Structural Characterisation of Predicted Helical Regions in the Chironex fleckeri CfTX-1 Toxin
Abstract
:1. Introduction
2. Results
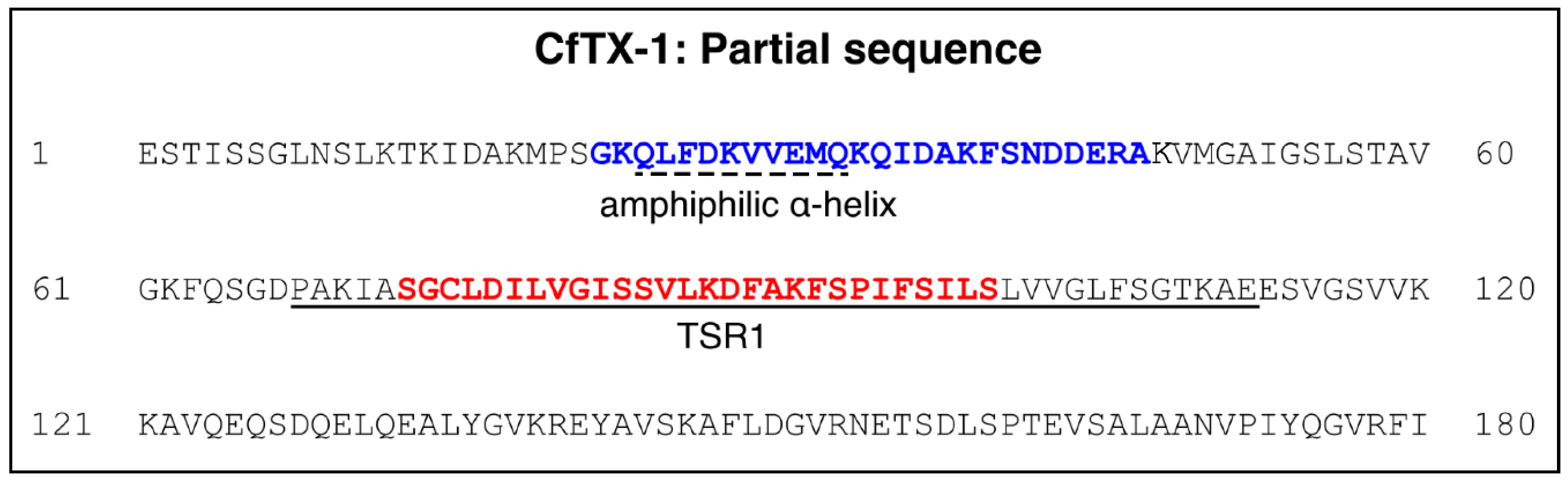
2.1. CfTX-1 Peptides—Design and Synthesis
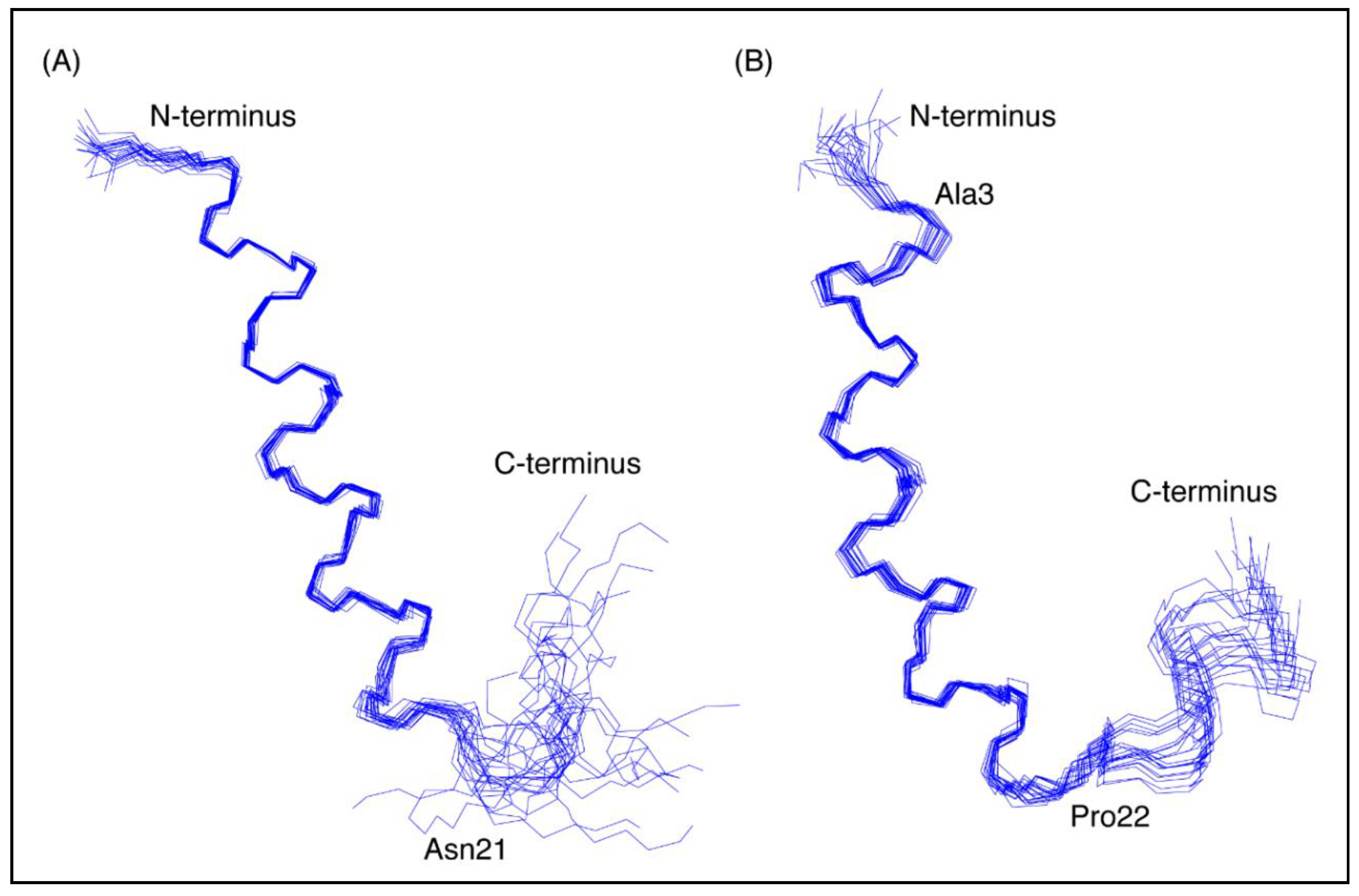
2.2. Structural Analysis Using NMR Spectroscopy
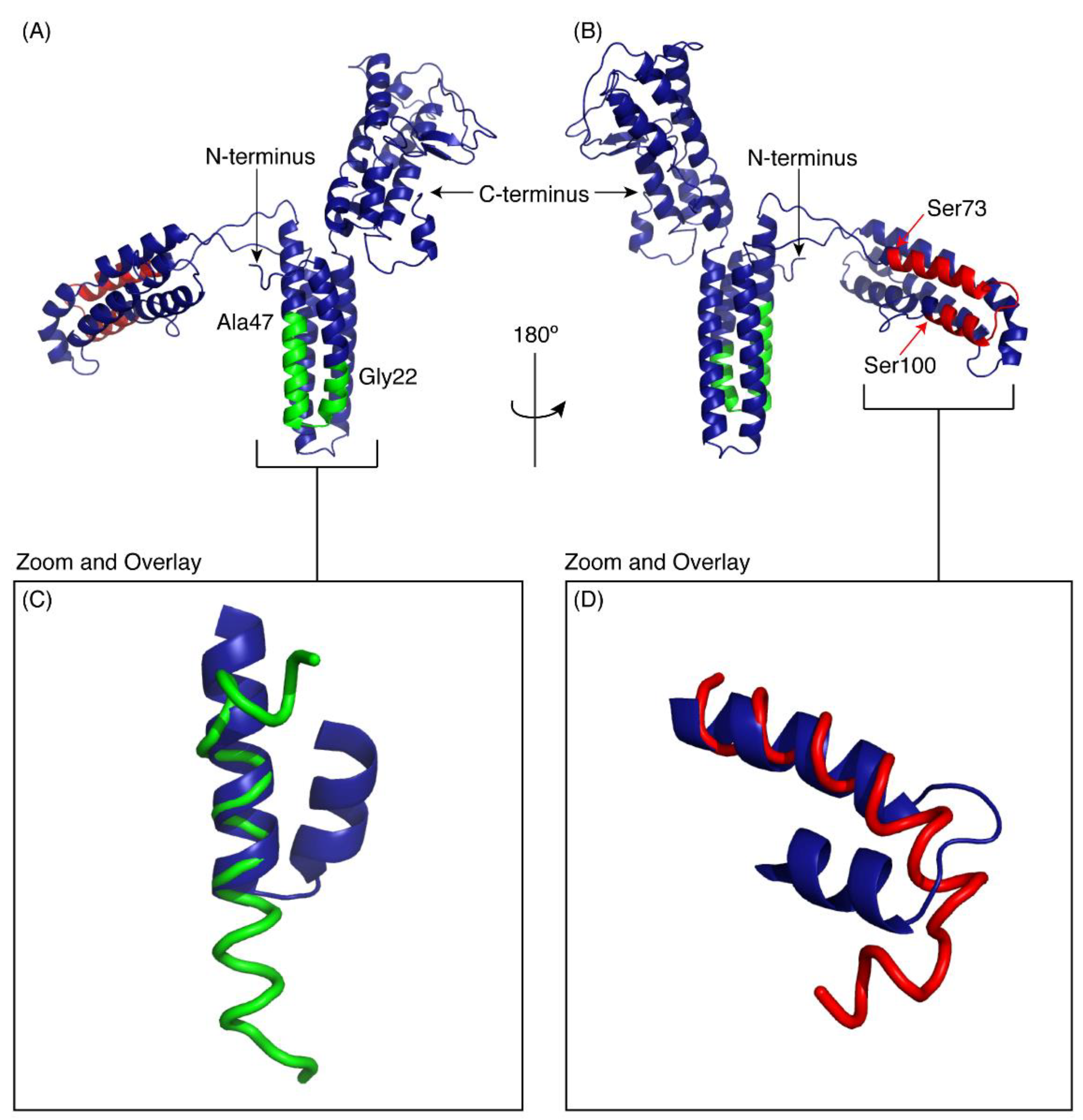
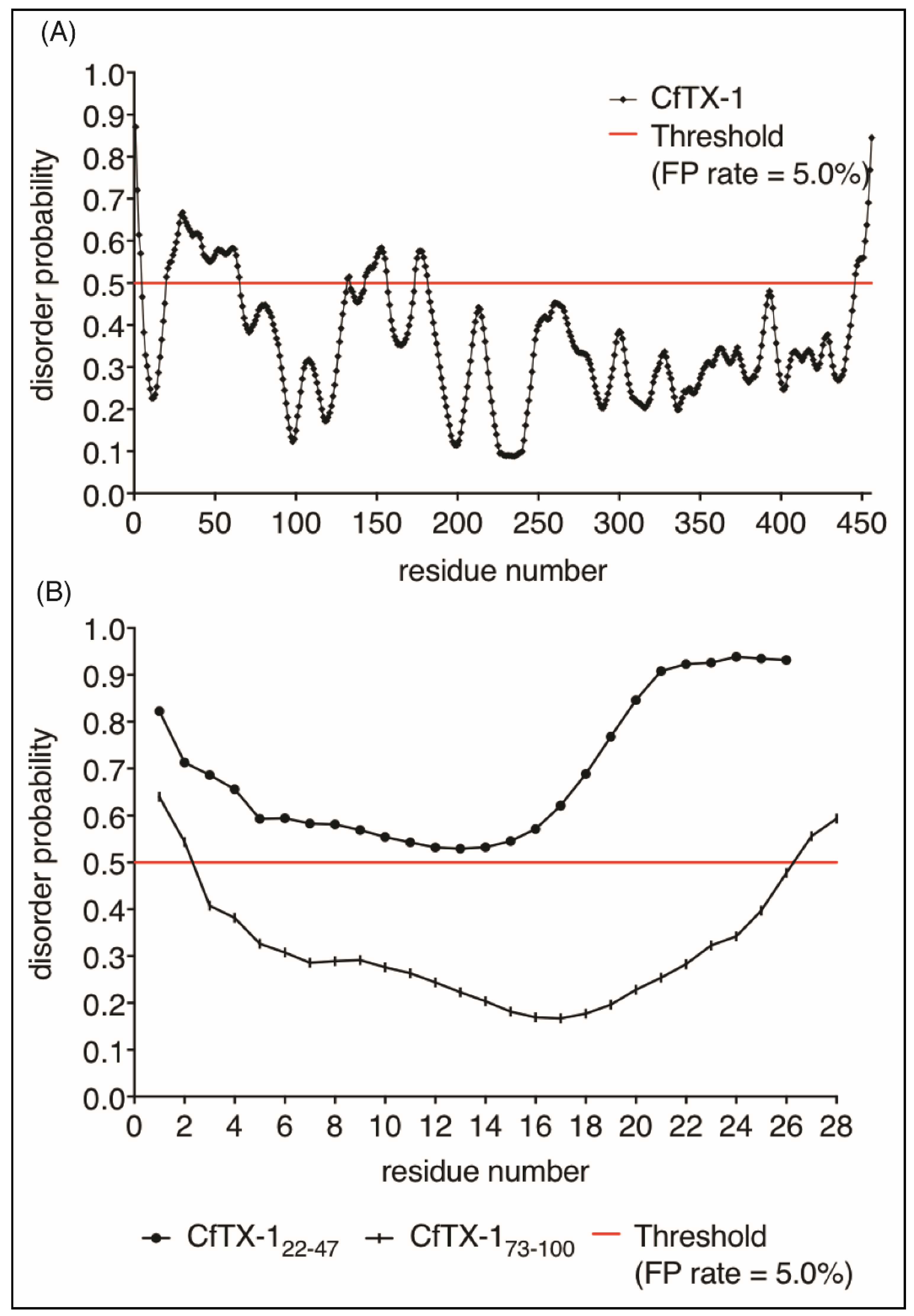
2.3. Structure Predictions for CfTX-1
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis and Purification
4.2. NMR Spectroscopy and Structure Determination
4.3. Structural Predictions for CfTX-1, CfTX-122–47, CfTX-173–100
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Brinkman, D.L.; Aziz, A.; Loukas, A.; Potriquet, J.; Seymour, J.; Mulvenna, J. Venom proteome of the box jellyfish Chironex fleckeri. PLoS ONE 2012, 7, e47866. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Burnell, J.N. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Seymour, J.E. In vitro effects on human heart and skeletal cells of the venom from two cubozoans, Chironex fleckeri and Carukia barnesi. Toxicon 2013, 76, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Jacups, S.P. Prospective study of Chironex fleckeri and other box jellyfish stings in the “Top End” of Australia’s Northern Territory. Med. J. Aust. 2005, 183, 631–636. [Google Scholar] [PubMed]
- O’Reilly, G.M.; Isbister, G.K.; Lawrie, P.M.; Treston, G.T.; Currie, B.J. Prospective study of jellyfish stings from tropical Australia, including the major box jellyfish Chironex fleckeri. Med. J. Aust. 2000, 175, 652–655. [Google Scholar]
- Beadnell, C.; Rider, T.; Williamson, J.; Fenner, P. Management of a major box jellyfish (Chironex fleckeri) sting. Lessons from the first minutes and hours. Med. J. Aust. 1992, 156, 655–658. [Google Scholar] [PubMed]
- Currie, B. Clinical implications of research on the box-jellyfish Chironex fleckeri. Toxicon 1994, 32, 1305–1313. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Jia, X.; Potriquet, J.; Kumar, D.; Dash, D.; Kvaskoff, D.; Mulvenna, J. Transcriptome and venom proteome of the box jellyfish Chironex fleckeri. BMC Genom. 2015, 16, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.; Burnell, J. Partial purification of cytolytic venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2008, 51, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.; Burnell, J. Identification, cloning and sequencing of two major venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2007, 50, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Bentlage, B.; Yanagihara, A.; Gillan, W.; Van Blerk, J.; Keil, D.P.; Bely, A.E.; Collins, A.G. Redescription of Alatina alata (Reynaud, 1830) (cnidaria: Cubozoa) from Bonaire, Dutch Caribbean. Zootaxa 2013, 3737, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and characterization of a novel protein toxin from the Hawaiian box jellyfish (sea wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A novel protein toxin from the deadly box jellyfish (sea wasp, habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Protze, J.; Piontek, J. Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin. Cell Dev. Biol. 2015, 42, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.J.; Saibil, H.R. The mechanism of pore formation by bacterial toxins. Curr. Opin. Struct. Biol. 2006, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; White, E.; Hongo, K.; Othman, I.; Orchard, C. The mechanism underlying the cardiotoxic effect of the toxin from the jellyfish Chironex fleckeri. Toxicol. Appl. Pharmacol. 1995, 133, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Sivaramakrishnan, S. Harnessing the unique structural properties of isolated α-helices. J. Biol. Chem. 2014, 289, 25460–25467. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hoang, H.N.; Liu, L.; Fairlie, D.P. Glucuronic acid as a helix-inducing linker in short peptides. Chem. Commun. 2018, 54, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.T.; Nielsen, N.C. VirtualSpectrum, a tool for simulating peak list for multidimensional NMR spectra. J. Biomol. NMR 2014, 60, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Bigam, C.G.; Holm, A.; Hodges, R.S.; Sykes, B.D. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J. Biomol. NMR 1995, 5, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Sykes, B.D.; Richards, F.M. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J. Mol. Biol. 1991, 222, 311–333. [Google Scholar] [CrossRef]
- Güntert, P. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 2004, 278, 353–378. [Google Scholar] [PubMed]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Zhang, C.; Mortuza, S.M.; He, B.; Wang, Y.; Zhang, Y. Template-based and free modeling of I-TASSER and quark pipelines using predicted contact maps in casp12. Proteins 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kinoshita, K. PrDOS: Prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 2007, 35, W460–W464. [Google Scholar] [CrossRef] [PubMed]
- Gottler, L.M.; Ramamoorthy, A. Structure, membrane orientation, mechanism, and function of pexiganan—A highly potent antimicrobial peptide designed from magainin. Biochim. Biophys. Acta 2009, 1788, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, B.-C.; Yu, Z.; Sun, M. Structural Insights into Bacillus thuringiensis Cry, Cyt and Parasporin Toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [PubMed]
- Bordag, N.; Keller, S. Α-helical transmembrane peptides: A “divide and conquer” approach to membrane proteins. Chem. Phys. Lipids 2010, 163, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.M.; Bakker, A.J.; Seymour, J.E.; Wilce, J.A. A functional comparison of the venom of three australian jellyfish–Chironex fleckeri, Chiropsalmus sp., and Carybdea xaymacana–on cytosolic Ca2+, haemolysis and artemia sp. Lethality. Toxicon 2005, 45, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, K. NMR of Proteins and Nucleic Acids; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 2009, 44, 213–223. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Peptide | |
|---|---|---|
| CfTX-122–47 | CfTX-173–100 | |
| Experimental Restraints | ||
| Interproton distance restraints | 263 | 437 |
| Intraresidue, |i − j| = 0 | 122 | 145 |
| Sequential, |i − j| = 1 | 82 | 158 |
| Medium range, 1 < |i − j| < 5 | 59 | 134 |
| Dihedral-angle restraints | 43 | 45 |
| R.M.S. Deviations from Mean Coordinate Structure (Å) | ||
| Backbone atoms (indicated) | 0.32 ± 0.12 (res. 4–19) | 0.43 ± 0.17 (res.3–20) |
| All heavy atoms (indicated) | 1.48 ± 0.23 (res. 4–19) | 1.06 ± 0.25 (res. 3–20) |
| Backbone atoms (all) | 2.19 ± 0.79 | 0.89 ± 0.32 |
| All heavy atoms (all) | 3.18 ± 0.87 | 1.36 ± 0.32 |
| Ramachandran (%) | ||
| Residues in most favoured regions | 85.8 | 91.3 |
| Residues in additionally allowed regions | 14.2 | 8.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreosso, A.; Bansal, P.S.; Smout, M.J.; Wilson, D.; Seymour, J.E.; Daly, N.L. Structural Characterisation of Predicted Helical Regions in the Chironex fleckeri CfTX-1 Toxin. Mar. Drugs 2018, 16, 201. https://doi.org/10.3390/md16060201
Andreosso A, Bansal PS, Smout MJ, Wilson D, Seymour JE, Daly NL. Structural Characterisation of Predicted Helical Regions in the Chironex fleckeri CfTX-1 Toxin. Marine Drugs. 2018; 16(6):201. https://doi.org/10.3390/md16060201
Chicago/Turabian StyleAndreosso, Athena, Paramjit S. Bansal, Michael J. Smout, David Wilson, Jamie E. Seymour, and Norelle L. Daly. 2018. "Structural Characterisation of Predicted Helical Regions in the Chironex fleckeri CfTX-1 Toxin" Marine Drugs 16, no. 6: 201. https://doi.org/10.3390/md16060201





