Characterization of a Novel PolyM-Preferred Alginate Lyase from Marine Vibrio splendidus OU02
Abstract
:1. Introduction
2. Results and Discussions
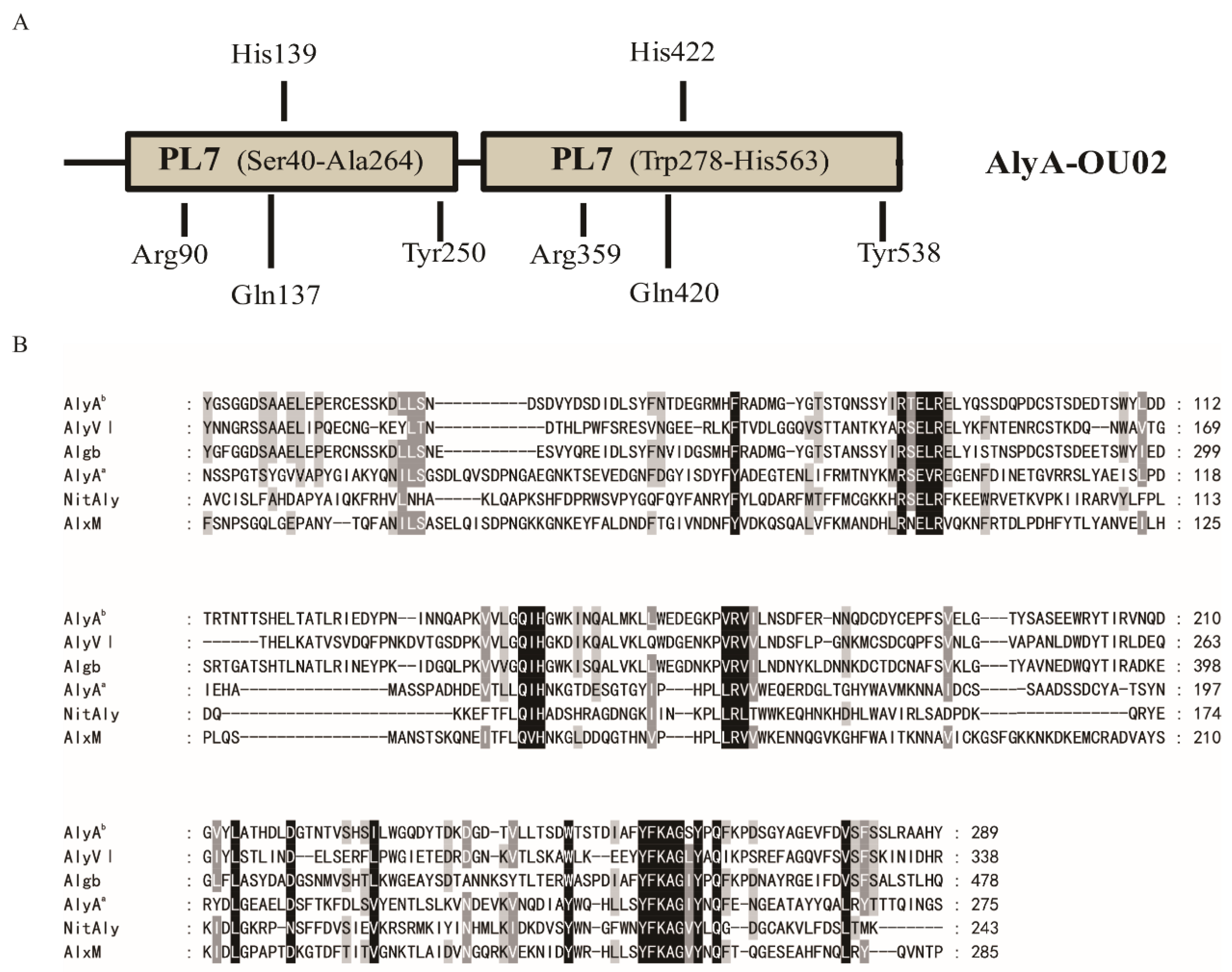
2.1. Sequence Analysis of AlyA-OU02
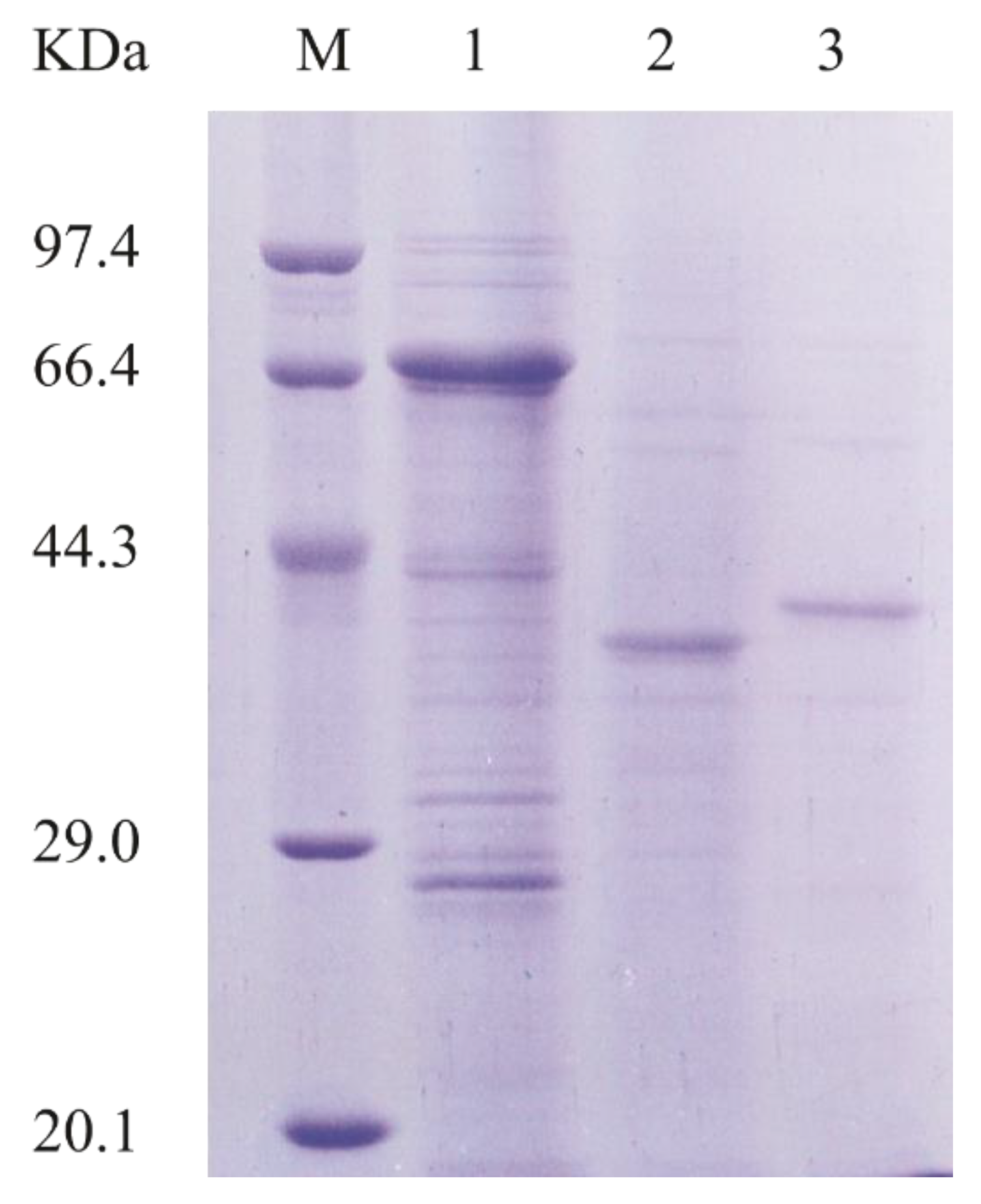
2.2. Cloning, Expression, and Purification of Different Versions of AlyA-OU02
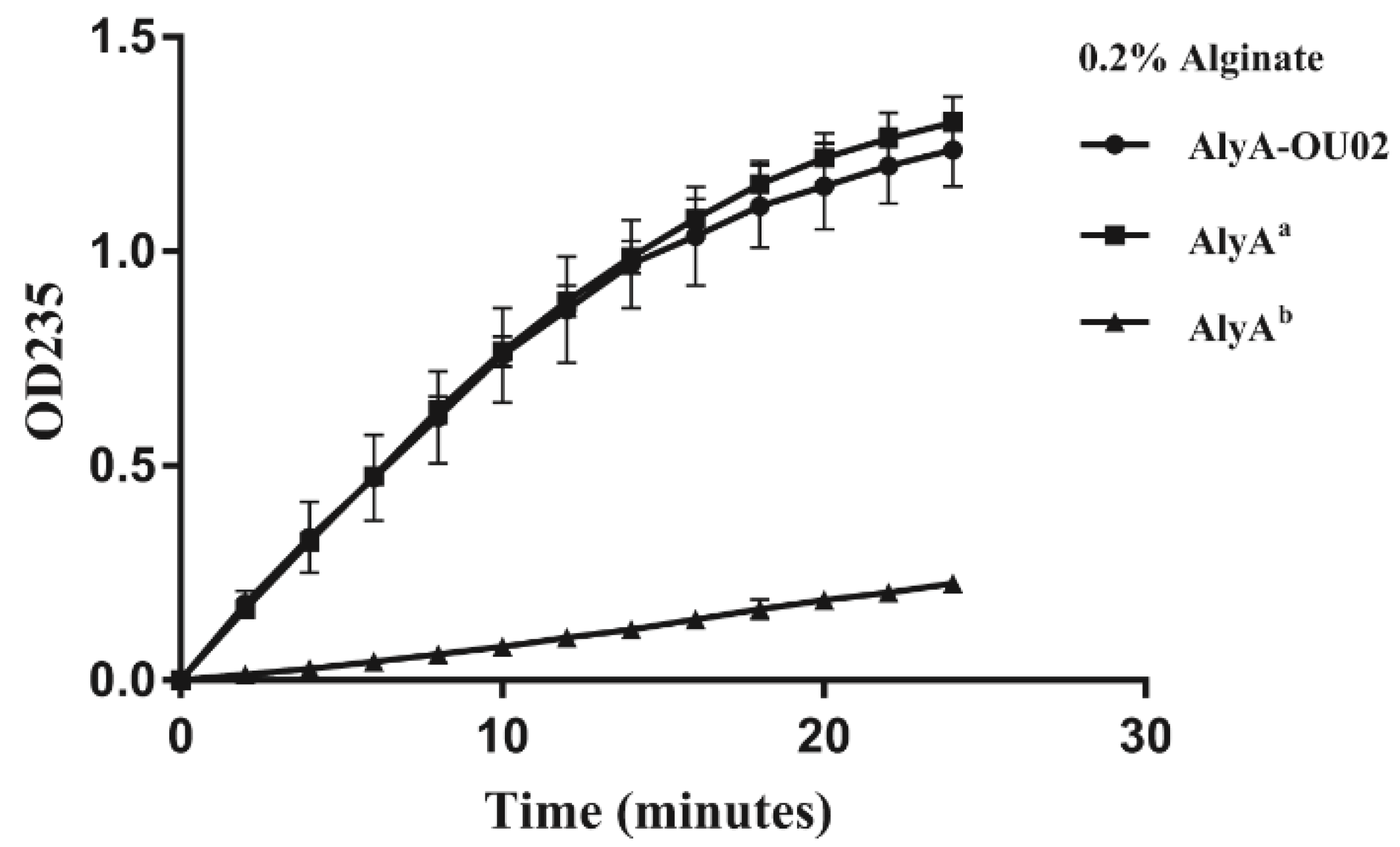
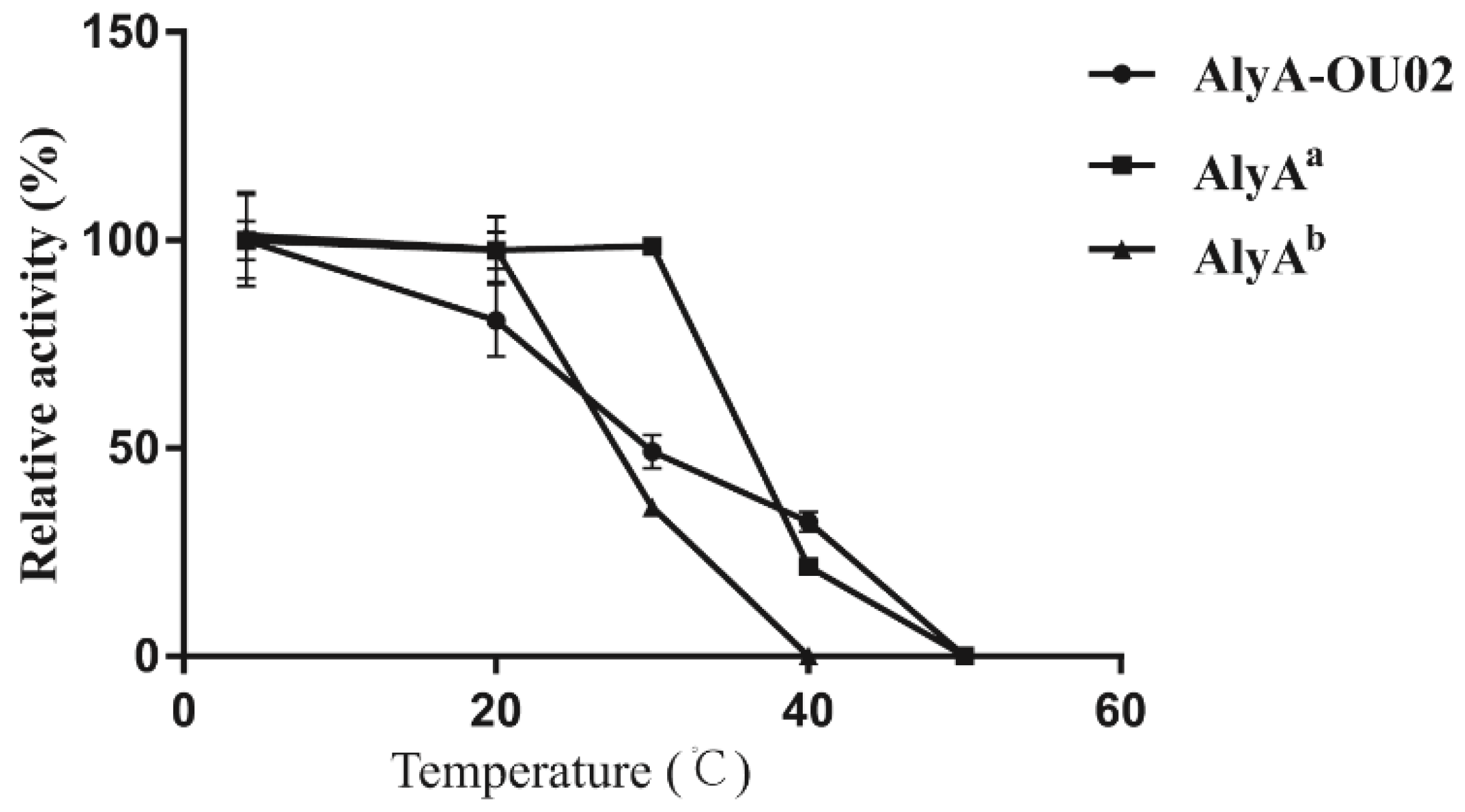
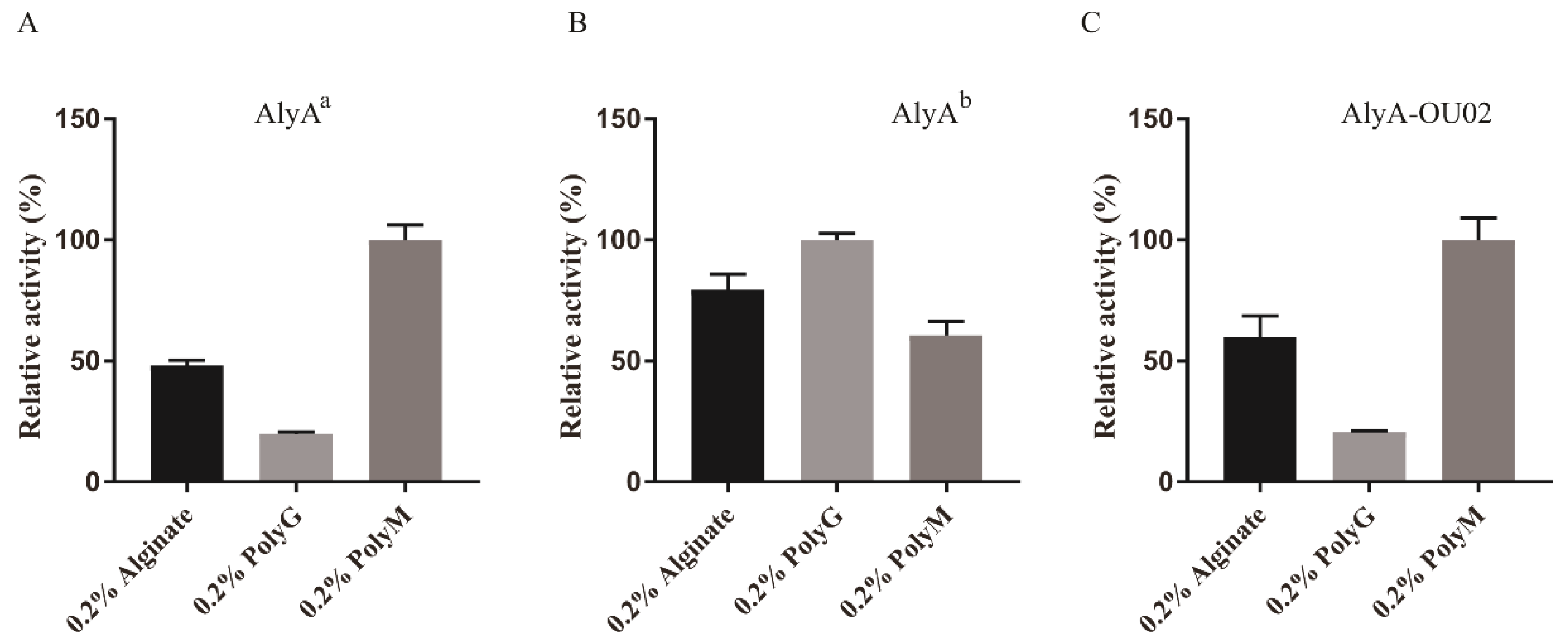
2.3. Enzymatic Activity Measurements for AlyA-OU02, AlyAa, and AlyAb
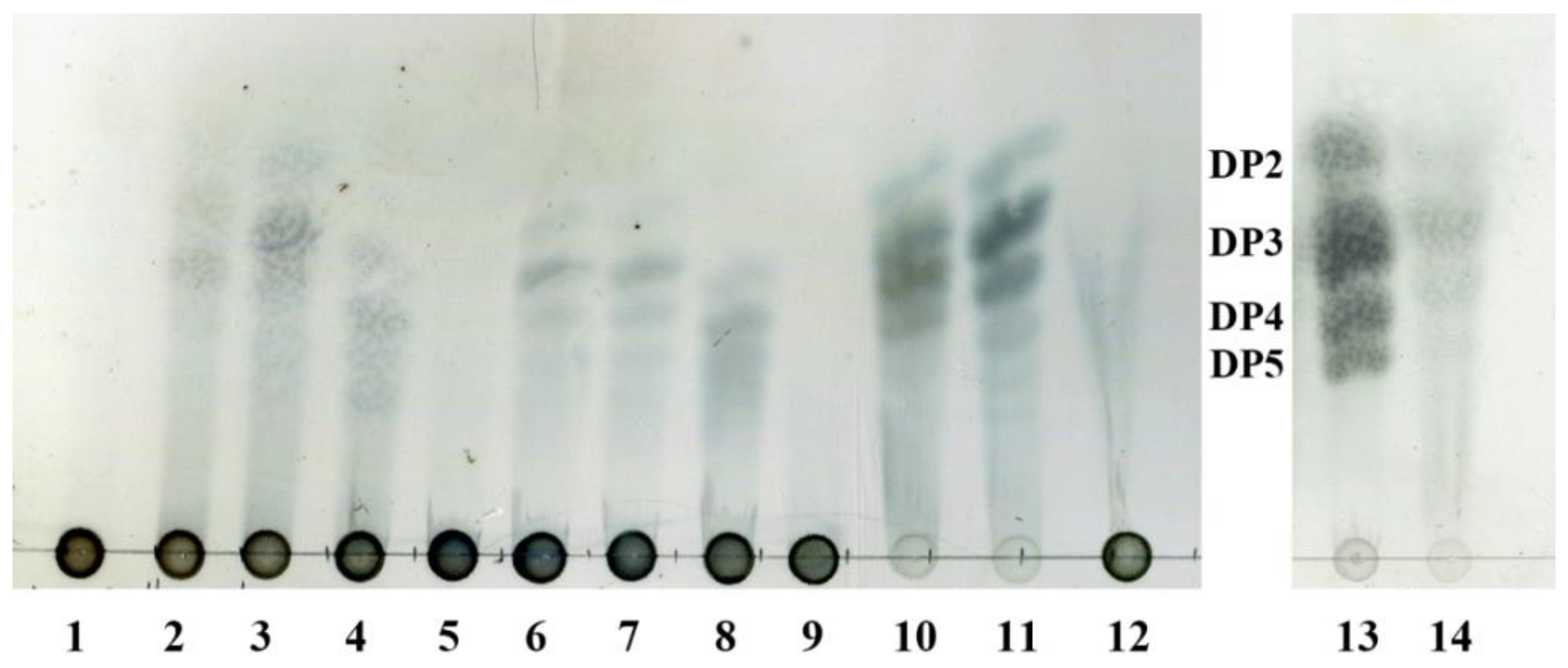
2.4. Thin-Layer Chromatography Analysis of the Degradation Products
3. Materials and Methods
3.1. Materials
3.2. Sequence Analysis of AlyA-OU02
3.3. Construction of Expression Vectors
3.4. Expression and Purification of AlyA-OU02, AlyAa and AlyAb
3.5. Enzymatic Activity Assay
3.6. TLC Analysis of the Degradation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- An, Q.D.; Zhang, G.L.; Wu, H.T.; Zhang, Z.C.; Zheng, G.S.; Luan, L.; Murata, Y.; Li, X. Alginate-deriving oligosaccharide production by alginase from newly isolated Flavobacterium sp. LXA and its potential application in protection against pathogens. J. Appl. Microbiol. 2009, 106, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Hu, Y.; Zhang, L.; Wang, Y.; Wang, S.; Zhang, Y.; Guo, H.; Ji, D.; Wang, Y. Alginate Oligosaccharide DP5 Exhibits Antitumor Effects in Osteosarcoma Patients following Surgery. Front. Pharmacol. 2017, 8, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertesvag, H. Alginate-modifying enzymes: Biological roles and biotechnological uses. Front. Microbiol. 2015, 6, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Suzuki, K.; Inoue, A.; Ojima, T. A novel oligoalginate lyase from abalone, Haliotis discus hannai, that releases disaccharide from alginate polymer in an exolytic manner. Carbohydr. Res. 2006, 341, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Zhang, K.; Zhu, Q.; Li, Z.; Liu, Y.; Fitzek, E.; Yohe, T.; Zhao, L.; Li, W.; Liu, T.; et al. Structural and biochemical characterization of a multidomain alginate lyase reveals a novel role of CBM32 in CAZymes. Biochim. Biophys. Acta 2018, 1862, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Yamasaki, M.; Mikami, B.; Hashimoto, W.; Murata, K. Substrate recognition by family 7 alginate lyase from Sphingomonas sp. A1. J. Mol. Biol. 2008, 380, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.M.; Miyakawa, T.; Inoue, A.; Nishiyama, R.; Nakamura, A.; Asano, A.; Ojima, T.; Tanokura, M. Structural basis for controlling the enzymatic properties of polymannuronate preferred alginate lyase FlAlyA from the PL-7 family. Chem. Commun. 2018, 54, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, Y.; Kawada, R.; Iwasaki, K.; Kimura, Y.; Oda, T.; Muramatsu, T. Cloning and sequence analysis of a gene (aly PG) encoding poly (alpha-L-guluronate) lyase from Corynebacterium sp. strain ALY-1. J. Biosci. Bioeng. 2000, 89, 199–202. [Google Scholar] [CrossRef]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyaseL: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.M.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, T.; Matsubara, Y.; Muramatsu, T.; Kimura, M.; Kakuta, Y. Crystal structure of the alginate (poly alpha-l-guluronate) lyase from Corynebacterium sp. at 1.2 A resolution. J. Mol. Biol. 2005, 345, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Badur, A.H.; Jagtap, S.S.; Yalamanchili, G.; Lee, J.K.; Zhao, H.; Rao, C.V. Alginate lyases from alginate-degrading Vibrio splendidus 12B01 are endolytic. Appl. Environ. Microbiol. 2015, 81, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Gong, Q.H.; Song, K.; Li, J.B.; Yu, W.G. Cloning, sequence analysis and expression of gene alyVI encoding alginate lyase from marine bacterium Vibrio sp. QY101. DNA Seq. 2004, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Anraku, M.; Nakagawa, S.; Ojima, T. Discovery of a Novel Alginate Lyase from Nitratiruptor sp. SB155-2 Thriving at Deep-sea Hydrothermal Vents and Identification of the Residues Responsible for Its Heat Stability. J. Biol. Chem. 2016, 291, 15551–15563. [Google Scholar] [CrossRef] [PubMed]
- Chavagnat, F.; Heyraud, A.; Colin-Morel, P.; Guinand, M.; Wallach, J. Catalytic properties and specificity of a recombinant, overexpressed D-mannuronate lyase. Carbohydr. Res. 1998, 308, 409–415. [Google Scholar] [CrossRef]
- Yamasaki, M.; Moriwaki, S.; Miyake, O.; Hashimoto, W.; Murata, K.; Mikami, B. Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7: A novel alginate lyase with a beta-sandwich fold. J. Biol. Chem. 2004, 279, 31863–31872. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Miyazaki, M.; Nogi, Y.; Kobayashi, T.; Horikoshi, K. Cloning and sequencing of alginate lyase genes from deep-sea strains of Vibrio and Agarivorans and characterization of a new Vibrio enzyme. Mar. Biotechnol. 2010, 12, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, X.; Zhang, L.; Yu, W.; Han, F. Cloning, Expression, and Characterization of a Cold-Adapted and Surfactant-Stable Alginate Lyase from Marine Bacterium Agarivorans sp. L11. J. Microbiol. Biotechnol. 2015, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, J.; Li, X.; Peng, Q.; Lu, H.; Du, Y. Characterization of a new alginate lyase from newly isolated Flavobacterium sp. S20. J. Ind. Microbiol. Biotechnol. 2013, 40, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Takadono, K.; Nishiyama, R.; Tajima, K.; Kobayashi, T.; Ojima, T. Characterization of an alginate lyase, FlAlyA, from Flavobacterium sp. strain UMI-01 and its expression in Escherichia coli. Mar. Drugs 2014, 12, 4693–4712. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Han, F.; Yu, W. Cloning, sequence analysis, and expression of gene alyPI encoding an alginate lyase from marine bacterium Pseudoalteromonas sp. CY24. Can. J. Microbiol. 2009, 55, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Miyake, O.; Ochiai, A.; Hashimoto, W.; Murata, K. Origin and diversity of alginate lyases of families PL-5 and -7 in Sphingomonas sp. strain A1. J. Bacteriol. 2004, 186, 2891–2896. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Lee, E.Y.; Kim, H.S. Cloning and characterization of alginate lyase from a marine bacterium Streptomyces sp. ALG-5. Mar. Biotechnol. 2009, 11, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Baik, K.S.; Park, S.C.; Choe, H.N.; Seong, C.N.; Shin, T.S.; Woo, H.C.; Cho, J.Y.; Kim, D. Characterization of alginate lyase gene using a metagenomic library constructed from the gut microflora of abalone. J. Ind. Microbiol. Biotechnol. 2012, 39, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, Q.; Lu, M.; Xu, C.; Li, F.; Zhang, R.; Liao, W.; Huang, S. AlgM4: A New Salt-Activated Alginate Lyase of the PL7 Family with Endolytic Activity. Mar. Drugs 2018, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, Y.; Ni, F.; Ning, L.; Yao, Z. Characterization of a new endo-type alginate lyase from Vibrio sp. NJU-03. Int. J. Biol. Macromol. 2018, 108, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Hu, F.; Yuan, H.; Sun, Y.; Yao, Z. Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07. Mar. Drugs 2018, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Isobe, N.; Itabashi, N.; Fujise, A.; Ohshiro, T. Characterization of a Long-Lived Alginate Lyase Derived from Shewanella Species YH1. Mar. Drugs 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; Shi, H.; Zhou, J.; Tan, Z.; Yuan, M.; Yao, P.; Liu, X. Characterization of a Novel Alginate Lyase from Marine Bacterium Vibrio furnissii H1. Mar. Drugs 2018, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Q.; Lu, D.; Han, W.; Li, F. A Novel Bifunctional Endolytic Alginate Lyase with Variable Alginate-Degrading Modes and Versatile Monosaccharide-Producing Properties. Front. Microbiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Mashino, C.; Uji, T.; Saga, N.; Mikami, K.; Ojima, T. Characterization of an Eukaryotic PL-7 Alginate Lyase in the Marine Red Alga Pyropia yezoensis. Curr. Biotechnol. 2015, 4, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Sun, Y. Purification and Characterization of a Novel Alginate Lyase from the Marine Bacterium Bacillus sp. Alg07. Mar. Drugs 2018, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Gu, J.; Cheng, Y.; Liu, H.; Li, Y.; Li, F. Novel Alginate Lyase (Aly5) from a Polysaccharide-Degrading Marine Bacterium, Flammeovirga sp. Strain MY04: Effects of Module Truncation on Biochemical Characteristics, Alginate Degradation Patterns, and Oligosaccharide-Yielding Properties. Appl. Environ. Microbiol. 2016, 82, 364–374. [Google Scholar] [CrossRef] [PubMed]


| Protein Name | Optimal pH/Temperature (°C) | Conserved Region QIH/QVH | Substrate Specificity | Reference |
|---|---|---|---|---|
| AlyL1 | 8.6/40 | QIH | PM, PG | [25] |
| Alg2A | 8.5/45 | QIH | PG | [26] |
| FlAlyA | 7.8/55 | QIH | PM | [27] |
| AlyPI | 7.0/40 | QIH | PM, PG | [28] |
| AI-II′ | 7.5/40 | QIH | PM, PG, PMG | [29] |
| ALG-5 | 8.0/30 | QIH | PG | [30] |
| AlyDW11 | 7.0/45 | QIH | PM | [31] |
| A9MT | 7.5/30 | QVH | PM | [24] |
| AlgM4 | 8.5/30 | QIH | PM, PG | [32] |
| AlgNJU-03 | 7.0/30 | QIH | PM, PG | [33] |
| AlgNJ-07 | 9.0/40 | - | PM | [34] |
| rAlgSV1-PL7 | 8.0/45 | QVH | PM, PG, PMG | [35] |
| AlyH1 | 7.5/40 | QIH | PM, PG | [36] |
| Aly2 | 6.0/40 | QIH | PG | [37] |
| Algb | 8.0/30 | QIH | PM, PG | [20] |
| PyAly | 8.0/35 | QVH | PM | [38] |
| AlyVI | 7.5/40 | QIH | PM, PG | [19] |
| NitAly | 6.0/70 | QIH | PM | [21] |
| AlxM | -/- | QVH | PM | [22] |
| Specific Activity (U/mg) | Alginate | PolyG | PolyM |
|---|---|---|---|
| AlyA-OU02 | 119 | 41 | 198 |
| AlyAa | 252 | 104.5 | 520 |
| AlyAb | 24 | 33 | 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, J.; Zhang, K.; Liu, X.; Liu, W.; Lyu, Q.; Ji, A. Characterization of a Novel PolyM-Preferred Alginate Lyase from Marine Vibrio splendidus OU02. Mar. Drugs 2018, 16, 295. https://doi.org/10.3390/md16090295
Zhuang J, Zhang K, Liu X, Liu W, Lyu Q, Ji A. Characterization of a Novel PolyM-Preferred Alginate Lyase from Marine Vibrio splendidus OU02. Marine Drugs. 2018; 16(9):295. https://doi.org/10.3390/md16090295
Chicago/Turabian StyleZhuang, Jingjing, Keke Zhang, Xiaohua Liu, Weizhi Liu, Qianqian Lyu, and Aiguo Ji. 2018. "Characterization of a Novel PolyM-Preferred Alginate Lyase from Marine Vibrio splendidus OU02" Marine Drugs 16, no. 9: 295. https://doi.org/10.3390/md16090295





