Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe
Abstract
:1. Introduction
2. Structural Characterization and Biological Activity
2.1. Asparagopsis
2.2. Caulerpa
2.3. Sargassum
2.4. Chromenes
2.5. Other Compounds
3. Biological Activity of Extracts
3.1. Asparagopsis sp.
3.2. Caulerpa sp.
3.3. Sargassum sp.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1A9 | human ovarian cancer |
| A549 | human lung carcinoma |
| Aβ25–35 | amyloid-peptide fragment 25–35 |
| CDC25B | cell division cycle 25 homolog B |
| DPPA | 1,1-diphenyl-2-picrylhydrazyl |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ECD | electronic circular dichroism |
| EC | Effective concentration |
| ED | effective dose |
| EGCG | epigallocatechin gallate |
| GSH | glutathione |
| HCT8 | human ilececal cancer |
| HIV- | human immunodeficiency virus |
| HL60 | promyelocytic leukemia cells |
| HOS | human bone tumor |
| HSV-1 | herpes simplex virus 1 |
| IC | inhibitory concentration |
| LAR | leukocyte antigen-related phosphatase |
| LXR | liver X receptor |
| MCF-7 | breast adenocarcinoma |
| MIC | minimum inhibitory concentration |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| P388 | mouse lymphocytic leukemia |
| PC3 | human prostate cancer |
| PPARs | Peroxisome proliferator-activated receptors |
| PTP1B | protein tyrosine phosphatase 1B |
| PTPs | protein phosphatases |
| ROS | reactive oxygen species |
| SHP-1 | src homology phosphatase-1 |
| SHP-2 | src homology phosphatase-2 |
| SH-SY5Y | neuroblastoma cell line |
| SK13 | Gram-positive spore-forming bacteria requiring Mn for growth |
| TCPTP | T-cell PTP |
References
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAS)—A Strategy and Practical Guide for Managers; IUCN Centre for Mediterranean Cooperation: Gland, Switzerland, 2013. [Google Scholar]
- Groeneveld, R.A.; Bartelings, H.; Börger, T.; Bosello, F.; Buisman, E.; Delpiazzo, E.; Eboli, F.; Fernandes, J.A.; Hamon, K.G.; Hattam, C.; et al. Economic impacts of marine ecological change: Review and recent contributions of the vectors project on european marine waters. Estuar. Coast. Shelf Sci. 2018, 201, 152–163. [Google Scholar] [CrossRef]
- Padilla, D.K.; Williams, S.L. Beyond ballast water: Aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2004, 2, 131–138. [Google Scholar] [CrossRef]
- Inderjit; Chapman, D.; Ranellet, M.; Kaushik, S. Invasive marine algae: An ecological perspective. Bot. Rev. 2006, 72, 153–178. [Google Scholar]
- Schaffelke, B.; Smith, J.E.; Hewitt, C.L. Introduced macroalgae—A growing concern. J. Appl. Phycol. 2006, 18, 529–541. [Google Scholar] [CrossRef]
- Schaffelke, B.; Hewitt, C.L. Impacts of introduced seaweeds. Bot. Mar. 2007, 50, 397–417. [Google Scholar] [CrossRef]
- Valentine, J.P.; Magierowski, R.H.; Johnson, C.R. Mechanisms of invasion: Establishment, spread and persistence of introduced seaweed populations. Bot. Mar. 2007, 50, 351–360. [Google Scholar] [CrossRef]
- Williams, S.L.; Grosholz, E.D. The invasive species challenge in estuarine and coastal environments: Marrying management and science. Estuar. Coasts 2008, 31, 3–20. [Google Scholar] [CrossRef]
- Anderson, L.W.J. Control of invasive seaweeds. Bot. Mar. 2007, 50, 418–437. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Bio-Technol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The mediterranean red alga Asparagopsis: A source of compounds against leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ní Chualáin, F.; Maggs, C.A.; Saunders, G.W.; Guiry, M.D. The invasive genus Asparagopsis (bonnemaisoniaceae, rhodophyta): Molecular systematics, morphology, and ecophysiology of falkenbergia isolates. J. Phycol. 2004, 40, 1112–1126. [Google Scholar] [CrossRef]
- Dijoux, L.; Viard, F.; Payri, C. The more we search, the more we find: Discovery of a new lineage and a new species complex in the genus Asparagopsis. PLoS ONE 2014, 9, e103826. [Google Scholar] [CrossRef] [PubMed]
- Burreson, B.J.; Moore, R.E.; Roller, P. Haloforms in essential oil of alga Asparagopsis-taxiformis (rhodophyta). Tetrahedron Lett. 1975, 473–476. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile halogen compounds in alga Asparagopsis-taxiformis (rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetamides, but-3-en-2-ols, and isopropanols from Asparagopsis taxiformis (delile) trev. Tetrahedron 1976, 32, 2843–2846. [Google Scholar] [CrossRef]
- McConnell, O.; Fenical, W. Halogen chemistry of red alga Asparagopsis. Phytochemistry 1977, 16, 367–374. [Google Scholar] [CrossRef]
- Combaut, G.; Bruneau, Y.; Teste, J.; Codomier, L. Halogen compounds from a red alga, Falkenbergia-rufolanosa, tetrasporophyte of Asparagopsis armata. Phytochemistry 1978, 17, 1661–1663. [Google Scholar] [CrossRef]
- Woolard, F.X.; Moore, R.E.; Roller, P.P. Halogenated acetic and acrylic acids from the red alga Asparagopsis taxiformis. Phytochemistry 1979, 18, 617–620. [Google Scholar] [CrossRef]
- Abrahamsson, K.; Ekdahl, A.; Collen, J.; Pedersen, M. Marine algae—A source of trichloroethylene and perchloroethylene. Limnol. Oceanogr. 1995, 40, 1321–1326. [Google Scholar] [CrossRef]
- Marshall, R.A.; Harper, D.B.; McRoberts, W.C.; Dring, M.J. Volatile bromocarbons produced by falkenbergia stages of Asparagopsis spp. (rhodophyta). Limnol. Oceanogr. 1999, 44, 1348–1352. [Google Scholar] [CrossRef]
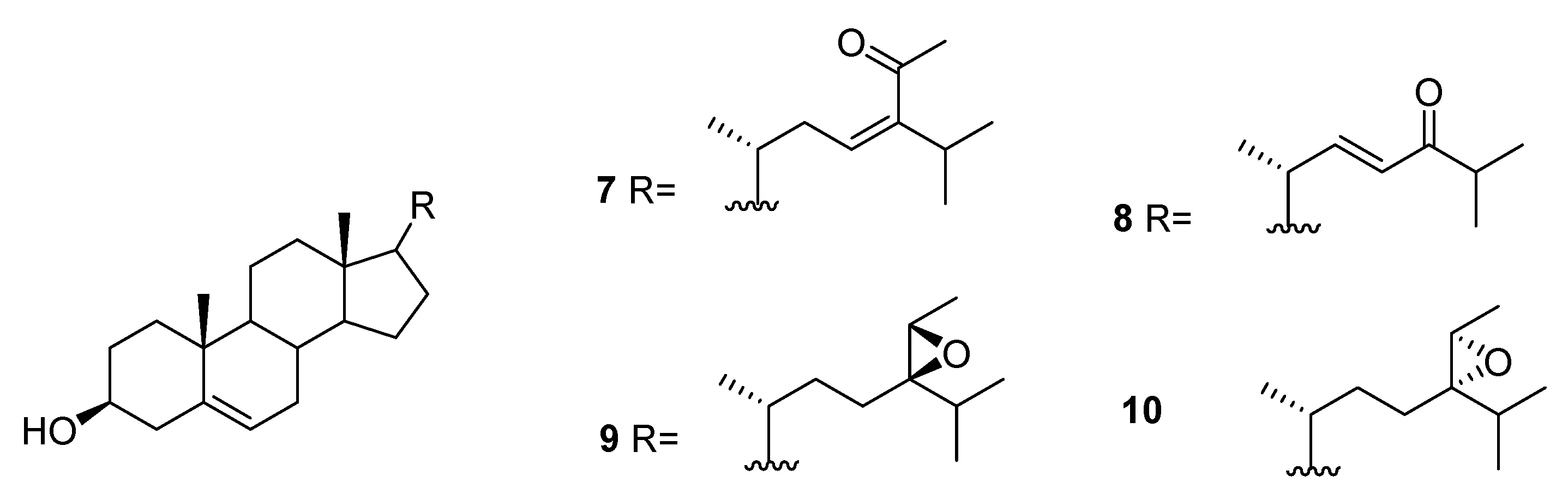
- Combaut, G.; Bruneau, Y.; Codomier, L.; Teste, J. Comparative sterols composition of the red alga Asparagopsis armata and its tetrasporophyte Falkenbergia rufolanosa. J. Nat. Prod. 1979, 42, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Bernardo, J.; Andrade, P.B.; Valentao, P.; Ferreres, F.; Mouga, T. Sterol profiles in 18 macroalgae of the portuguese coast. J. Phycol. 2011, 47, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Francisco, C.; Combaut, G.; Teste, J.; Tarchini, C.; Djerassi, C. Side chain-hydroxylated sterols of the red alga Asparagopsis armata—Significant products or artifacts due to autoxidation. Steroids 1979, 34, 163–169. [Google Scholar] [CrossRef]
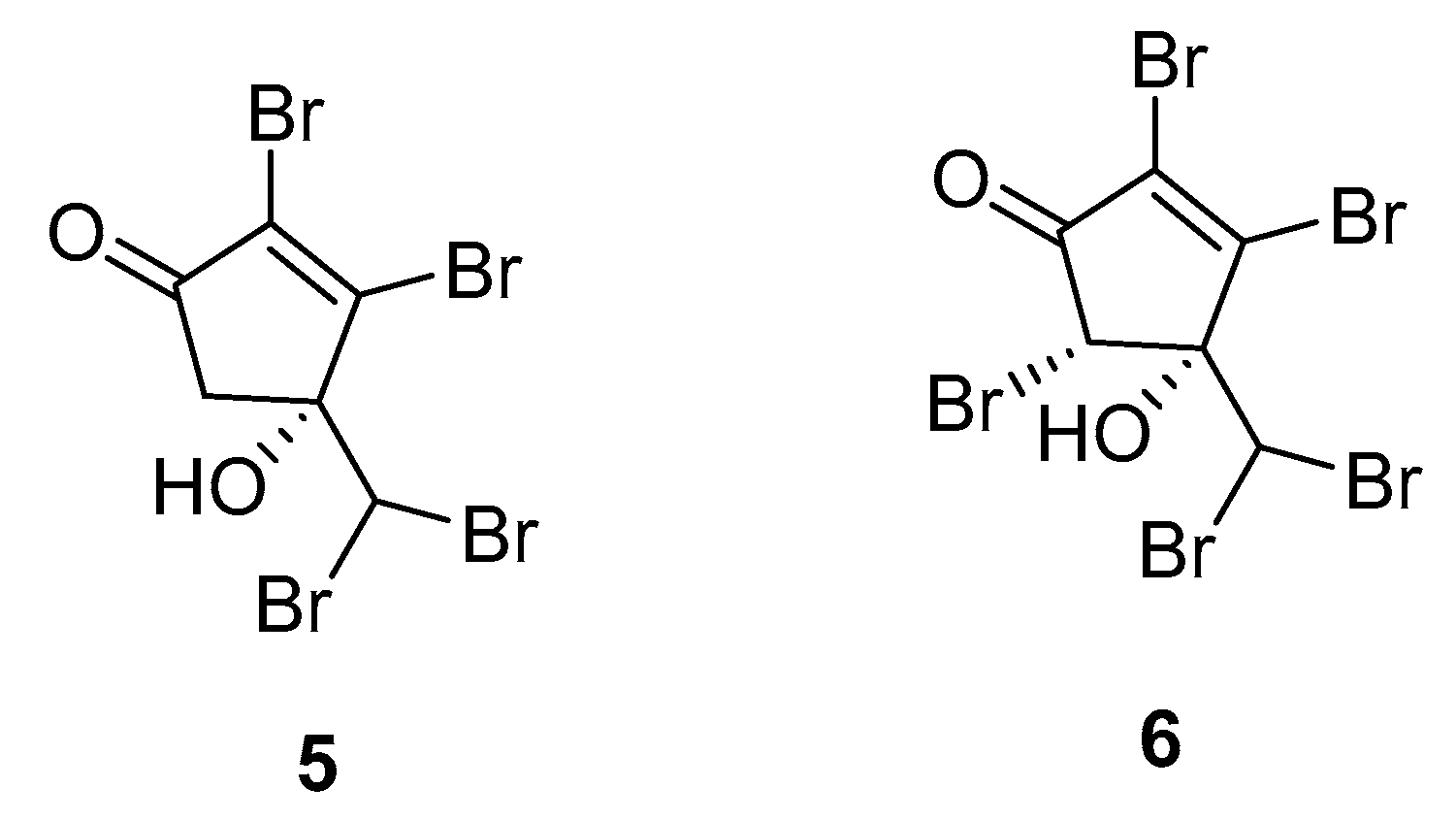
- Greff, S.; Zubia, M.; Genta-Jouve, G.; Massi, L.; Perez, T.; Thomas, O.P. Mahorones, highly brominated cyclopentenones from the red alga Asparagopsis taxiformis. J. Nat. Prod. 2014, 77, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Žuljević, A.; Thibaut, T.; Despalatović, M.; Cottalorda, J.-M.; Nikolić, V.; Cvitković, I.; Antolić, B. Invasive alga Caulerpa racemosa var. Cylindracea makes a strong impact on the mediterranean sponge sarcotragus spinosulus. Biol. Invasions 2011, 13, 2303–2308. [Google Scholar] [CrossRef]
- Kersting, D.K.; Ballesteros, E.; De Caralt, S.; Linares, C. Invasive macrophytes in a marine reserve (columbretes islands, nw mediterranean): Spread dynamics and interactions with the endemic scleractinian coral cladocora caespitosa. Biol. Invasions 2014, 16, 1599–1610. [Google Scholar] [CrossRef]
- Klein, J.; Verlaque, M. The Caulerpa racemosa invasion: A critical review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Denapoli, L.; Fattorusso, E.; Magno, S.; Mayol, L. 3 squalene derivatives from Caulerpa prolifera. Phytochemistry 1982, 21, 782–784. [Google Scholar] [CrossRef]
- Aliya, R.; Shameel, M. Marine natural products of Caulerpa (siphonocladophyceae). Pak. J. Bot. 2003, 35, 659–669. [Google Scholar]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-H.; Liu, D.-Q.; Liang, T.-J.; Yu, X.-Q.; Feng, M.-T.; Yao, L.-G.; Fang, Y.; Wang, B.; Feng, L.-H.; Zhang, M.-X.; et al. Caulerprenylols a and b, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorg. Med. Chem. Lett. 2013, 23, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Q.; Mao, S.-C.; Zhang, H.-Y.; Yu, X.-Q.; Feng, M.-T.; Wang, B.; Feng, L.-H.; Guo, Y.-W. Racemosins a and b, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Santos, G. Caulerpin, a new red pigment from green algae of genus Caulerpa. J. Chem. Soc. C Org. 1970, 6, 842–843. [Google Scholar] [CrossRef]
- Capon, R.J.; Ghisalberti, E.L.; Jefferies, P.R. New sesquiterpenes from Caulerpa flexilis var. Muelleri. Aust. J. Chem. 1981, 34, 1775–1778. [Google Scholar] [CrossRef]
- Guerriero, A.; Meinesz, A.; D’Ambrosio, M.; Pietra, F. Isolation of toxic and potentially toxic sesqui- and monoterpenes from the tropical green seaweed Caulerpa taxifolia which has invaded the region of cap martin and monaco. Helv. Chim. Acta 1992, 75, 689–695. [Google Scholar] [CrossRef]
- Amico, V.; Oriente, G.; Piattelli, M.; Trinyali, C.; Fattorusso, E.; Mayno, S.; Mayo, L. Caulerpenyne, an unusual sequiterpenoid from the green alga Caulerpa prolifera. Tetrahedron Lett. 1978, 38, 3593–3596. [Google Scholar] [CrossRef]
- Paul, V.J.; Fenlcal, W. Toxic feeding deterrents from the tropical marine alga cavlerpa bikinensis (chlorophyta). Tetrahedron Lett. 1982, 23, 5017–5020. [Google Scholar] [CrossRef]
- Paul, V.J.; Littler, M.M.; Littler, D.S.; Fenical, W. Evidence for chemical defense in tropical green alga Caulerpa ashmeadii (caulerpaceae: Chlorophyta): Isolation of new bioactive sesquiterpenoids. J. Chem. Ecol. 1987, 13, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Ghisalberti, E.L.; Jefferies, P.R. Metabolites of the green algae, caulerpa species. Phytochemistry 1983, 22, 1465–1467. [Google Scholar] [CrossRef]
- Paul, V.J.; Fenical, W. Diterpenoid metabolites from pacific marine algae of the order caulerpales (chlorophyta). Phytochemistry 1985, 24, 2239–2243. [Google Scholar] [CrossRef]
- Handley, J.T.; Blackman, A.J. Secondary metabolites from the marine alga Caulerpa brownii (chlorophyta). Aust. J. Chem. 2005, 58, 39–46. [Google Scholar] [CrossRef]
- Knoepfflerpeguy, M.; Belsher, T.; Boudouresque, C.F.; Lauret, M. Sargassum-muticum begins to invade the mediterranean. Aquat. Bot. 1985, 23, 291–295. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the mediterranean sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Critchley, A.T. Sargassum-muticum—A taxonomic history including world-wide and western pacific distributions. J. Mar. Biol. Assoc. U. K. 1983, 63, 617–625. [Google Scholar] [CrossRef]
- Critchley, A.T.; Dijkema, R. On the presence of the introduced brown alga Sargassum muticum, attached to commercially imported ostrea-edulis in the sw netherlands. Bot. Mar. 1984, 27, 211–216. [Google Scholar] [CrossRef]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of sargassum species. Pharmacogn. Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.-E.N.; Sowellim, S.Z.A.; El-Hosini, M.S.; Abo-Atia, A. The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium. Z. Naturforsch. Sect. C J. Biosci. 2003, 58c, 333–336. [Google Scholar]
- Tang, H.F.; Yi, Y.H.; Yao, X.S.; Xu, Q.Z.; Zhang, S.Y.; Lin, H.W. Bioactive steroids from the brown alga Sargassum carpophyllum. J. Asian Nat. Prod. Res. 2002, 4, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.-H.; Quan, Y.-C.; Jiang, H.-Y.; Wen, Z.-S.; Qu, Y.-L.; Guan, L.-P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(s)-saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective lxr beta agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Permeh, P.; Saeidnia, S.; Mashinchian-Moradi, A.; Gohari, A.R. Sterols from Sargassum oligocystum, a brown algae from the persian gulf, and their bioactivity. Nat. Prod. Res. 2012, 26, 774–777. [Google Scholar] [CrossRef] [PubMed]
- He, W.-F.; Yao, L.-G.; Liu, H.-L.; Guo, Y.-W. Thunberol, a new sterol from the chinese brown alga Sargassum thunbergii. J. Asian Nat. Prod. Res. 2014, 16, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Ragubeer, N.; Limson, J.L.; Beukes, D.R. Electrochemistry-guided isolation of antioxidant metabolites from Sargassum elegans. Food Chem. 2012, 131, 286–290. [Google Scholar] [CrossRef]
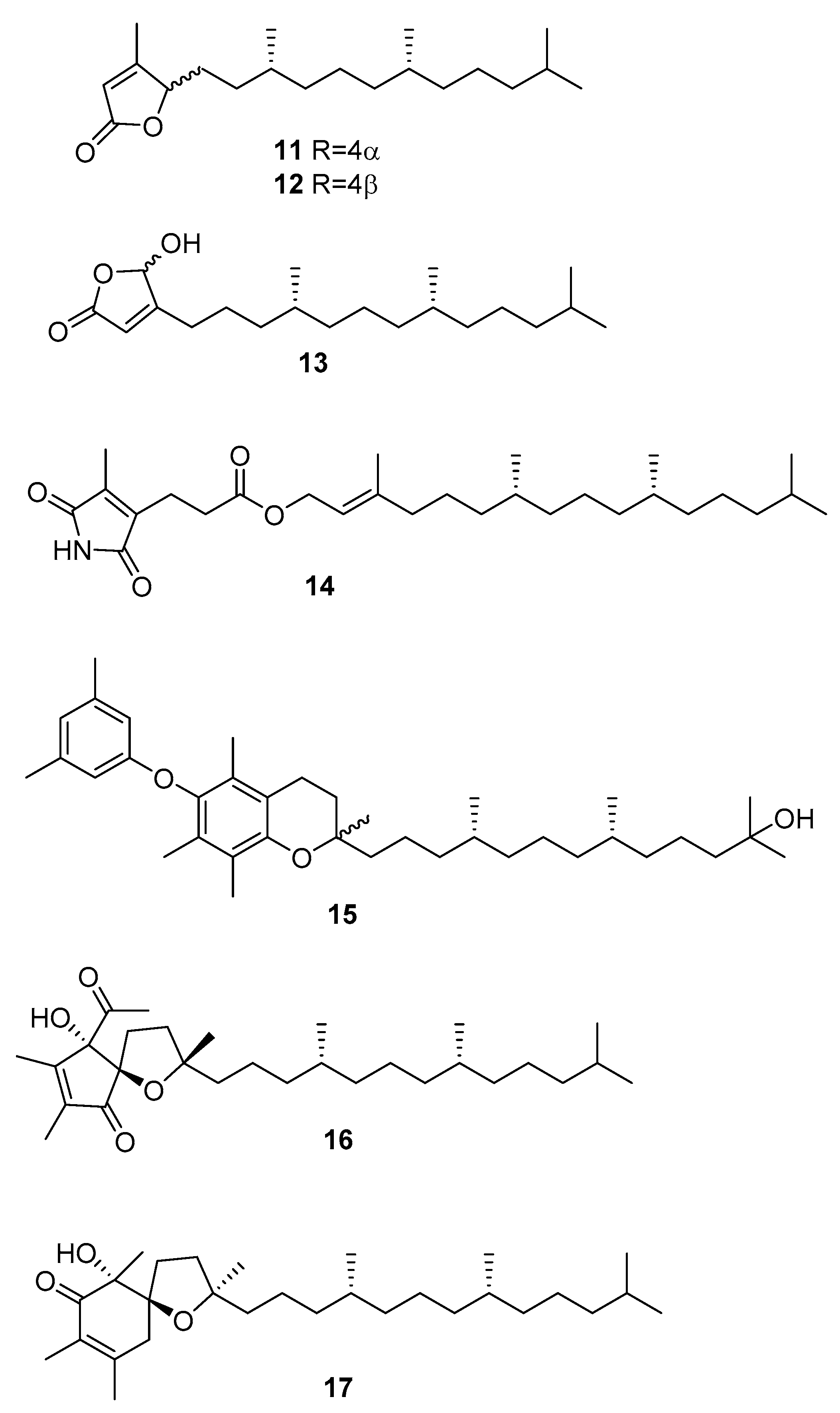
- Reddy, P.; Urban, S. Meroditerpenoids from the southern australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.F.; Bolton, J.J.; Lategan, C.A.; Smith, P.J.; Beukes, D.R. Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite plasmodium falciparum. Z. Naturforsch. Sect. C J. Biosci. 2008, 63, 848–852. [Google Scholar] [CrossRef]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Brkljaca, R.; Urban, S. Chemical profiling (hplc-nmr & hplc-ms), isolation, and identification of bioactive meroditerpenoids from the southern australian marine brown alga Sargassum paradoxum. Mar. Drugs 2015, 13, 102–127. [Google Scholar] [CrossRef]
- Segawa, M.; Shirahama, H. New plastoquinones from the brown alga Sargassum sagamianum var yezoense. Chem. Lett. 1987, 1365–1366. [Google Scholar] [CrossRef]
- Kusumi, T.; Shibata, Y.; Ishitsuka, M.; Kinoshita, T.; Kakisawa, H. Structures of new plastoquinones from the brown alga Sargassum serratifolium. Chem. Lett. 1979, 277–278. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.-B.; Shin, J. Meroditerpenoids from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.-J.; Yoo, J.-S.; Ahn, J.-W.; Lee, B.-J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Karadeniz, F.; Ahn, B.-N.; Kwon, M.S.; Mun, O.-J.; Bae, M.J.; Seo, Y.; Kim, M.; Lee, S.-H.; Kim, Y.Y.; et al. Bioactive quinone derivatives from the marine brown alga Sargassum thunbergii induce anti-adipogenic and pro-osteoblastogenic activities. J. Sci. Food Agric. 2016, 96, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Ryu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Lee, H.; Kim, Y.; Lee, B.-H.; Shin, J.; Kim, T.-Y. Sargaquinoic acid and sargachromenol, extracts of Sargassum sagamianum, induce apoptosis in hacat cells and mice skin: Its potentiation of uvb-induced apoptosis. Eur. J. Pharmacol. 2008, 582, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, M.; Kusumi, T.; Nomura, Y.; Konno, T.; Kakisawa, H. New geranylgeranylbenzoquinone derivatives from Sargassum tortile. Chem. Lett. 1979, 1269–1272. [Google Scholar] [CrossRef]
- Kim, S.-N.; Choi, H.Y.; Lee, W.; Park, G.M.; Shin, W.S.; Kim, Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through ppar alpha/gamma activation in 3t3-l1 cells. FEBS Lett. 2008, 582, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kwon, H.C.; Kim, S.N.; Kim, H.S.; Um, B.H. Plastoquinones from Sargassum yezoense; chemical structures and effects on the activation of peroxisome proliferator-activated receptor gamma. Chem. Pharm. Bull. 2011, 59, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-J.; Han, S.-C.; Yoon, W.-J.; Koh, Y.-S.; Hyun, J.-W.; Kang, H.-K.; Cho, J.Y.; Yoo, E.-S. Sargaquinoic acid isolated from Sargassum siliquastrum inhibits lipopolysaccharide-induced nitric oxide production in macrophages via modulation of nuclear factor-kappa b and c-jun n-terminal kinase pathways. Immunopharmacol. Immunotoxicol. 2012, 35, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.-J.; Heo, S.-J.; Han, S.-C.; Lee, H.-J.; Kang, G.-J.; Kang, H.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S. Anti-inflammatory effect of sargachromanol g isolated from Sargassum siliquastrum in raw 264.7 cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Seo, Y. Chromanols from Sargassum siliquastrum and their antioxidant activity in ht 1080 cells. Chem. Pharm. Bull. 2011, 59, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jang, J.; Ye, B.-R.; Kim, M.-S.; Yoon, W.-J.; Oh, C.; Kang, D.-H.; Lee, J.-H.; Kang, M.-C.; Jeon, Y.-J.; et al. Chromene suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated raw 264.7 cells. Food Chem. Toxicol. 2014, 67, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Lee, B.H.; Choi, B.W.; Lee, H.S.; Shin, J. Chromenes from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2005, 68, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kumanireng, A.S.; Ichinose, I.; Kitahara, Y.; Kakinuma, Y.; Nishihira, M.; Kato, M. Active components of Sargassum tortile effecting settlement of swimming larvae of coryne-uchidai. Experientia 1975, 31, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Mori, Y.; Yokoi, T.; Nakazawa, S.; Kuroda, H.; Masada, Y.; Kitamura, K.; Kuriyama, K. Structure and absolute-configuration of sargatriol, a new isoprenoid chromenol from a brown alga, Sargassum tortile C. AGARDH. Chem. Pharm. Bull. 1983, 31, 106–113. [Google Scholar] [CrossRef]
- Kikuchi, T.; Mori, Y.; Yokoi, T.; Nakazawa, S.; Kuroda, H.; Masada, Y.; Kitamura, K.; Umezaki, I. Structure of sargatriol, a new isoprenoid chromenol from a marine alga—Sargassum tortile. Chem. Pharm. Bull. 1975, 23, 690–692. [Google Scholar] [CrossRef]
- Cho, S.H.; Cho, J.Y.; Kang, S.E.; Hong, Y.K.; Ahn, D.H. Antioxidant activity of mojabanchromanol, a novel chromene, isolated from brown alga Sargassum siliquastrum. J. Environ. Biol. 2008, 29, 479–484. [Google Scholar] [PubMed]
- Tsuchiya, N.; Sato, A.; Haruyama, H.; Watanabe, T.; Iijima, Y. Nahocols and isonahocols, endothelin antagonists from the brown alga, Sargassum autumnale. Phytochemistry 1998, 48, 1003–1011. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, H.; Fukuoka, Y.; Matsuo, A.; Soga, O.; Nakayama, M. Structure of sargassumlactam, a new beta,gamma-unsaturated-gamma-lactam, from the marine alga Sargassum kjellmanianum. Chem. Lett. 1980, 1453–1454. [Google Scholar] [CrossRef]
- Nakayama, M.; Fukuoka, Y.; Nozaki, H.; Matsuo, A.; Hayashi, S. Structure of (+)-kjellmanianone, a highly oxygenated cyclopentenone from the marin alga, Sargassum kjellmanianum. Chem. Lett. 1980, 1243–1246. [Google Scholar] [CrossRef]
- Cai, Y.-P.; Xie, C.-B.; Wang, B.-C.; Li, P.-L.; Li, B.-F. Two new resorcinols from Sargassum thunbergii. J. Asian Nat. Prod. Res. 2010, 12, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Ganti, V.S.; Kim, K.H.; Bhattarai, H.D.; Shin, H.W. Isolation and characterisation of some antifouling agents from the brown alga Sargassum confusum. J. Asian Nat. Prod. Res. 2006, 8, 309–315. [Google Scholar] [CrossRef] [PubMed]
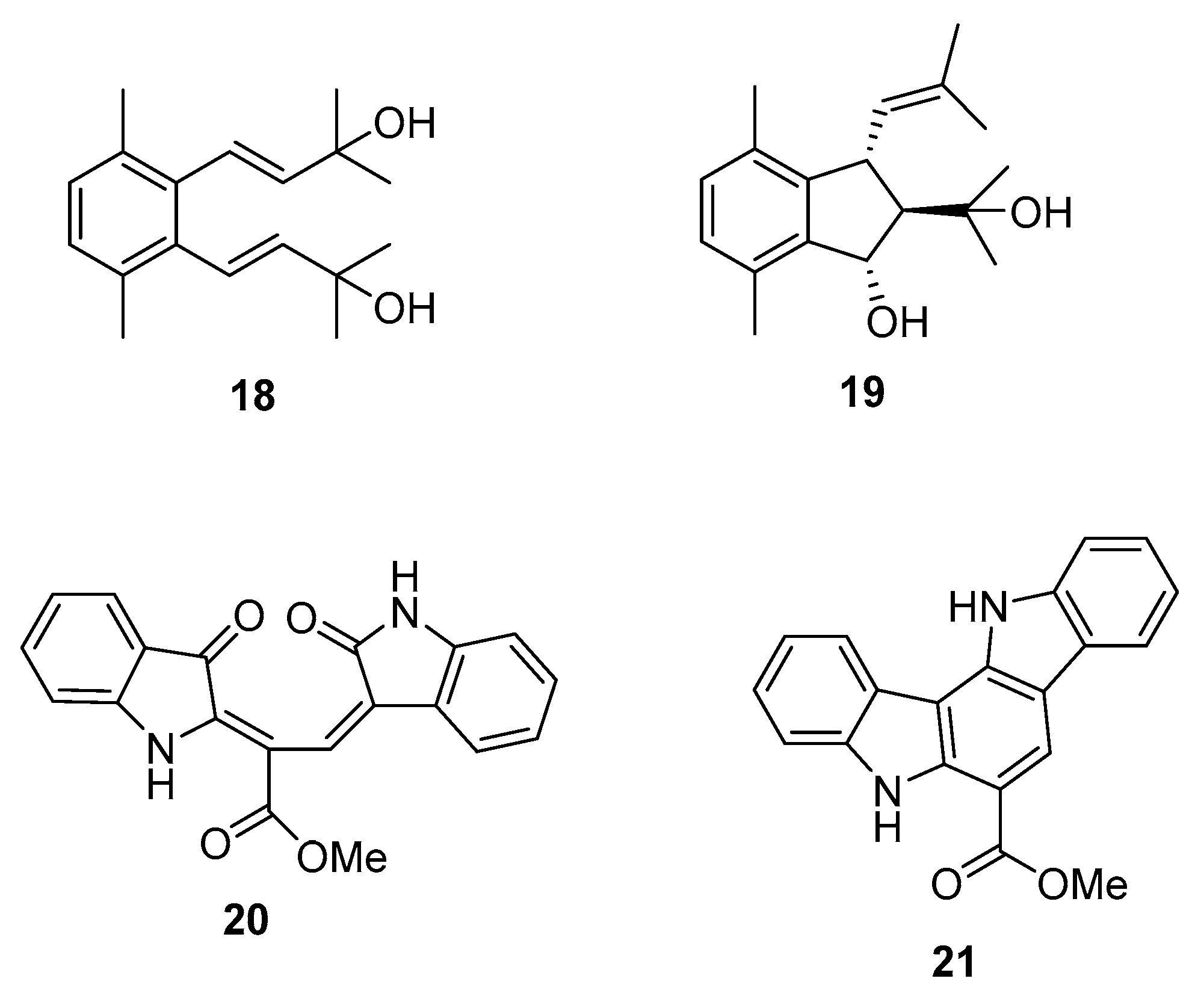
- Shizuri, Y.; Matsukawa, S.; Ojika, M.; Yamada, K. 2 new farnesylacetone derivatives from the brown alga Sargassum micracanthum. Phytochemistry 1982, 21, 1808–1809. [Google Scholar] [CrossRef]
- Kusumi, T.; Ishitsuka, M.; Nomura, Y.; Konno, T.; Kakisawa, H. New farnesylacetone derivatives from Sargassum micracanthum. Chem. Lett. 1979, 8, 1181–1184. [Google Scholar] [CrossRef]
- Ryu, G.; Park, S.H.; Kim, E.S.; Choi, B.W.; Ryu, S.Y.; Lee, B.H. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch. Pharm. Res. 2003, 26, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-G.; Kwon, S.-C.; Park, G.-M.; Ham, J.; Shin, W.-S.; Lee, S. Vasodilatation effect of farnesylacetones, active constituents of Sargassum siliquastrum, on the basilar and carotid arteries of rabbits. Bioorg. Med. Chem. Lett. 2008, 18, 6324–6326. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Watanabe, R.; Suenaga, K.; Yamada, K.; Uemura, D. Isolation and structures of hedaols a, b, and c, new bisnorditerpenes from a japanese brown alga. J. Nat. Prod. 2001, 64, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Salvador, N.; Gomez Garreta, A.; Lavelli, L.; Antonia Ribera, M. Antimicrobial activity of iberian macroalgae. Sci. Mar. 2007, 71, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Manilal, A.; Sujith, S.; Kiran, G.S.; Selvin, J.; Shakir, C.; Gandhimathi, R.; Lipton, A.P. Antimicrobial potential and seasonality of red algae collected from the southwest coast of india tested against shrimp, human and phytopathogens. Ann. Microbiol. 2009, 59, 207–219. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; Nathalie, B. Antiviral activity of the extracts of rhodophyceae from morocco. Afr. J. Biotechnol. 2010, 9, 7968–7975. [Google Scholar]
- Pinteus, S.; Alves, C.; Monteiro, H.; Araujo, E.; Horta, A.; Pedrosa, R. Asparagopsis armata and sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J. Microbiol. Biotechnol. 2015, 31, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vedhagiri, K.; Manilal, A.; Valliyammai, T.; Shanmughapriya, S.; Sujith, S.; Selvin, J.; Natarajaseenivasan, K. Antimicrobial potential of a marine seaweed Asparagopsis taxiformis against leptospira javanica isolates of rodent reservoirs. Ann. Microbiol. 2009, 59, 431–437. [Google Scholar] [CrossRef]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spano, A.; Morabito, M.; Maugeri, T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the straits of messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Selvin, J.; George, S. In vivo therapeutic potentiality of red seaweed, Asparagopsis (bonnemaisoniales, rhodophyta) in the treatment of vibriosis in penaeus monodon fabricius. Saudi J. Biol. Sci. 2012, 19, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Leitner, S.; Minicante, S.A.; Lass-Floerl, C. The mediterranean red alga Asparagopsis taxiformis has antifungal activity against aspergillus species. Mycoses 2013, 56, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, M.A.; Shameel, M. In vitro nematicidal activities of seaweed extracts from karachi coast. Pak. J. Bot. 2006, 38, 1245–1248. [Google Scholar]
- Manilal, A.; Sujith, S.; Sabarathnam, B.; Kiran, G.S.; Selvin, J.; Shakir, C.; Lipton, A.P. Bioactivity of the red algae Asparagopsis taxiformis collected from the southwestern coast of india. Braz. J. Oceanogr. 2010, 58, 93–100. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.-S.; Kerjean, V.; Deslandes, E. Antioxidant and cytotoxic activities of some red algae (rhodophyta) from Brittany Coasts (France). Bot. Mar. 2009, 52, 268–277. [Google Scholar] [CrossRef]
- Al-Saif, S.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from jeddah coast of red sea, saudi arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.M.; de Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Guimaraes, T.D.; Bittencourt, F.; Gouvea, L.P.; Aresi, C.; de Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-infective potential of marine invertebrates and seaweeds from the brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Ganesapandian, S.; Singh, M.; Sangeetha, N.; Kumaraguru, A.K. Antimicrobial activity of seaweeds against multi drug resistant strains. Int. J. Pharmacol. 2011, 7, 522–526. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Souza, E.T.; de Queiroz, A.C.; de Miranda, G.E.C.; Lorenzo, V.R.; da Silva, E.F.; Freire-Dias, T.L.M.; Cupertino-Silva, Y.K.; Melo, G.M.D.; Santos, B.V.O.; Chaves, M.C.D.; et al. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (caulerpaceae). Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2009, 19, 115–120. [Google Scholar] [CrossRef]
- Da Matta, C.B.B.; de Souza, E.T.; de Queiroz, A.C.; de Lira, D.P.; de Araujo, M.V.; Cavalcante-Silva, L.H.A.; de Miranda, G.E.C.; de Araujo, J.X.; Barbosa, J.M.; Santos, B.V.D.; et al. Antinociceptive and anti-inflammatory activity from algae of the genus caulerpa. Mar. Drugs 2011, 9, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Iyer, V.V. Antioxidant activity and gas chromatographic-mass spectrometric analysis of extracts of the marine algae, Caulerpa peltata and Padina gymnospora. Indian J. Pharm. Sci. 2014, 76, 548–552. [Google Scholar] [PubMed]
- Koishi, A.C.; Zanello, P.R.; Bianco, E.M.; Bordignon, J.; dos Santos, C.N.D. Screening of dengue virus antiviral activity of marine seaweeds by an in situ enzyme-linked immunosorbent assay. PLoS ONE 2012, 7, e51089. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.A.O.; Dantas, G.R.; Lira, D.P.; Barbosa, J.M.; de Miranda, G.E.; Santos, B.V.D.; Souto, J.T. Aqueous and methanolic extracts of Caulerpa mexicana suppress cell migration and ear edema induced by inflammatory agents. Mar. Drugs 2011, 9, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Silkina, A.; Bazes, A.; Mouget, J.-L.; Bourgougnon, N. Comparative efficiency of macroalgal extracts and booster biocides as antifouling agents to control growth of three diatom species. Mar. Pollut. Bull. 2012, 64, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Lee, K.-W.; Kim, S.-K.; Jeon, Y.-J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.-W.; Youn, H.-Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (turner) c. Agardh on lipopolysaccharide-simulated macrophage activation via nf-kappa b pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Kang, S.-E.; Cho, J.-Y.; Kim, A.-R.; Park, S.-M.; Hong, Y.-K.; Ahn, D.-H. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J. Med. Food 2007, 10, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.N.; Cheung, P.C.K.; Ooi, V.E.C.; Ang, P.O. Evaluation of antioxidative activity of extracts from a brown seaweed, Sargassum siliquastrum. J. Agric. Food Chem. 2002, 50, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Sathivel, A.; Devaki, T. Antioxidant effect of Sargassum polycystum (phaeophyceae) against acetaminophen induced changes in hepatic mitochondrial enzymes during toxic hepatitis. Chemosphere 2005, 61, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Ramirez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp and Porphyra sp.) in human subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm. Biol. 2013, 51, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Matsunaga, T.; Takahashi, S.; Hasegawa, C.; Saito, H. Inhibitory activity on lipid peroxidation of extracts from marine brown alga. Phytother. Res. 2003, 17, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Ha, Y.-M.; Joo, C.-U.; Cho, K.K.; Kim, S.-J.; Choi, I.S. Inhibition of oral pathogens and collagenase activity by seaweed extracts. J. Environ. Biol. 2012, 33, 115–121. [Google Scholar] [PubMed]
- Kang, J.Y.; Khan, M.N.A.; Park, N.H.; Cho, J.Y.; Lee, M.C.; Fujii, H.; Hong, Y.K. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J. Ethnopharmacol. 2008, 116, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Joung, E.-J.; Kwon, M.-S.; Lee, B.; Utsuki, T.; Oh, C.-W.; Kim, H.-R. Anti-inflammatory effect of ethanolic extract of Sargassum serratifolium in lipopolysaccharide-stimulated bv2 microglial cells. J. Med. Food 2016, 19, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Samee, H.; Li, Z.-x.; Lin, H.; Khalid, J.; Guo, Y.-c. Anti-allergic effects of ethanol extracts from brown seaweeds. J. Zhejiang Univ. Sci. B 2009, 10, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-N.; Lee, W.; Bae, G.-U.; Kim, Y.K. Anti-diabetic and hypolipidemic effects of Sargassum yezoense in db/db mice. Biochem. Biophys. Res. Commun. 2012, 424, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Toulabi, P.B.; Abai, M.R.; Sadati, N.; Hadjiakhoondi, F.; Hadjiakhoondi, A.; Vatandoost, H. Larvicidal activity of marine algae, Sargassum swartzii and Chondria dasyphylla, against malaria vector Anopheles stephensi. J. Vector Borne Dis. 2011, 48, 241–244. [Google Scholar] [PubMed]
- Gamal-Eldeen, A.M.; Abo-Zeid, M.A.M.; Ahmed, E.F. Anti-genotoxic effect of the Sargassum dentifolium extracts: Prevention of chromosomal aberrations, micronuclei, and DNA fragmentation. Exp. Toxicol. Pathol. 2013, 65, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.S.; Rajkumar, M.; Srinivasan, R.; Harikumar, R.P.; Suresh, S.; Kumar, S. Antitumour activity of Sargassum wightii (greville) extracts against dalton’s ascites lymphoma. Pak. J. Biol. Sci. PJBS 2013, 16, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Muthuraman, M.S.; Prabhu, A.R.; Priyadharshini, R.R.; Parthiban, S. Evaluation of antitumor and antioxidant activity of Sargassum tenerrimum against ehrlich ascites carcinoma in mice. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in b16f10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-K.; Kim, M.-J.; Kim, K.-B.-W.-R.; Ahn, D.-H. In vivo and in vitro inhibitory activity of an ethanolic extract of Sargassum fulvellum and its component grasshopper ketone on atopic dermatitis. Int. Immunopharmacol. 2016, 40, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Kang, J.-Y.; Hong, Y.-K.; Lee, H.; Chowdhury, M.T.H.; Choi, J.-S.; Choi, I.S.; Moon, I.S. A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis In Vitro. Cell. Dev. Biol. Anim. 2012, 48, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.; Iniyan, A.M.; Vincent, S.G.P. Chemical genetic effects of Sargassum wightii during embryonic development in zebrafish. Indian J. Pharmacol. 2015, 47, 195–198. [Google Scholar] [CrossRef] [PubMed]


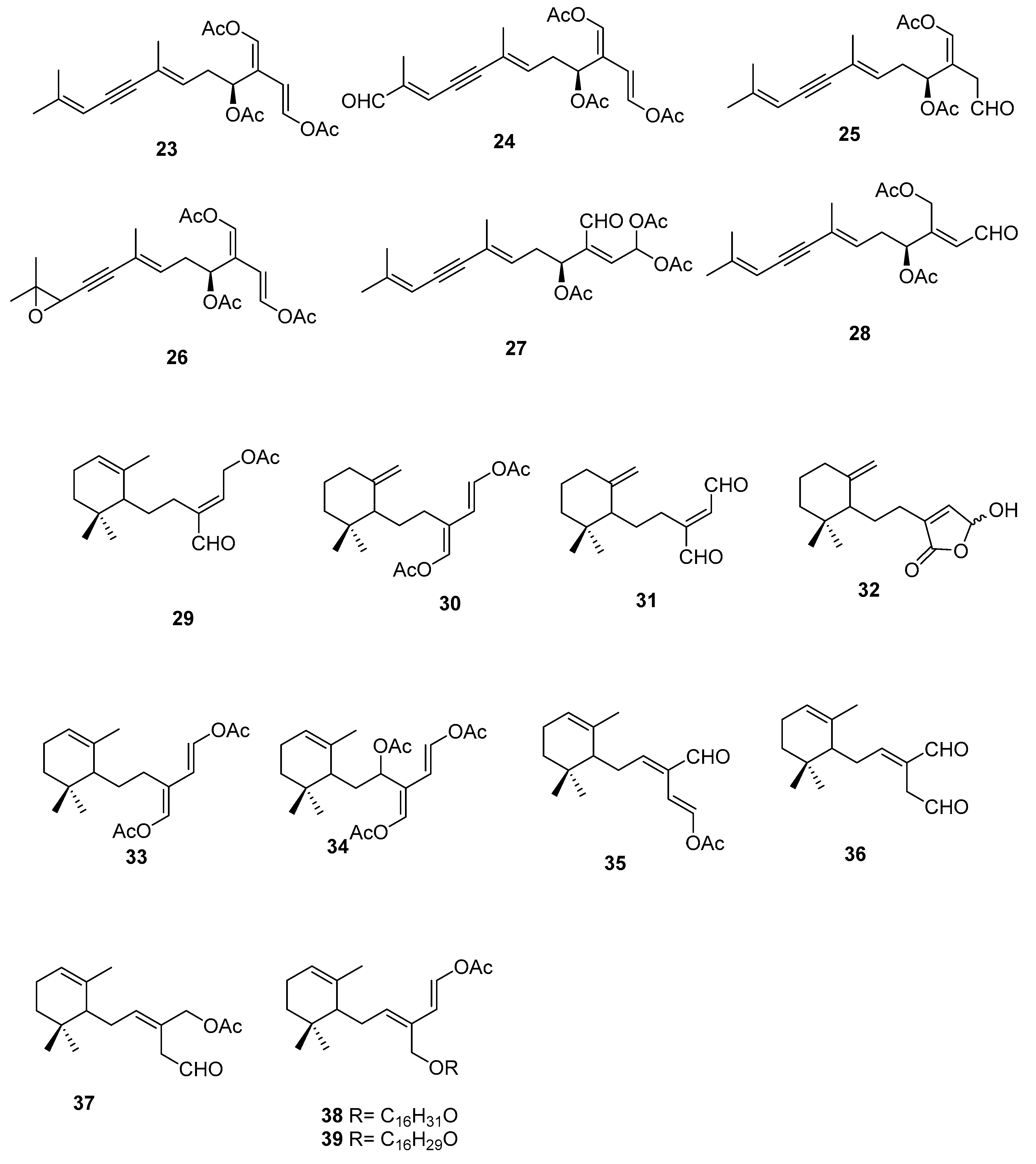
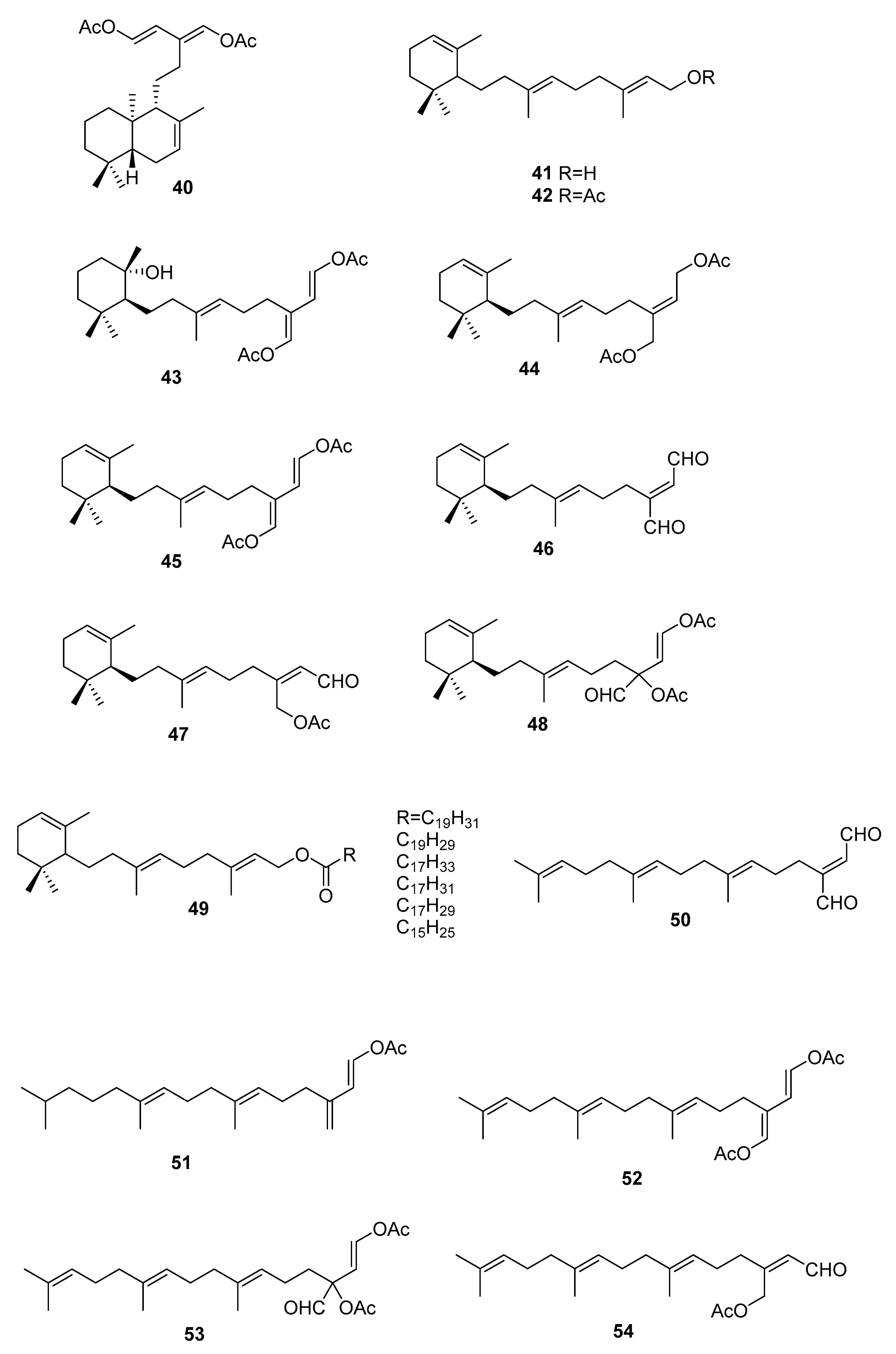
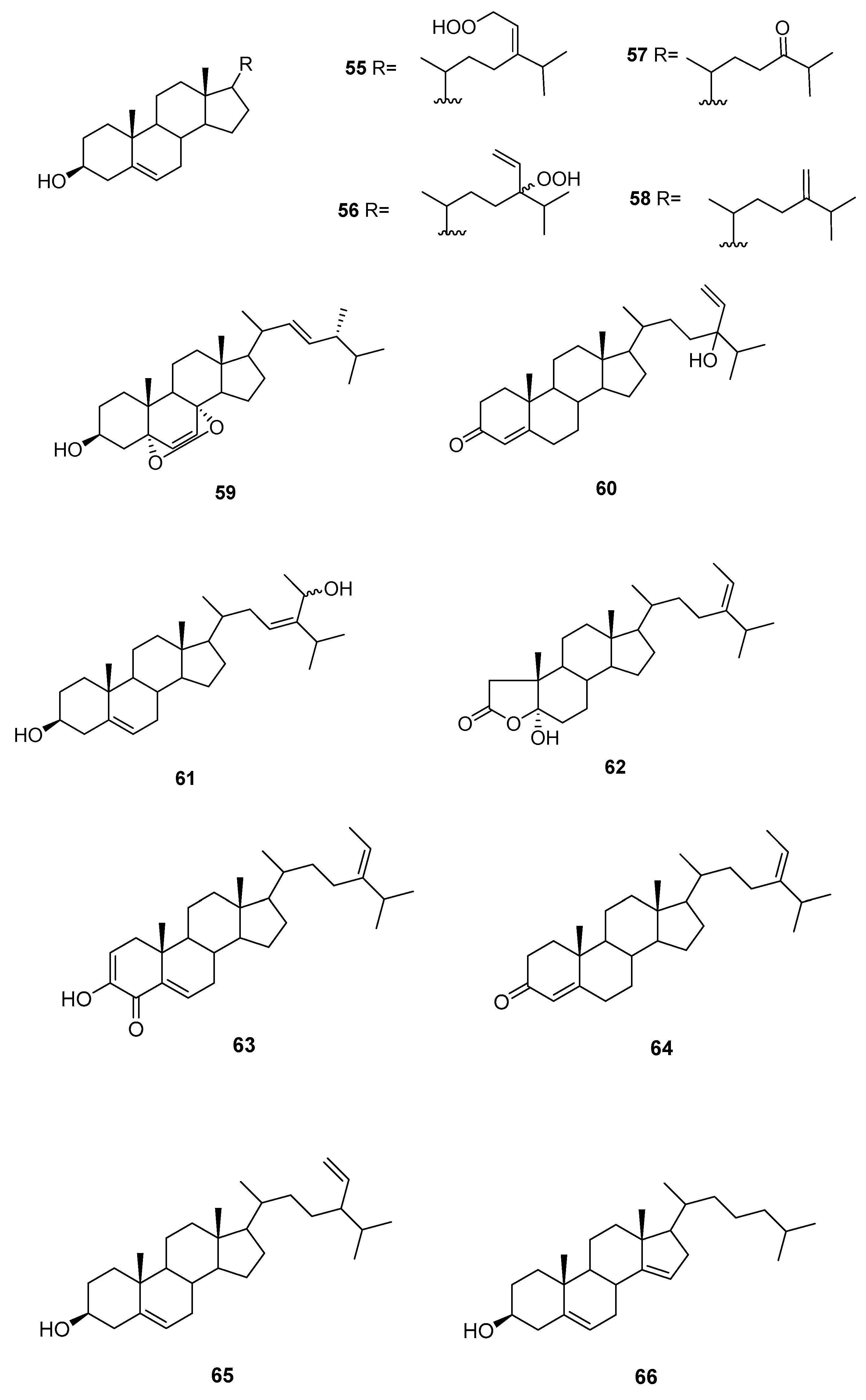
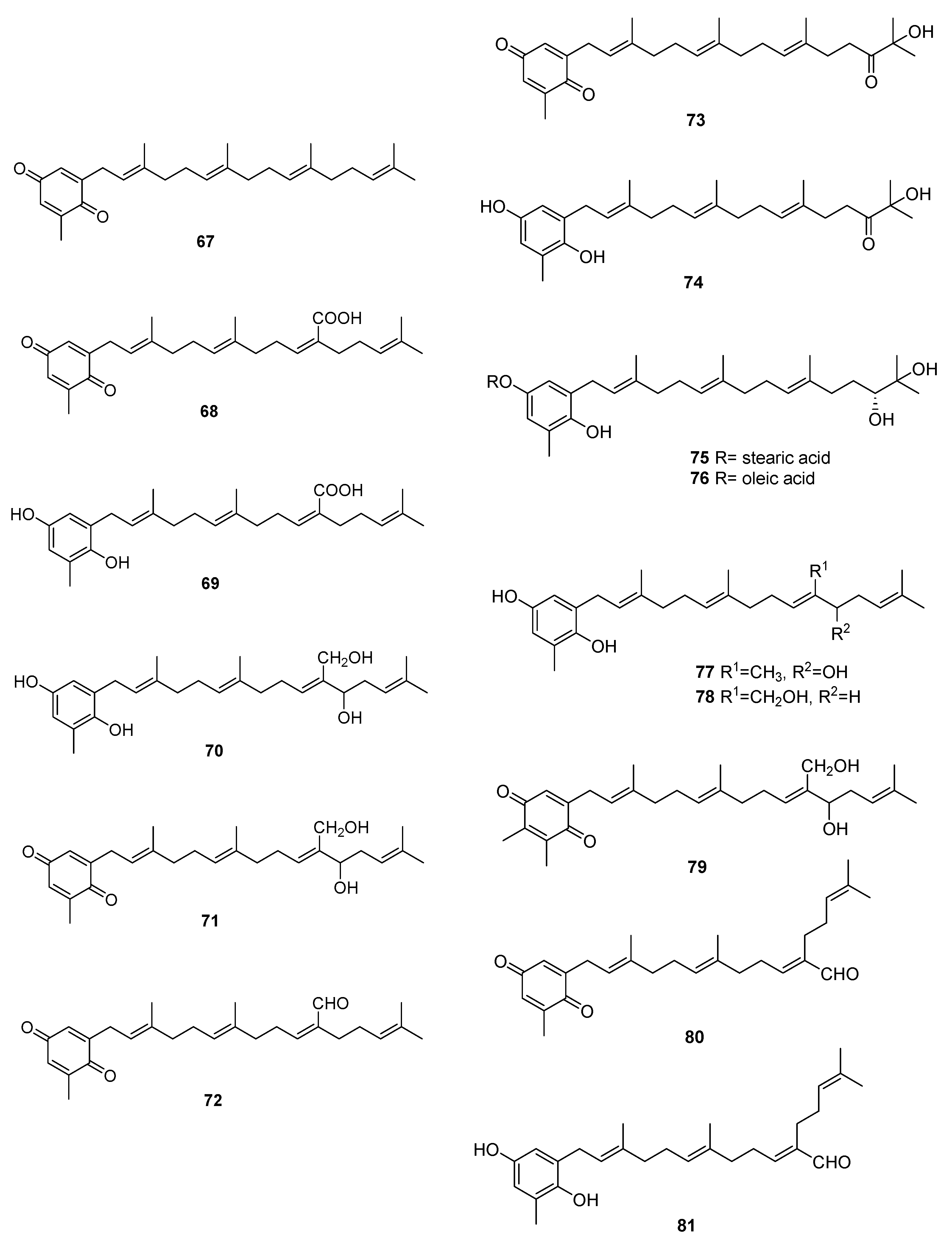



| Species | Compounds | Biological Activity |
|---|---|---|
| C. ashmeadii [39] | 34–39 | Feeding preference, antimicrobial, ichthyotoxicity |
| C. bikinensis [38] | 30–32 | Feeding deterrents |
| C. flexilis var. muelleri [35] | 29, 33 | - |
| C. prolifera [37] | 25 | - |
| C. taxifolia [36] | 26–28 | - |
| Species | Compounds | Biological Activity |
|---|---|---|
| S. elegans [54] | 68,69,72 | Antioxidants |
| S. fallax [55] | 67–71 | Antitumour against P388 |
| S. herophyllum [56] | 67,69,72 | Antiplasmodial activity |
| S. michranthum [57] | 73–76 | Antioxidants, radical scaveging, inhibitory effect on lipid peroxidation, antiproliferative against 26-L5, cytotoxicity |
| S. paradoxum [58] | 67–71,77–83 | Antibacterial |
| S. sagamium var. yezoense [59] | 68,69,80,84 | - |
| S. sagamium [64,65] | 68 | Anticholinesterase activity, proapoptotic, and anti-inflammatory |
| S. serratifolium [60] | 68,80 | - |
| S. siliquastrum [61] | 96,97 | Radical scavenging |
| S. thunbergii [62,63] | 68,69 | Osteoblastogenesis-enhancing abilities |
| S. tortile [66] | 67,89–95 | - |
| S. yezoense [67,68] | 68,69,85–88 | Transcriptional activity of PPARs (Peroxisome proliferator-activated receptors), antidiabetic potential |
| Species | Compounds | Biological Activity |
|---|---|---|
| S. paradoxum [58] | 98 | - |
| S. serratifolium [60] | 99 | - |
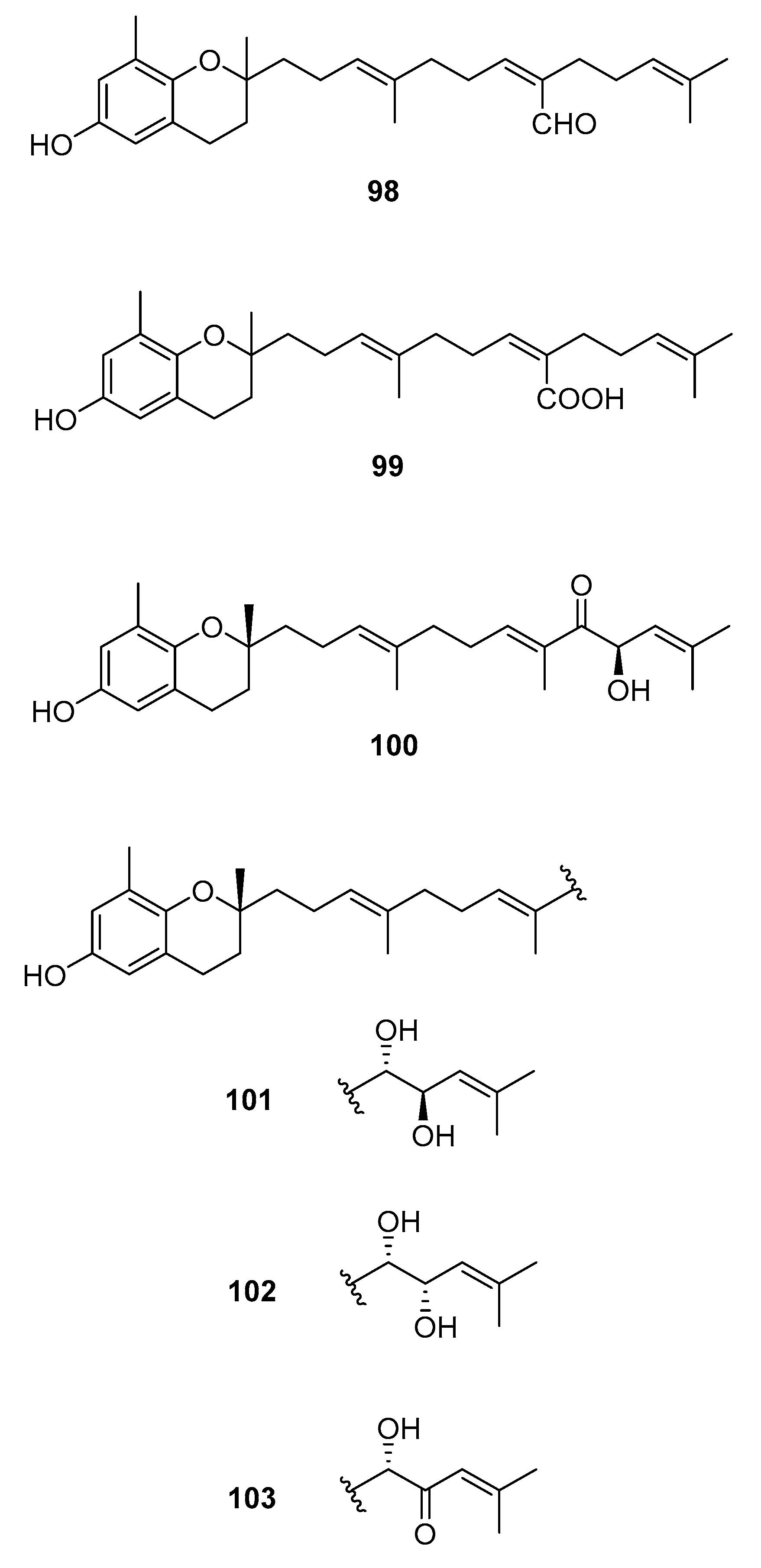
| S. siliquastrum Yoon [70,71,72,73,77] | 100–120,127 | Anti-inflammatory, antioxidant, radical-scavenging activity, inhibition of butylcholine esterase |
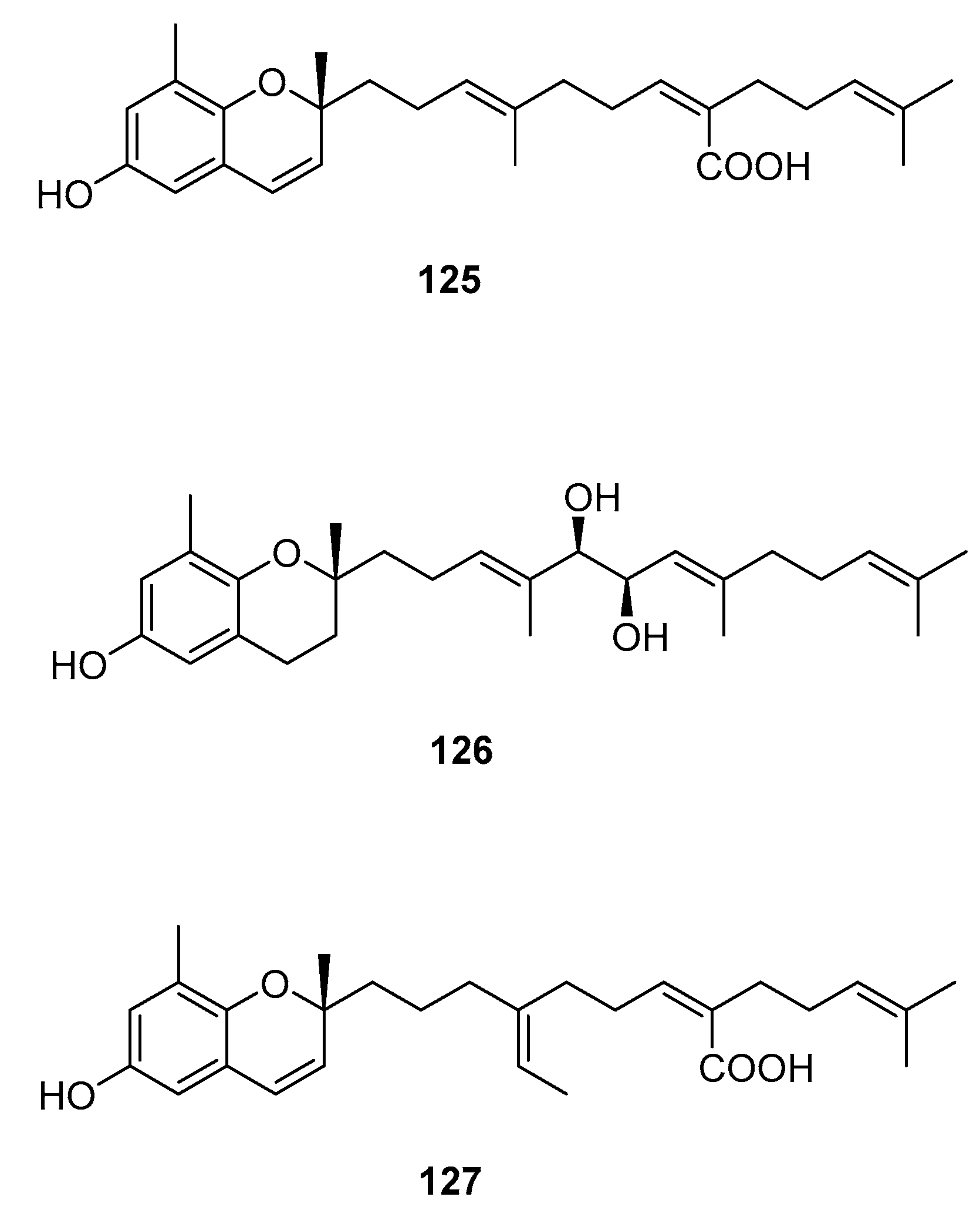
| S. sagamianum [64,65] | 125 | Proapoptotic activity, anticholinesterase activity |
| S. thunbergii [62] | 121,122,125 | Radical scavenging |
| S. tortile [74,75,76] | 123,124,126 | Larval attractants |
| Species | Compounds | Biological Activity |
|---|---|---|
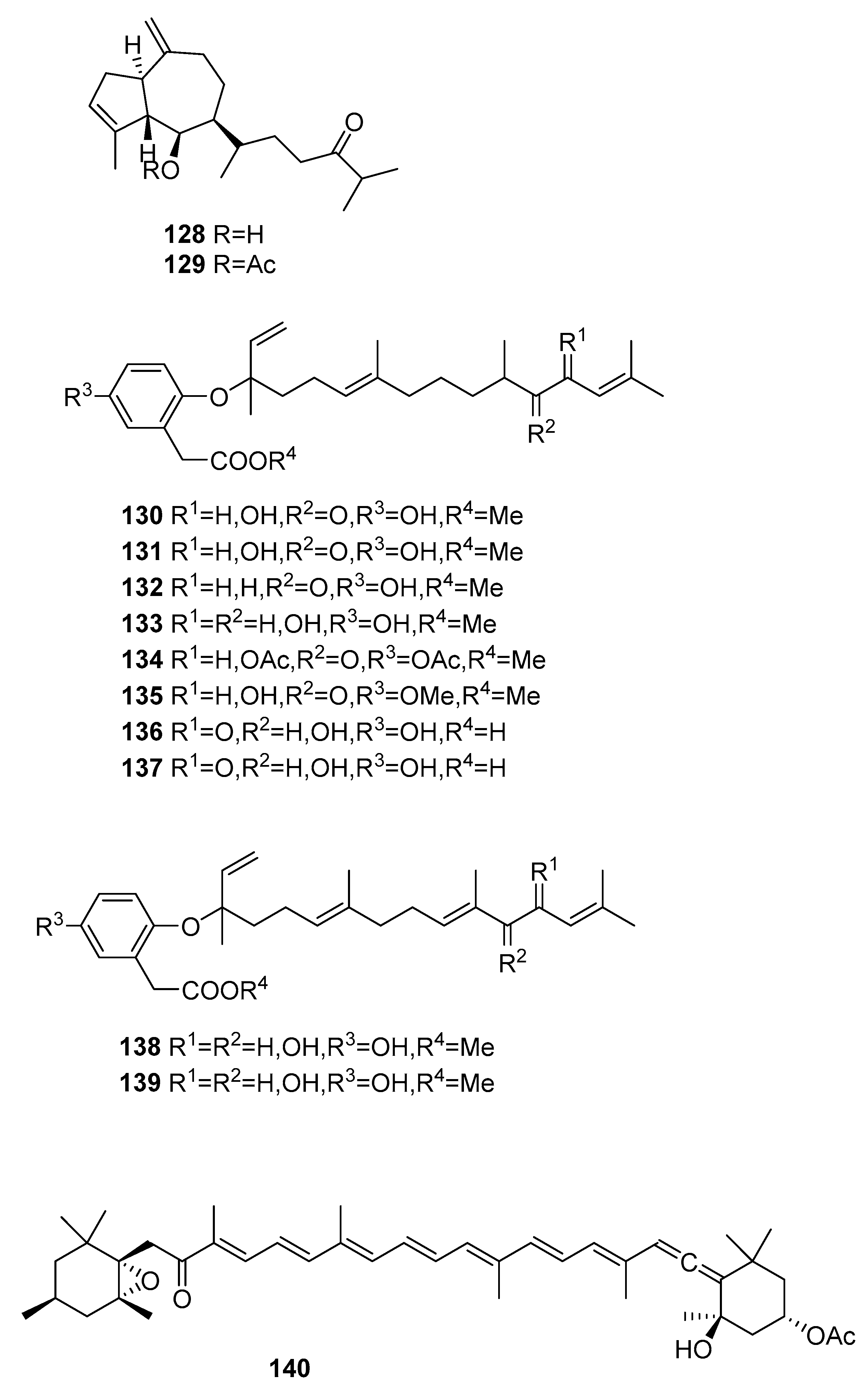
| S. asperifolium [48] | 128,129 | - |
| S. autumnale [78] | 130–139 | Endothelin antagonists |
| S. elegans [54] | 140 | Antioxidant |
| S. fusiformis [79] | 140 | - |
| S. heterophyllum [56] | 140 | Antiplasmodial, cytotoxicity |
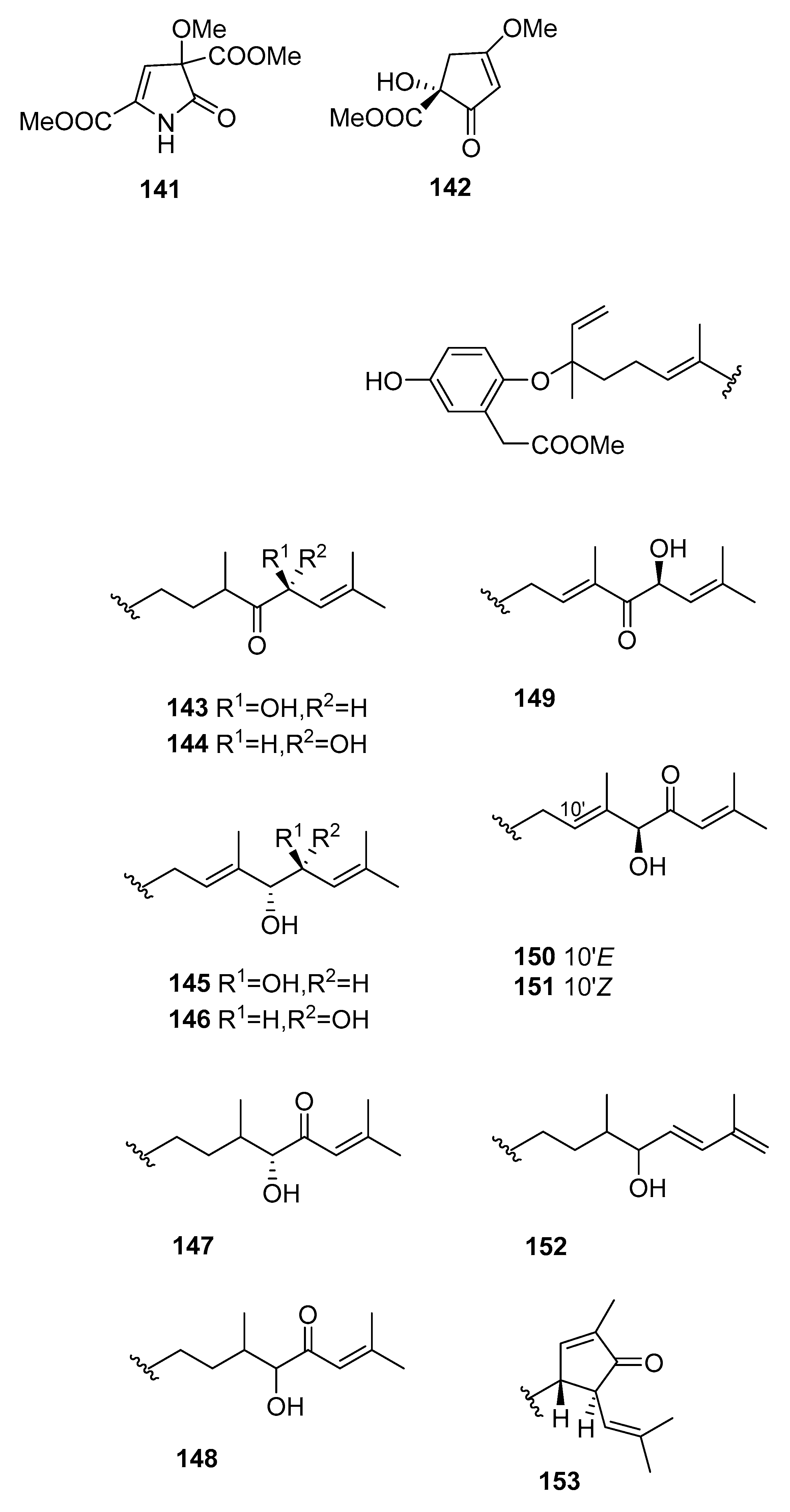
| S. Kjellmanium [80,81] | 141,142 | - |
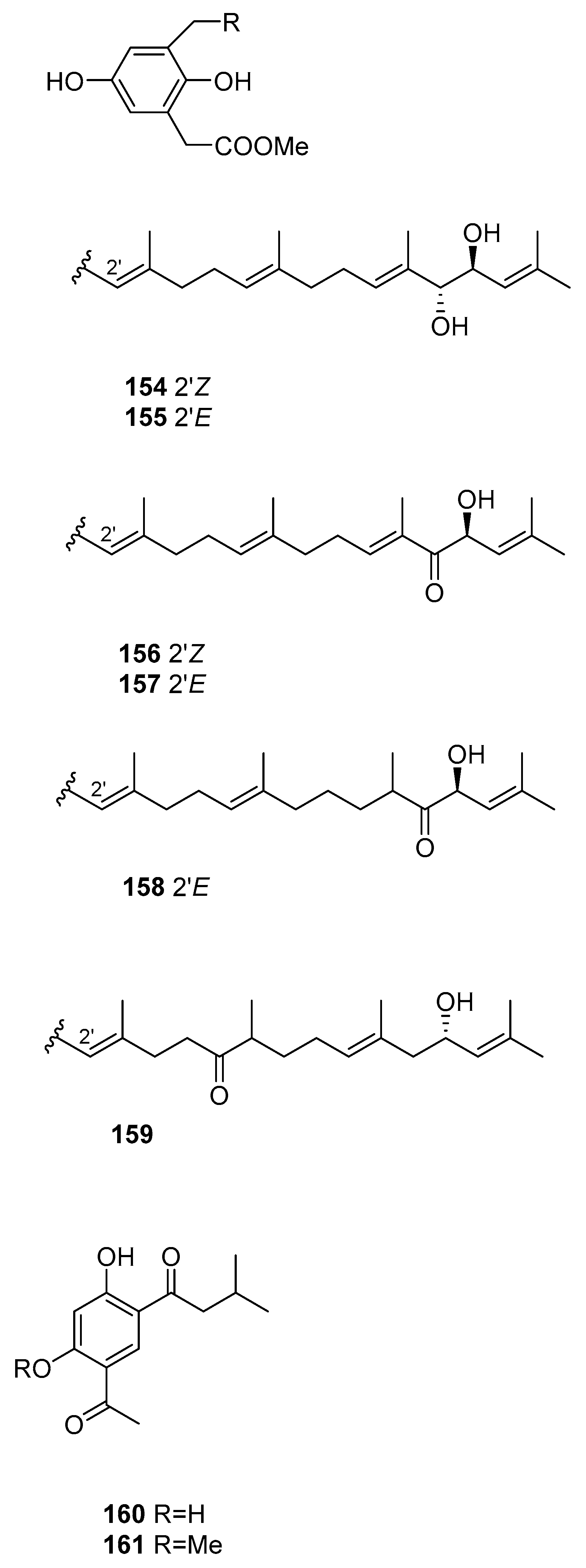
| S. siliquastrum [61] | 143–159 | Radical scaveging, active against isocitrate lyase |
| S. thunbergii [82] | 160,161 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe. Mar. Drugs 2018, 16, 265. https://doi.org/10.3390/md16080265
Máximo P, Ferreira LM, Branco P, Lima P, Lourenço A. Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe. Marine Drugs. 2018; 16(8):265. https://doi.org/10.3390/md16080265
Chicago/Turabian StyleMáximo, Patrícia, Luísa M. Ferreira, Paula Branco, Pedro Lima, and Ana Lourenço. 2018. "Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe" Marine Drugs 16, no. 8: 265. https://doi.org/10.3390/md16080265






