d, J = 8.2 Hz, 4H, Ar-H), 6.69 (d, J = 8.2 Hz, 4H, Ar-H), 6.50 (s, 8H, Ar-H), 4.04–3.90 (m, 4H, CH2), 3.82 (d, J = 16.7 Hz, 2H, CH2), 3.62 (s, 6H, O-CH3), 3.57 (s, 6H, N-CH3), 3.26 (d, J = 16.7 Hz, 2H, CH2), 2.80 (s, 6H, N-CH3); 13C NMR (100 MHz, DMSO-d6) δ 163.7, 160.5, 157.3, 155.9, 153.2, 146.8, 132.0, 129.9, 129.0, 128.4, 128.3, 127.7, 115.4, 113.0, 54.7, 30.2, 29.9, 27.4, 24.0; HRMS (ESI) calcd. for C46H45N10O8Zn+ [M + H]+ 929.2708, found 929.2709.