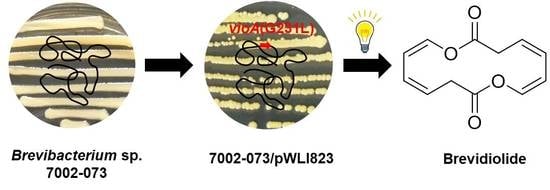
Heterologous Expression of a VioA Variant Activates Cryptic Compounds in a Marine-Derived Brevibacterium Strain
Abstract
:1. Introduction
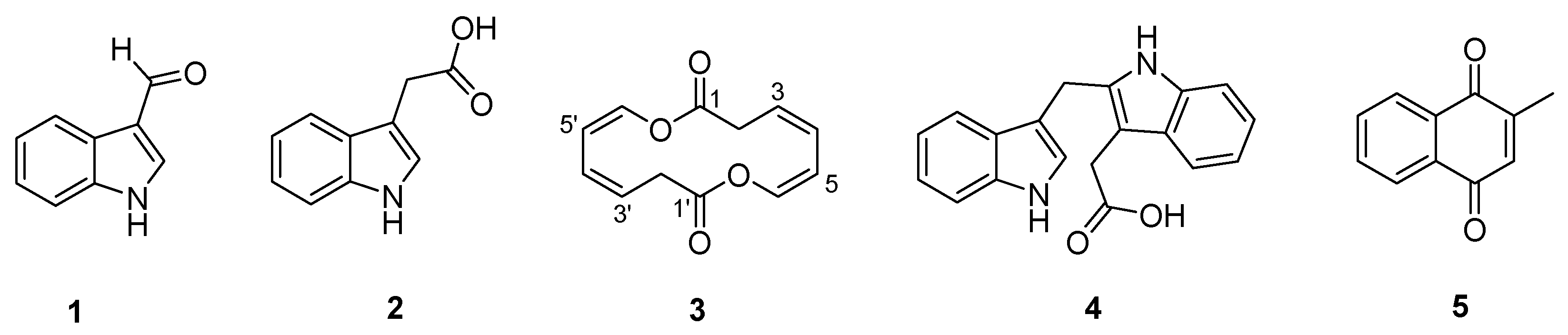
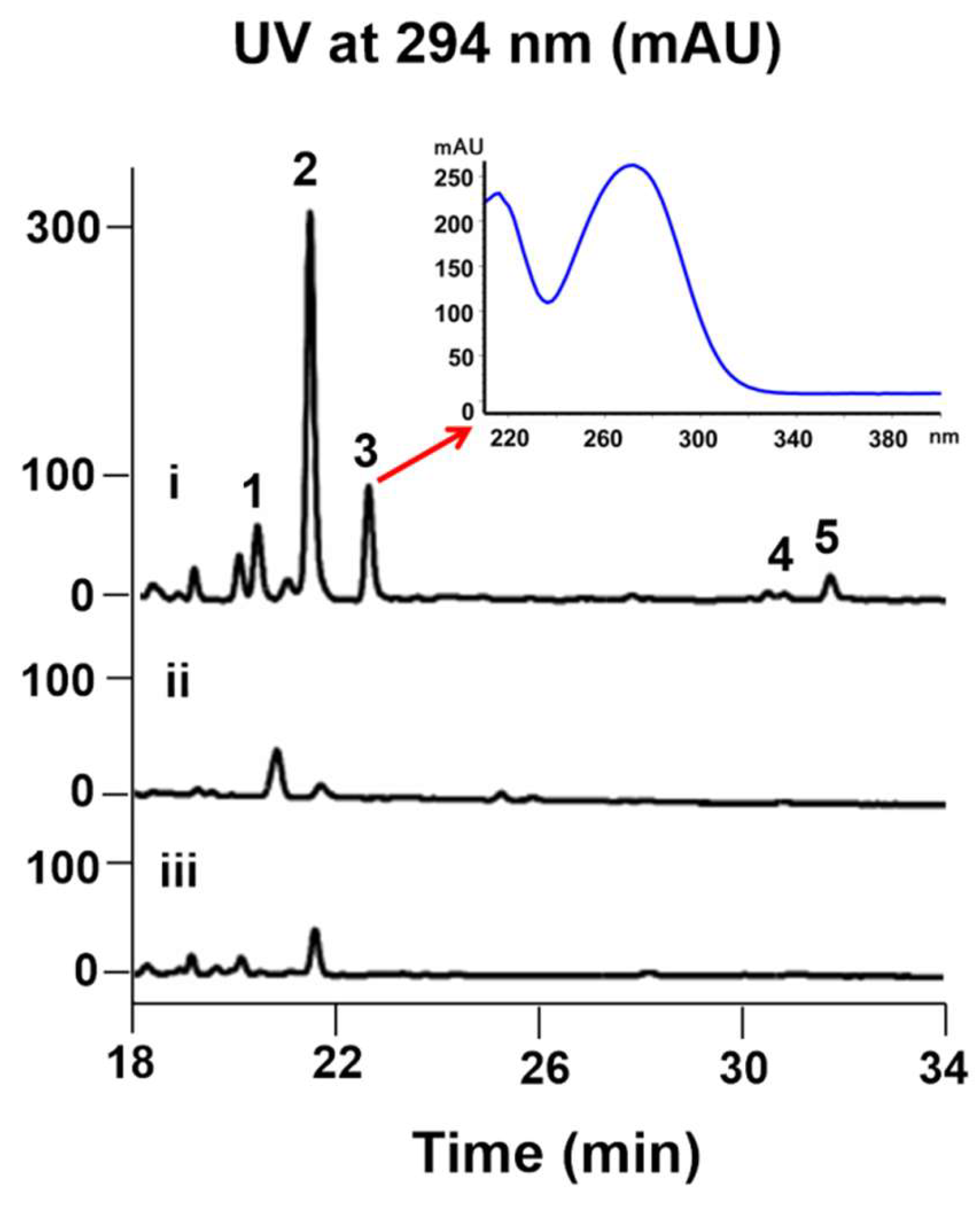
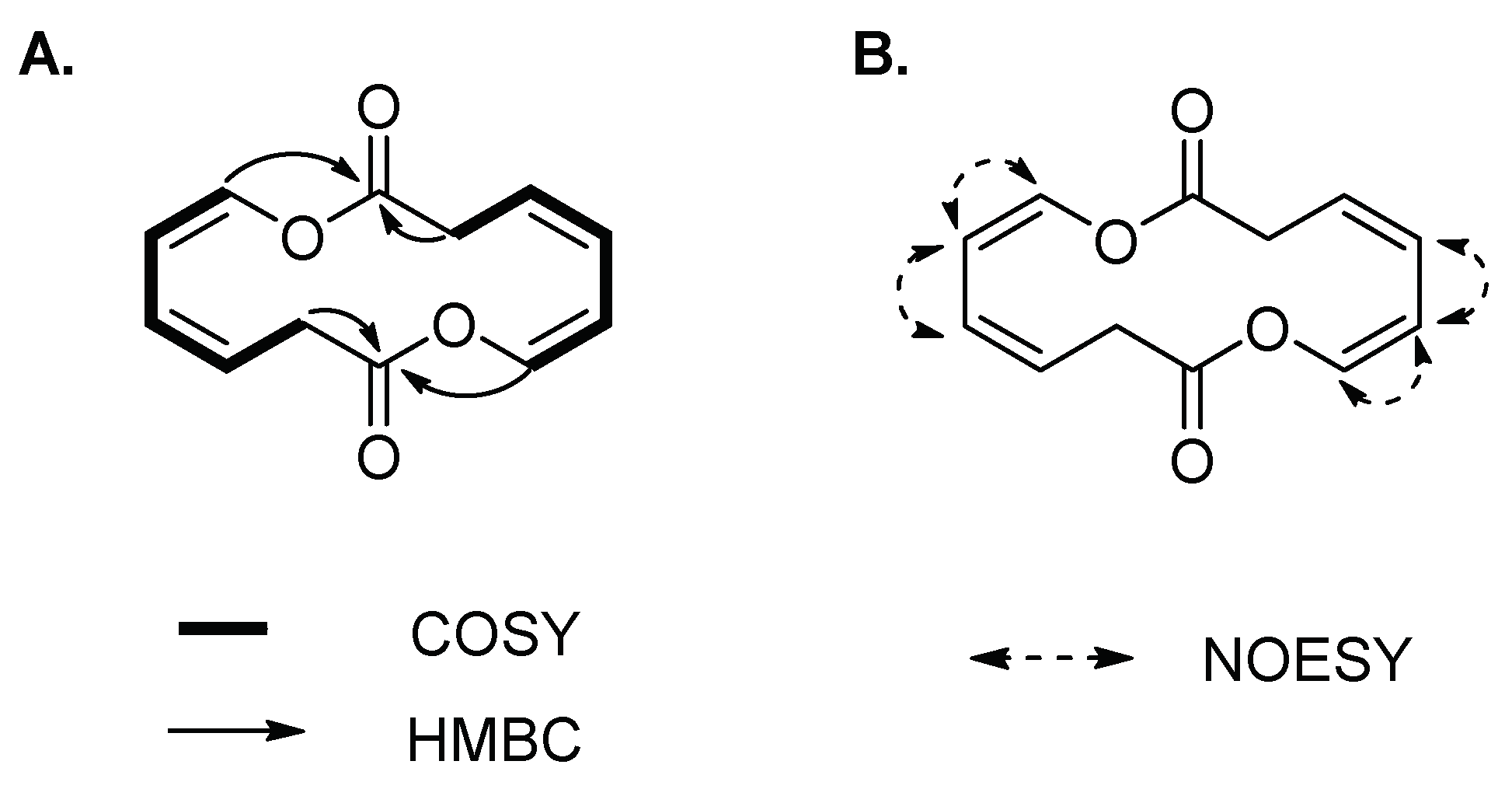
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strains, Plasmids, and Culture Conditions
3.3. Transformation Procedures
3.4. Isolation and Purification
3.5. Antibacterial Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Subramani, R.; Aalbersberg, W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012, 167, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.U.; Shaikh, A.L. Marine actinobacteria as a drug treasure house. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopwood, D.A.; Chater, K.F. Fresh approaches to antibiotic production. Philos. Trans. R. Soc. B Biol. Sciet. 1980, 290, 313–328. [Google Scholar] [CrossRef]
- Challis, G.L. Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 2008, 154, 1555–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.; Surette, M.G. Communication in bacteria: An ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; Traxler, M.F.; López, D.; Kolter, R. Antibiotics as signal molecules. Chem. Rev. 2011, 111, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hou, L.; Li, H.; Qiu, Y.; Ju, J.; Li, W. Activation of a plasmid-situated type III PKS gene cluster by deletion of awblgene in deepsea-derived Streptomyces somaliensis SCSIO ZH66. Microb. Cell Fact. 2016, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B.; Heermann, R. Pyrones as bacterial signaling molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Rattray, F.P.; Fox, P.F. Aspects of enzymology and biochemical properties of Brevibacterium linens relevant to cheese ripening: A review. J. Dairy Sci. 1999, 82, 891–909. [Google Scholar] [CrossRef]
- Xu, D.; Tan, Y.; Huan, X.; Hu, X.; Wang, X. Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J. Microbiol. Methods 2010, 80, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Guo, Q.; Peng, G.T.; He, X.X.; Chen, X.J.; Lei, L.F.; Deng, Y.; Su, X.J.; Zhang, C.X. New cyclic tetrapeptide from the coral-derived endophytic bacteria Brevibacterium sp. L-4 collected from the South China Sea. Nat. Prod. Res. 2016, 30, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Lipton, A.N.; Priyadharshini, S.; Anitha, K.; Suárez, L.E.; Arasu, M.V.; Choi, K.C.; Selvin, J.; Al-Dhabi, N.A. Antiadhesive activity of poly-hydroxy butyrate biopolymer from a marine Brevibacterium casei MSI04 against shrimp pathogenic vibrios. Microb. Cell Fact. 2014, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Sabarathnam, B.; Selvin, J. Biofilm disruption potential of a glycolipid biosurfactant from marine Brevibacterium casei. FEMS Immunol. Med. Microbiol. 2010, 59, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Huang, H.; Li, H.; Wang, S.; Ju, J.; Li, W. Overexpression of a type III PKS gene affording novel violapyrones with enhanced anti-influenza A virus activity. Microb. Cell Fact. 2018, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.A.; Elkhayat, E.S.; Ebel, R.; Edrada, R.; Proksch, P. Indole alkaloid from the red sea sponge Hyrtios erectus. Arkivoc 2007, 2007, 225–231. [Google Scholar]
- Lei, X.; Qiu, F.; Sun, H.; Bai, L.; Wang, W.X.; Xiang, W.; Xiao, H. A self-assembled oligopeptide as a versatile NMR alignment medium for the measurement of residual dipolar couplings in methanol. Angew. Chem. Int. Ed. Engl. 2017, 56, 12857–12861. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kawarada, A. Products of peroxidase catalyzed oxidation of indolyl-3-acetic acid. Agric. Biol. Chem. 1978, 42, 1315–1321. [Google Scholar]
- Lebrasseur, N.; Fan, G.J.; Oxoby, M.; Looney, M.A.; Quideau, S. λ3-Iodane-mediated arenol dearomatization. Synthesis of five-membered ring-containing analogues of the aquayamycin ABC tricyclic unit and novel access to the apoptosis inducer menadione. Tetrahedron 2005, 61, 1551–1562. [Google Scholar] [CrossRef]
- Wang, T.T.; Wei, Y.J.; Ge, H.M.; Jiao, R.H.; Tan, R.X. Acaulins A and B, trimeric macrodiolides from Acaulium sp. H-JQSF. Org. Lett. 2018, 20, 2490–2493. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Andrianasolo, E.H.; Shin, W.K.; Goeger, D.E.; Yokochi, A.; Schemies, J.; Jung, M.; France, D.; Cornell-kennon, S.; Lee, E.; et al. Structural and synthetic investigations of tanikolide dimer, a SIRT2 selective inhibitor, and tanikolide seco-acid from the madagascar marine cyanobacterium Lyngbya majuscula. J. Org. Chem. 2009, 74, 5267–5275. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhou, H.; Zhang, P.; Wang, X.; Li, W.; Zhang, W.; Liu, X.; Liu, H.W.; Keller, N.P.; An, Z.; et al. Polyketide production of pestaloficiols and macrodiolide ficiolides revealed by manipulations of epigenetic regulators in an endophytic fungus. Org. Lett. 2016, 18, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Grabley, S.; Hammann, P.; Thiericke, R.; Wink, J.; Philipps, S.; Zeeck, A. Secondary metabolites by chemical screening. 21. Clonostachydiol, a novel anthelmintic macrodiolide from the fungus Clonostachys cylindrospora (strain FH-A 6607). J. Antibiot. 1993, 46, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.; Sextius, P.; Bonnarme, P.; Spinnler, H.E.; Monnet, V.; Irlinger, F. Genetic transformation of Brevibacterium linens strains producing high amounts of diverse sulphur compounds. J. Dairy Res. 2005, 72, 179–187. [Google Scholar] [CrossRef] [PubMed]
| Position | δH (J in Hz) | δC |
|---|---|---|
| 1 | 167.4 | |
| 2 | 3.66, m | 29.8 |
| 3 | 5.95, m | 127.3 |
| 4 | 6.04, dd (6.6, 9.0) | 126.1 |
| 5 | 5.48, t (6.6) | 107.5 |
| 6 | 6.60, d (7.2) | 142.4 |
| 1′ | 167.4 | |
| 2′ | 3.66, m | 29.8 |
| 3′ | 5.95, m | 127.3 |
| 4′ | 6.04, m | 126.1 |
| 5′ | 5.48, t (6.6) | 107.5 |
| 6′ | 6.60, d (7.2) | 142.4 |
| Strains | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Staphylococcus aureus | >12.5 | >12.5 | >12.5 | >12.5 | 3.12 |
| Escherichia coli | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| Enterococcus faecalis | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| Enterococcus faecium | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| Salmonella typhimurium | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| Acinetobacter baumannii | >12.5 | >12.5 | >12.5 | >12.5 | >12.5 |
| Klebsiella pneumoniae | >12.5 | >12.5 | >12.5 | >12.5 | 3.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Hou, L.; Hou, J.; Zhang, Y.; Li, H.; Li, W. Heterologous Expression of a VioA Variant Activates Cryptic Compounds in a Marine-Derived Brevibacterium Strain. Mar. Drugs 2018, 16, 191. https://doi.org/10.3390/md16060191
Han X, Hou L, Hou J, Zhang Y, Li H, Li W. Heterologous Expression of a VioA Variant Activates Cryptic Compounds in a Marine-Derived Brevibacterium Strain. Marine Drugs. 2018; 16(6):191. https://doi.org/10.3390/md16060191
Chicago/Turabian StyleHan, Xiao, Lukuan Hou, Jing Hou, Yongyu Zhang, Huayue Li, and Wenli Li. 2018. "Heterologous Expression of a VioA Variant Activates Cryptic Compounds in a Marine-Derived Brevibacterium Strain" Marine Drugs 16, no. 6: 191. https://doi.org/10.3390/md16060191