Diterpenes from the Marine Algae of the Genus Dictyota
Abstract
:1. Introduction
2. Diterpenes of Group I
2.1. Prenylated-Guaiane Diterpenes
2.2. Other Diterpenes of Group 1
3. Diterpenes of Group 1I
3.1. Dolabellane Diterpenes
3.2. Dolastane Diterpenes
3.3. Secodolastane Diterpenes
3.4. Dictyoxetane Diterpenes
4. Diterpenes of Group III
4.1. Xenicane Diterpenes
4.2. Crenulidane Diterpenes
4.3. Dichotomane Diterpenes
4.4. Crenulane Diterpenes
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Teixeira, V.L.; Kelecom, A. A chemotaxonomic study of diterpenes from marine brown algae of the genus Dictyota. Sci. Total Environ. 1988, 75, 271–283. [Google Scholar] [CrossRef]
- Taylor, R.B.; Lindquist, N.; Kubanek, J.; Hay, M.E. Intraspecific variation in palatability and defensive chemistry of brown seaweeds: Effects on herbivore fitness. Oecologia 2003, 136, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. I. Estimation of absolute polyphenol content of Ascophyllum nodosum (L.) le jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1977, 30, 209–221. [Google Scholar] [CrossRef]
- Bouzidi, N.; Daghbouche, Y.; El Hattab, M.; Aliche, Z.; Culioli, G.; Piovetti, L.; Garrigues, S.; de la Guardia, M. Determination of total sterols in brown algae by fourier transform infrared spectroscopy. Anal. Chim. Acta 2008, 616, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Yokoya, N.S.; Colepicolo, P. Biochemical modulation by carbon and nitrogen addition in cultures of Dictyota menstrualis (dictyotales, phaeophyceae) to generate oil-based bioproducts. Mar. Biotechnol. 2016, 18, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, A.F.; Hussein, M.D.; Fouad, S.T. Carbohydrates of the brown seaweed Dictyota dichotoma. Phytochemistry 1978, 17, 741–743. [Google Scholar] [CrossRef]
- Gaysinski, M.; Ortalo-Magné, A.; Thomas, O.P.; Culioli, G. Extraction, purification, and NMR analysis of terpenes from brown algae. Methods Mol. Biol. 2015, 1308, 207–223. [Google Scholar] [PubMed]
- Duh, C.Y.; Sheu, H.R. Cytotoxic cembranoids from the soft corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod. 1996, 59, 595–598. [Google Scholar] [CrossRef]
- Reyes, F.; Ardá, A.; Martín, R.; Fernández, R.; Rueda, A.; Montalvo, D.; Gómez, C.; Jiménez, C.; Rodríguez, J.; Ma, S.J. New cytotoxic cembranes from the Sea Pen Gyrophyllum sibogae. J. Nat. Prod. 2004, 67, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Iwashima, M.; Matsumoto, Y.; Takahashi, H.; Iguchi, K. New marine cembrane-type diterpenoids from the okinawan soft coral Clavularia koellikeri. J. Nat. Prod. 2000, 63, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, J.C.; Bueno, L.B.; Cavalcanti, D.N.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Diterpenes from the brown alga Dictyota crenulata. Molecules 2008, 13, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, M.O.; Kusumi, T.; Kakisawa, H. Antitumor xenicane and norxenicane lactones from the brown alga Dictyota dichotoma. J. Org. Chem. 1988, 53, 5010–5013. [Google Scholar] [CrossRef]
- Barbosa, J.P.; Teixeira, V.L.; Pereira, R.C. A dolabellane diterpene from the brown alga Dictyota pfaffii as chemical defense against herbivores. Bot. Mar. 2004, 47, 147–151. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrin, Y.; Robledo, D. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.E.; Makki, M.S.; Al-Kayal, N.S.; Basaif, S.A.; El-Foty, K.O.; Asiri, A.M.; Alarif, W.M.; Badria, F.A. Cytotoxic and protective DNA damage of three new diterpenoids from the brown alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014, 77, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-scale purification of pachydictyol a from the brown alga Dictyota dichotoma obtained from algal wash and evaluation of its antifouling activity against the freshwater mollusk Limnoperna fortunei. J. Appl. Phycol. 2017, 30, 629–636. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Zavodnik, N. Hydroazulenoid diterpenes from the brown alga Dictyota dichotoma var. Implexa. Phytochemistry 1986, 25, 2179–2181. [Google Scholar] [CrossRef]
- Nagle, D.G.; Sultana, G.N.N.; Schrader, K.K.; Hossain, C.F.; Stanikunaite, R.; Hamann, M.T.; Rajbandari, I. Secondary metabolites from plants and marine organisms as selective anti-cyanobacterial agents. ACS Symp. 2003, 848, 179–194. [Google Scholar]
- Simas, D.L.R.; Kaiser, C.R.; Gestinari, L.M.; Duarte, H.M.; de Paula, J.C.; Soares, A.R. Diterpenes from the brown seaweed Dictyota caribaea (dictyotaceae, phaeophyceae): The ecological and taxonomic significance. Biochem. Syst. Ecol. 2014, 52, 33–37. [Google Scholar] [CrossRef]
- Palermo, J.; Bernardo, J.; Seldes, A. In Dictyol-d-2-beta-acetate and other diterpenoids from the brown alga Dictyota dichotoma. An. Asoc. Quim. Argent. 1994, 82, 355–358. [Google Scholar]
- Abou-El-Wafa, G.S.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols b and c: New diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef] [PubMed]
- Othmani, A.; Bouzidi, N.; Viano, Y.; Alliche, Z.; Seridi, H.; Blache, Y.; El Hattab, M.; Briand, J.-F.; Culioli, G. Anti-microfouling properties of compounds isolated from several Mediterranean Dictyota spp. J. Appl. Phycol. 2013, 26, 1573–1584. [Google Scholar] [CrossRef]
- Choi, B.W.; Lee, H.S.; Lee, K.B.; Lee, B.H. Isolation of diacyl glycerol acyl transferase (DGAT) inhibitors from Pachydictyon coriaceum. Phytother. Res. 2011, 25, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Sticher, O.; Rüegger, H. Four new hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Planta Med. 1993, 59, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C.; Andersen, R.J. Diterpenoids from the brown alga Dictyota binghamiae. Can. J. Chem. 1984, 62, 1666–1671. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. New xenicane and hydroazulenoid diterpenes from an Australian collection of Dictyota divaricata. Tetrahedron 1991, 22, 1399–1410. [Google Scholar] [CrossRef]
- Alarado, A.B.; Gerwick, W.H. Dictyol h, a new tricyclic diterpenoid from the brown seaweed Dictyota dentata. J. Nat. Prod. 2004, 48, 132–134. [Google Scholar] [CrossRef]
- Kim, J.Y.; Alamsjah, M.A.; Hamada, A.; Fujita, Y.; Ishibashi, F. Algicidal diterpenes from the brown alga Dictyota dichotoma. Biosci. Biotechnol. Biochem. 2014, 70, 2571–2574. [Google Scholar] [CrossRef]
- Pereira, R.C.; Lourenco, A.L.; Terra, L.; Abreu, P.A.; Laneuville Teixeira, V.; Castro, H.C. Marine diterpenes: Molecular modeling of thrombin inhibitors with potential biotechnological application as an antithrombotic. Mar. Drugs 2017, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Koenig, G.M.; Sticher, O. New and highly oxidised hydroazulenoid diterpenes from the tropical marine brown alga Dictyota volubilis. Tetrahedron 1993, 49, 571–580. [Google Scholar] [CrossRef]
- Jongaramruong, J.; Kongkam, N. Novel diterpenes with cytotoxic, anti-malarial and anti-tuberculosis activities from a brown alga Dictyota sp. J. Asian Nat. Prod. Res. 2007, 9, 743–751. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Nys, R.D.; Sticher, O. A diterpene from the marine brown alga Dictyota bartayresii. Phytochemistry 1992, 31, 2541–2542. [Google Scholar] [CrossRef]
- Gedara, S.R.; Abdelhalim, O.B.; Elsharkawy, S.H.; Salama, O.M.; Shier, T.W.; Halim, A.F. Cytotoxic hydroazulene diterpenes from the brown alga Dictyota dichotoma. Z. Naturforsch. C. Biosci. 2003, 58, 17–22. [Google Scholar] [CrossRef]
- Hardt, I.H.; Fenical, W.; Cronin, G.; Hay, M.E. Acutilols, potent herbivore feeding deterrents from the tropical brown alga, Dictyota acutiloba. Phytochemistry 1996, 43, 71–73. [Google Scholar] [CrossRef]
- Ayyad, S.E.; Abdel-Halim, O.B.; Shier, W.T.; Hoye, T.R. Cytotoxic hydroazulene diterpenes from the brown alga Cystoseira myrica. Z. Naturforsch. C Biosci. 2003, 58, 33–38. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Lyakhova, E.G.; Kalinovsky, A.I.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Stonik, V.A. Diterpenoid hydroperoxides from the far-eastern brown alga Dictyota dichotoma. Aust. J. Chem. 2009, 62, 1185–1188. [Google Scholar] [CrossRef]
- Siamopoulou, P.; Bimplakis, A.; Iliopoulou, D.; Vagias, C.; Cos, P.; Vanden Berghe, D.; Roussis, V. Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 2004, 65, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Fenical, W. Hydroxydilophol, a new monocyclic diterpenoid from the brown alga Dictyota masonii. J. Org. Chem. 1979, 10, 1354–1356. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Sticher, O. Diterpenes from the brown alga Dictyota divaricata. Phytochemistry 1991, 30, 3679–3682. [Google Scholar] [CrossRef]
- Viano, Y.; Bonhomme, D.; Camps, M.; Briand, J.F.; Ortalomagné, A.; Blache, Y.; Piovetti, L.; Culioli, G. Diterpenoids from the mediterranean brown alga Dictyota sp. Evaluated as antifouling substances against a marine bacterial biofilm. J. Nat. Prod. 2009, 72, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, M.O.; Ichikawa, A.; Kusumi, T.; Kakisawa, H. Existence of a termite soldier diterpene-like substances in the dictyotaceae algae. Symp. Chem. Natl. Prod. 1988, 188–195. [Google Scholar] [CrossRef]
- Kolesnikova, S.A.; Kalinovsky, A.I.; Fedorov, S.N.; Shubina, L.K.; Stonik, V.A. Diterpenes from the far-eastern brown alga Dictyota dichotoma. Phytochemistry 2006, 67, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Currenti, R.; Oriente, G.; Piattelli, M.; Tringali, C. 18-hydroxy 3,7-dolabelladiene from the brown alga, Dictyota dichotoma. Phytochemistry 1981, 20, 848–849. [Google Scholar] [CrossRef]
- Tringali, C.; Oriente, G.; Piattelli, M.; Nicolosi, G. Structure and conformation of two new dolabellane-based diterpenes from Dictyota sp. J. Nat. Prod. 1984, 47, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.R.; Cirne-Santos, C.C.; de Palmer Paixao, I.C.; Ramos, F.A.; Jimenez, C.; Rodriguez, J.; Resende, J.A.; Teixeira, V.L.; et al. Dolabelladienols a-c, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M. An update of terpenoids, steroids and biodiversity of seaweeds from the coasts of Pakistan. J. Chem. Soc. Pak. 2010, 32, 379–395. [Google Scholar]
- Ireland, C.; Faulkner, D.J. Diterpenes from Dolabella californica. J. Org. Chem. 1977, 42, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Wang, Y.Y.; Zhao, P.J.; Li, Y.; Luo, X.D. Dolabellane diterpenoids from Aglaia odorata. Phytochemistry 2010, 71, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Bouaïcha, N.; Tringali, C.; Pesando, D.; Malléa, M.; Roussakis, C.; Verbist, J.F. Bioactive diterpenoids isolated from Dilophus ligulatus. Planta Med. 1993, 59, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.B.; Pullaiah, K.C.; Surapaneni, R.K.; Sullivan, B.W.; Albizati, K.F.; Faulkner, D.J.; He, C.; Clardy, J. The diterpenes of Dictyota dichotoma from the Indian ocean. J. Org. Chem. 1987, 18, 2736–2742. [Google Scholar] [CrossRef]
- Rao, C.B.; Trimurtulu, G.; Sreedhara, C.; Rao, D.V.; Bobzin, S.C.; Faulkner, D.J. Diterpenes from the brown alga Dictyota bartayresiana. Phytochemistry 1994, 37, 509–513. [Google Scholar] [CrossRef]
- Wright, A.D.; König, G.M.; Sticher, O. Two new dolabellane derivatives from the brown alga Dictyota pardarlis. Tetrahedron 1990, 46, 3851–3858. [Google Scholar] [CrossRef]
- Wright, A.D.; König, G.M.; Sticher, O. New dolabellane derivatives from the brown alga Dictyota pardalis. Helv. Chim. Acta 1991, 74, 1801–1807. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D. New dolabellanes from the marine alga Dictyota pardalis f. Pseudohamata. Tetrahedron 1994, 50, 8011–8018. [Google Scholar] [CrossRef]
- Stephens, P.R.S.; Cirne-Santos, C.C.; de Souza Barros, C.; Teixeira, V.L.; Carneiro, L.A.D.; Amorim, L.D.S.C.; Ocampo, J.S.P.; Castello-Branco, L.R.R.; de Palmer Paixão, I.C.N. Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J. Appl. Phycol. 2016, 29, 775–780. [Google Scholar] [CrossRef]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rebello, M.A.; Frugulhetti, I.C.; Texeira, V.L. In vitro antiviral diterpenes from the brazilian brown alga Dictyota pfaffii. Planta Med. 2004, 70, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; de Palmer Paixao, I.C.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl. Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Quesada, A.; Rahman, M.M.; Gibbons, S.; Vagias, C.; Roussis, V. Dolabellanes with antibacterial activity from the brown alga Dilophus spiralis. J. Nat. Prod. 2011, 74, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Klein, T.E.; Hogue, E.R.; Myers, B.L. Tricyclic diterpenes from the brown marine algae Dictyota divaricata and Dictyota linearis. J. Org. Chem. 1982, 13, 811–815. [Google Scholar] [CrossRef]
- Kelecom, A.; Teixeira, V.L. Dolastane diterpenes from the marine brown alga Dictyota cervicornis. Phytochemistry 1988, 27, 2907–2909. [Google Scholar] [CrossRef]
- Ali, M.S.; Pervez, M.K. Ring-a hydroxylated dolastanes from the marine brown alga Dictyota dichotoma (HUDS.) lamour. Nat. Prod. Res. 2003, 17, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ahma, V.U.; Parveen, S.; Bano, S.; Shaikha, W.; Shameela, M. Dolastane diterpenoids from the brown alga Dictyota indica. Phytochemistry 1991, 30, 1015–1018. [Google Scholar] [CrossRef]
- Garcia, D.G.; Bianco, E.M.; Santos Mda, C.; Pereira, R.C.; Faria, M.V.; Teixeira, V.L.; Burth, P. Inhibition of mammal Na+K+-ATPase by diterpenes extracted from the Brazilian brown alga Dictyota cervicornis. Phytother. Res. 2009, 23, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.O.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.; Pereira, R.C.; Nakamura, C.V. 4-acetoxydolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis as antileishmanial agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef] [PubMed]
- Bianco, É.M.; Rogers, R.; Teixeira, V.L.; Pereira, R.C. Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J. Appl. Phycol. 2008, 21, 341–346. [Google Scholar] [CrossRef]
- Bianco, E.M.; Teixeira, V.L.; Pereira, R.C.; de Souza, A.M.; Nucci, P.; Afonso, I.F.; Rodrigues, C.R.; Castro, H.C. Brown seaweed defensive chemicals: A structure-activity relationship approach for the marine environment. Nat. Prod. Commun. 2009, 4, 173–178. [Google Scholar] [PubMed]
- De Souza Barros, C.; Cirne-Santos, C.C.; Garrido, V.; Barcelos, I.; Stephens, P.R.S.; Giongo, V.; Teixeira, V.L.; de Palmer Paixão, I.C.N. Anti-HIV-1 activity of compounds derived from marine alga Canistrocarpus cervicornis. J. Appl. Phycol. 2015, 28, 2523–2527. [Google Scholar] [CrossRef]
- Sun, H.H.; Mcconnell, O.J.; Fenical, W.; Hirotsu, K.; Clardy, J. Tricyclic diterpenoids of the dolastane ring system from the marine alga Dictyota divaricata. Tetrahedron 1981, 37, 1237–1242. [Google Scholar] [CrossRef]
- Ochi, M.; Watanabe, M.; Kido, M.; Ichikawa, Y.; Miura, I.; Tokoroyama, T. Amijidictyol, a new diterpenoid from the brown seaweed Dictyota linearis: X-ray crystal and molecular structure. Chem. Lett. 1980, 16, 1233–1234. [Google Scholar] [CrossRef]
- Begley, M.J.; Pattenden, G.; Robertson, G.M. Synthetic radical chemistry. Total synthesis of (±)-isoamijiol. J. Chem. Soc. Perkin Trans. 1988, 46, 1085–1094. [Google Scholar] [CrossRef]
- Dunlop, R.W.; Ghisalberti, E.L.; Jefferies, P.R.; Skelton, B.W.; White, A.H. Structure of a new dolastane diterpene from Dictyota furcellata. Aust. J. Chem. 1989, 42, 315–319. [Google Scholar] [CrossRef]
- González, A.G.; Martiń, J.D.; Norte, M.; Rivera, P.; Perales, A.; Fayos, J. Structure and absolute configurations of Dictyota sp. Diterpenes. Tetrahedron 1983, 39, 3355–3357. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Tomassini, T.; Kelecom, A. Cervicol, a further secodolastane diterpene from the marine brown alga Dictyota cervicornis küzing (Phaeophyceae, Dictyotaceae). Bull. Soc. Chim. Belg. 1986, 95, 263–268. [Google Scholar] [CrossRef]
- Bano, S.; Parveen, S.; Ahmad, V.U. Marine natural products, XIV secodolastane diterpenoids of Dictyota indica from the Arabian sea. J. Nat. Prod. 1990, 53, 492–495. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, S.; Yuan, W.; Dong, J.; Huang, K.; Sun, Z.; Yan, P. Further new xenicanes from a Chinese collection of the brown alga Dictyota plectens. Chem. Pharm. Bull. 2015, 63, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Finer, J.; Clardy, J.; Fenical, W.; Minale, L.; Riccio, R.; Battaile, J.; Kirkup, M.; Moore, R.E. Structures of dictyodial and dictyolactone, unusual marine diterpenoids. J. Org. Chem. 1979, 44, 2044–2047. [Google Scholar] [CrossRef]
- Enoki, N.; Ishida, R.; Matsumoto, T. Structures and conformations of new nine-membered ring diterpenoids from the marine alga Dictyota dichotoma. Chem. Lett. 1982, 11, 1749–1752. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T. Hydroxydictyodial, a new antifeedant diterpene from the brown alga Dictyota spinulosa. Chem. Lett. 1984, 2, 231–232. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Bakkas, S.; Villani, G.; Varcamonti, M.; Zanfardino, A.; Gavagnin, M. Diterpene content of the alga Dictyota ciliolata from a Moroccan lagoon. Phytochem. Lett. 2009, 2, 211–215. [Google Scholar] [CrossRef]
- Patil, A.D.; Berry, D.; Brooks, D.P.; Hemling, M.E.; Kumar, N.V.; Mitchell, M.P.; Ohlstein, E.H.; Westley, J.W. A diterpene epoxide from the marine brown alga Dictyota sp.: Possible vasopressin V1 receptor antagonist. Phytochemistry 1993, 33, 1061–1064. [Google Scholar] [CrossRef]
- Viano, Y.; Bonhomme, D.; Ortalo-Magné, A.; Thomas, O.P.; Hattab, M.E.; Piovetti, L.; Blache, Y.; Culioli, G. Dictyotadimer a, a new dissymmetric bis-diterpene from a brown alga of the genus Dictyota. Tetrahedron Lett. 2011, 52, 1031–1035. [Google Scholar] [CrossRef]
- Kusumi, T.; Muanza-Nkongolo, D.; Goya, M.; Ishitsuka, M.; Iwashita, T.; Kakisawa, H. Structures of crenulacetals a, b, c, and d. The new diterpenoids from the brown algae of Dictyotaceae. J. Org. Chem. 1986, 17, 384–387. [Google Scholar] [CrossRef]
- Takikawa, M.; Uno, K.; Ooi, T.; Kusumi, T.; Akera, S.; Muramatsu, M.; Mega, H.; Horita, C. Crenulacetal c, a marine diterpene, and its synthetic mimics inhibiting Polydora websterii, a harmful lugworm damaging pearl cultivation. Chem. Pharm. Bull. 1998, 29, 462–466. [Google Scholar] [CrossRef]
- Wang, T.Z.; Emmanuel Pinard, A.; Paquette, L.A. Asymmetric synthesis of the diterpenoid marine toxin (+)-acetoxycrenulide. J. Am. Chem. Soc. 1996, 118, 1309–1318. [Google Scholar] [CrossRef]
- Pereira, H.S.; Leao-Ferreira, L.R.; Moussatche, N.; Teixeira, V.L.; Cavalcanti, D.N.; Costa, L.J.; Diaz, R.; Frugulhetti, I.C. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2004, 64, 69–76. [Google Scholar] [CrossRef]
- Abrantes, J.L.; Barbosa, J.; Cavalcanti, D.; Pereira, R.C.; Frederico Fontes, C.L.; Teixeira, V.L.; Moreno Souza, T.L.; Paixao, I.C. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Cavalcanti, D.N.; Teixeira, V.L. Effects of secondary metabolites from the tropical Brazilian brown alga Dictyota menstrualis on the amphipod Parhyale hawaiensis. Mar. Ecol. Prog. Ser. 2000, 205, 95–100. [Google Scholar] [CrossRef]
- Fonseca, R.R.; Filho, A.P.; Villaça, R.C.; Teixeira, V.L. Inhibitory effects against pasture weeds in Brazilian amazonia of natural products from the marine brown alga Dictyota menstrualis. Nat. Prod. Commun. 2013, 8, 1669–1672. [Google Scholar] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. R. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
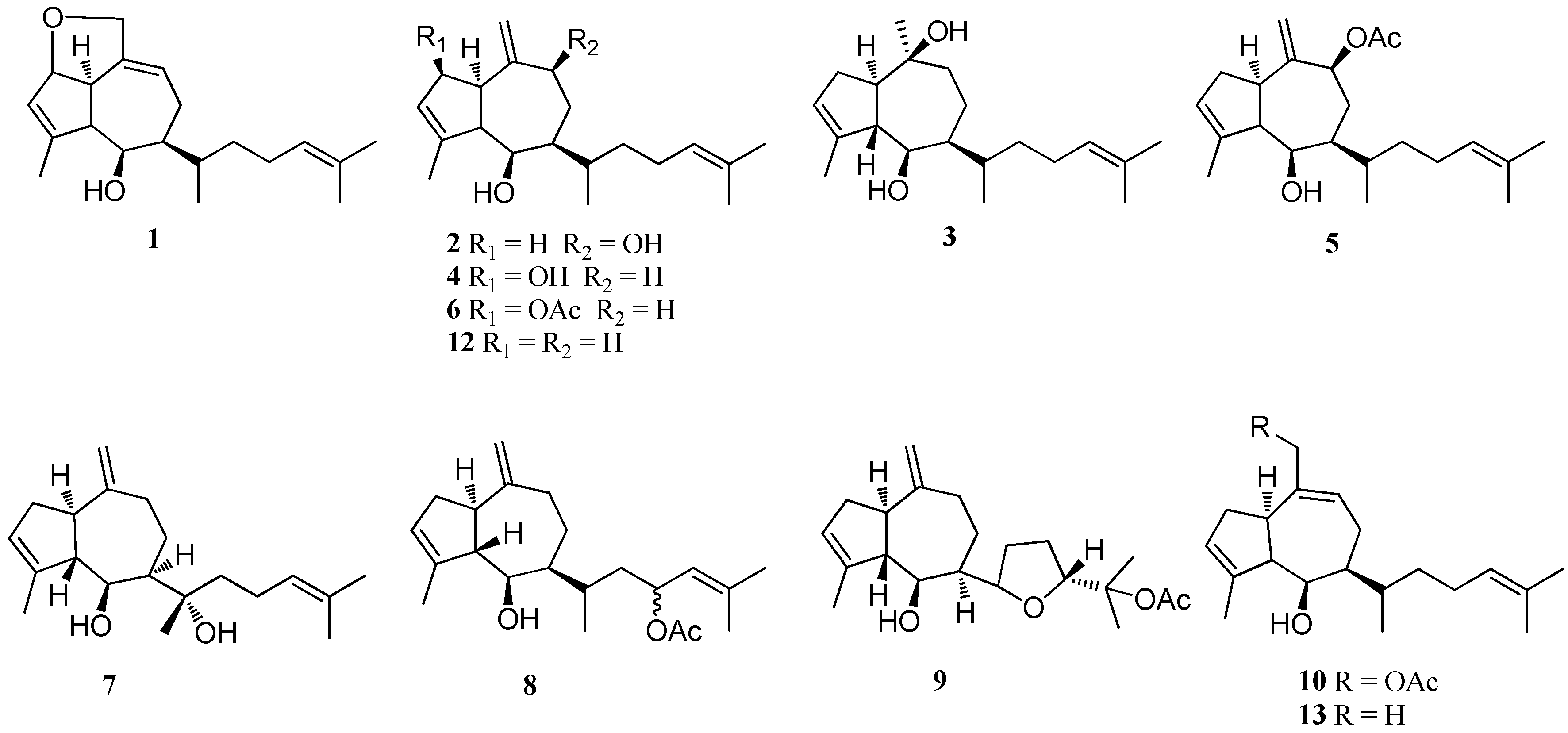
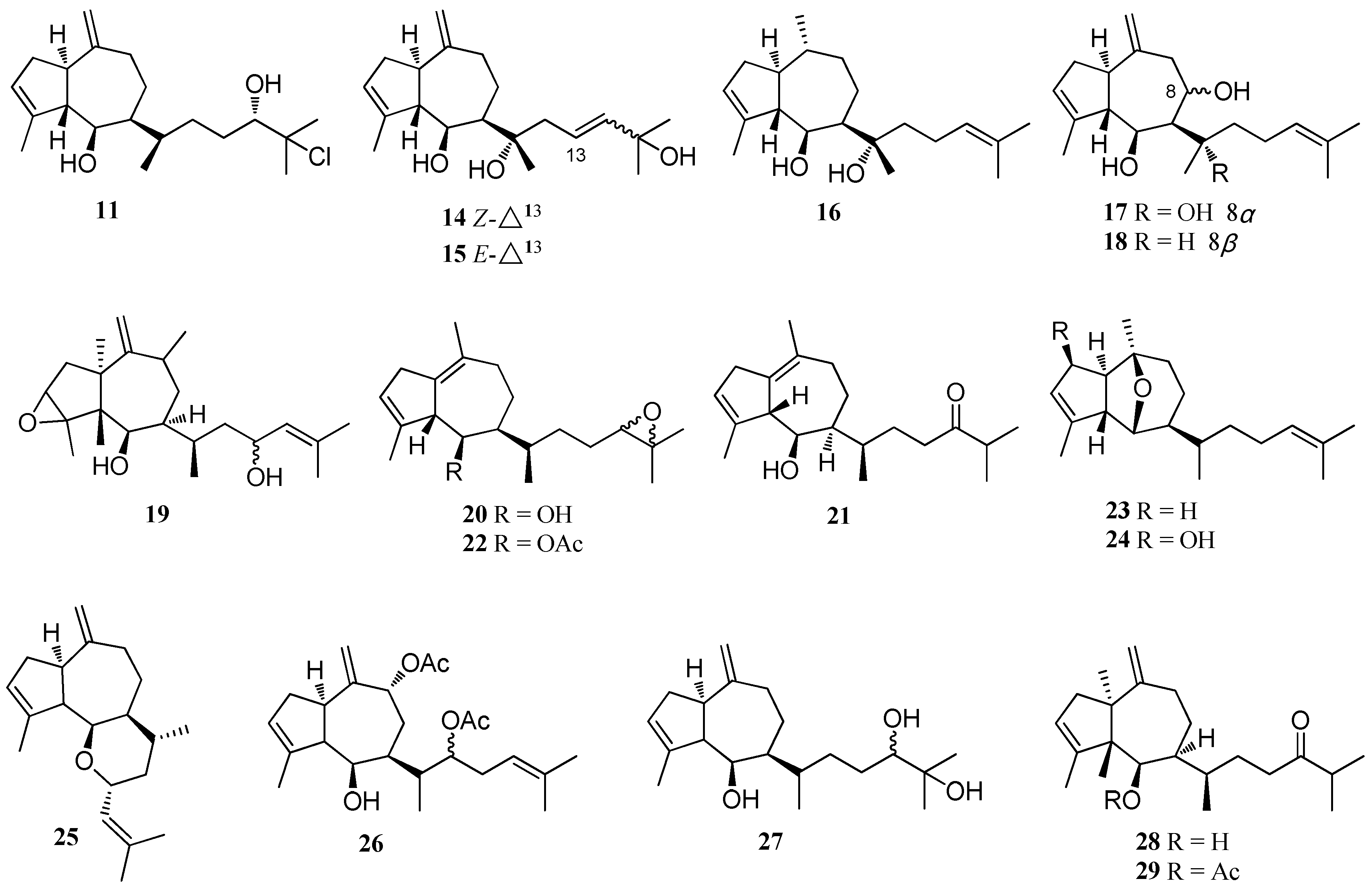



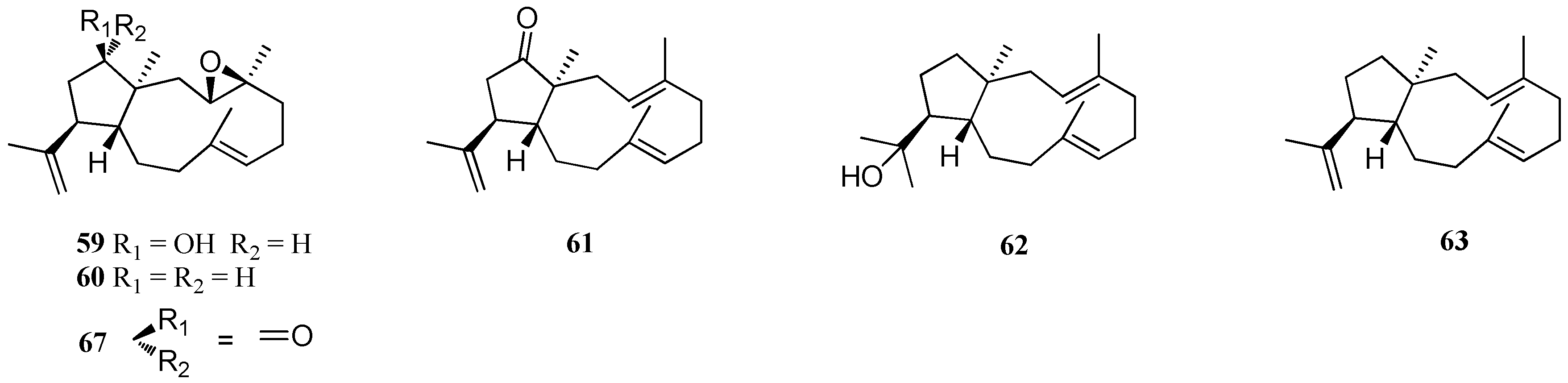
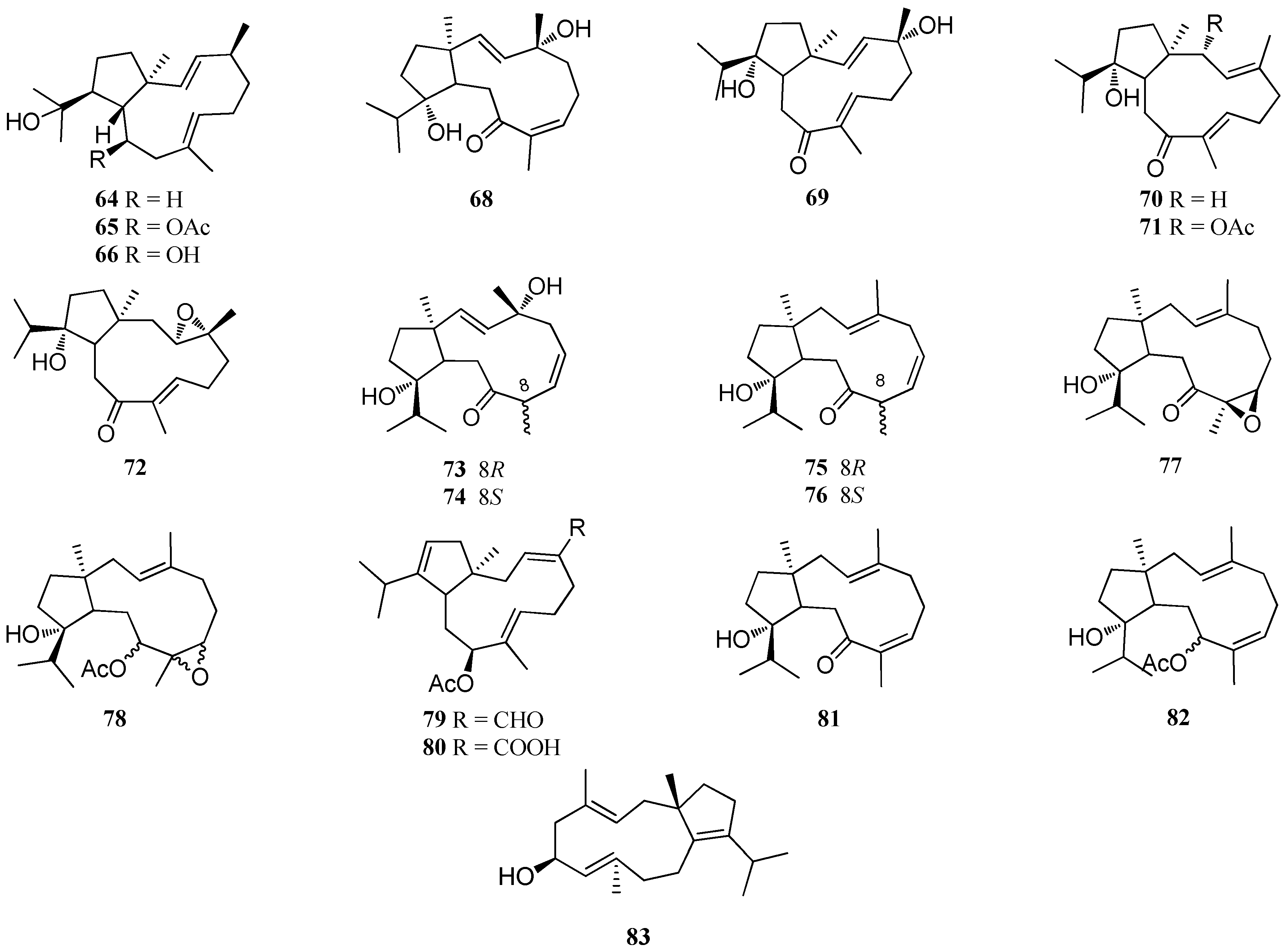
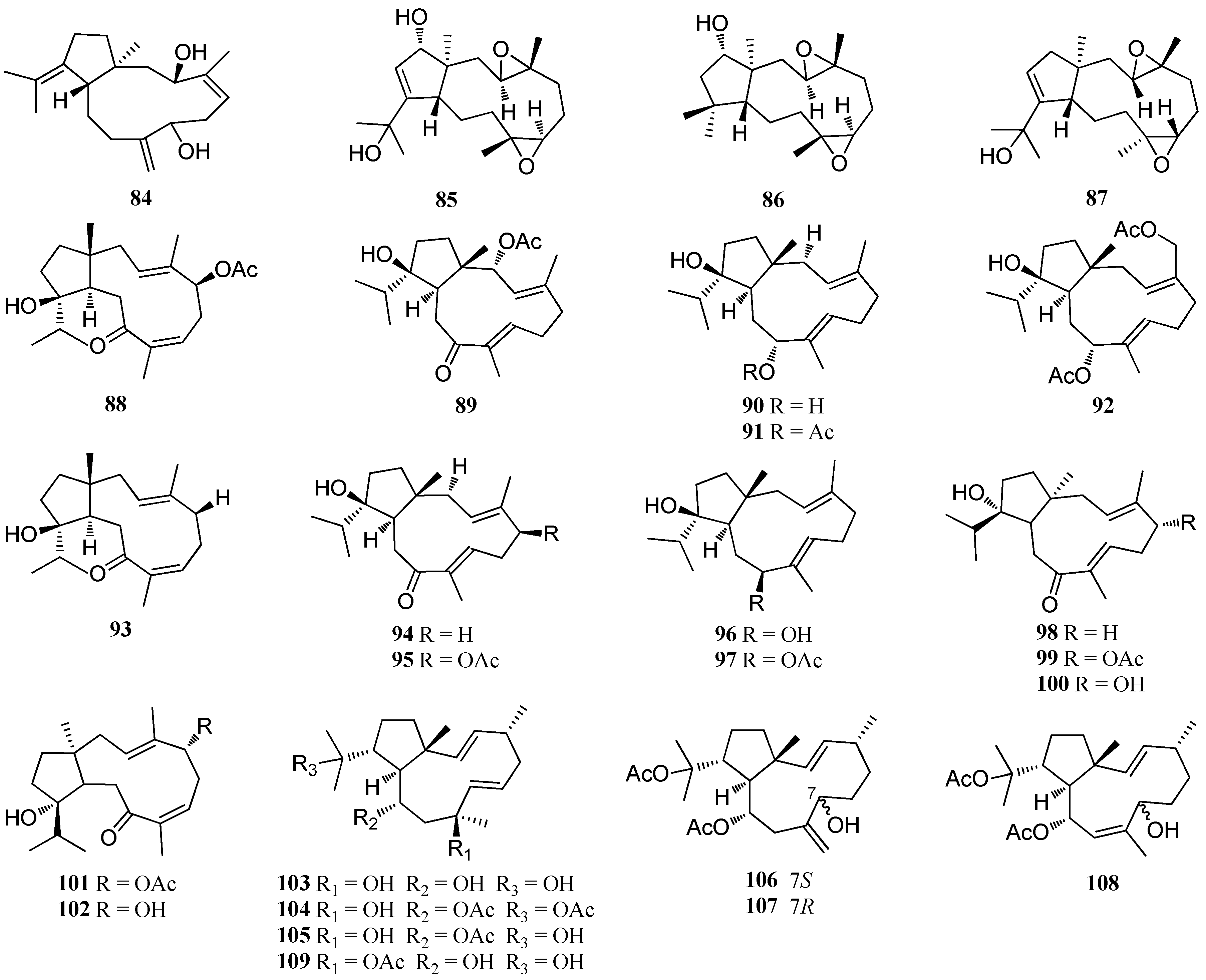
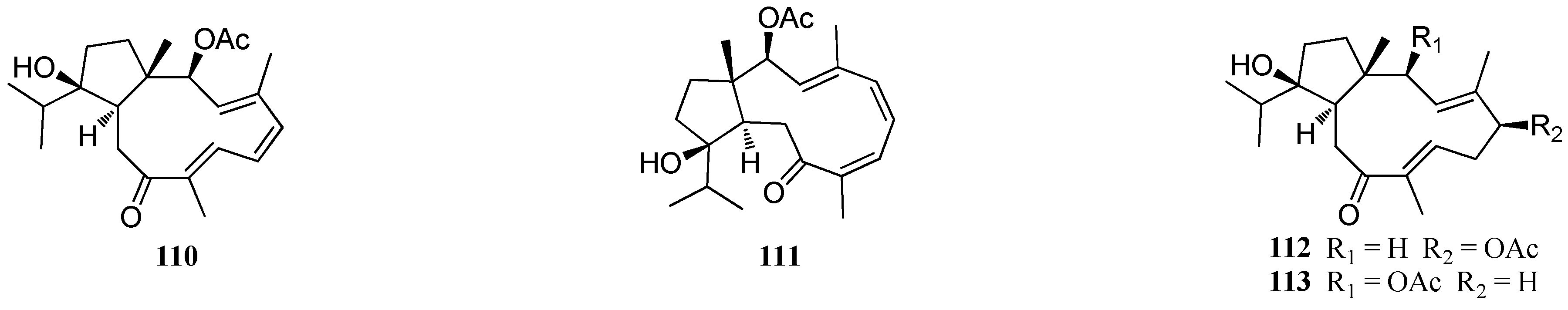
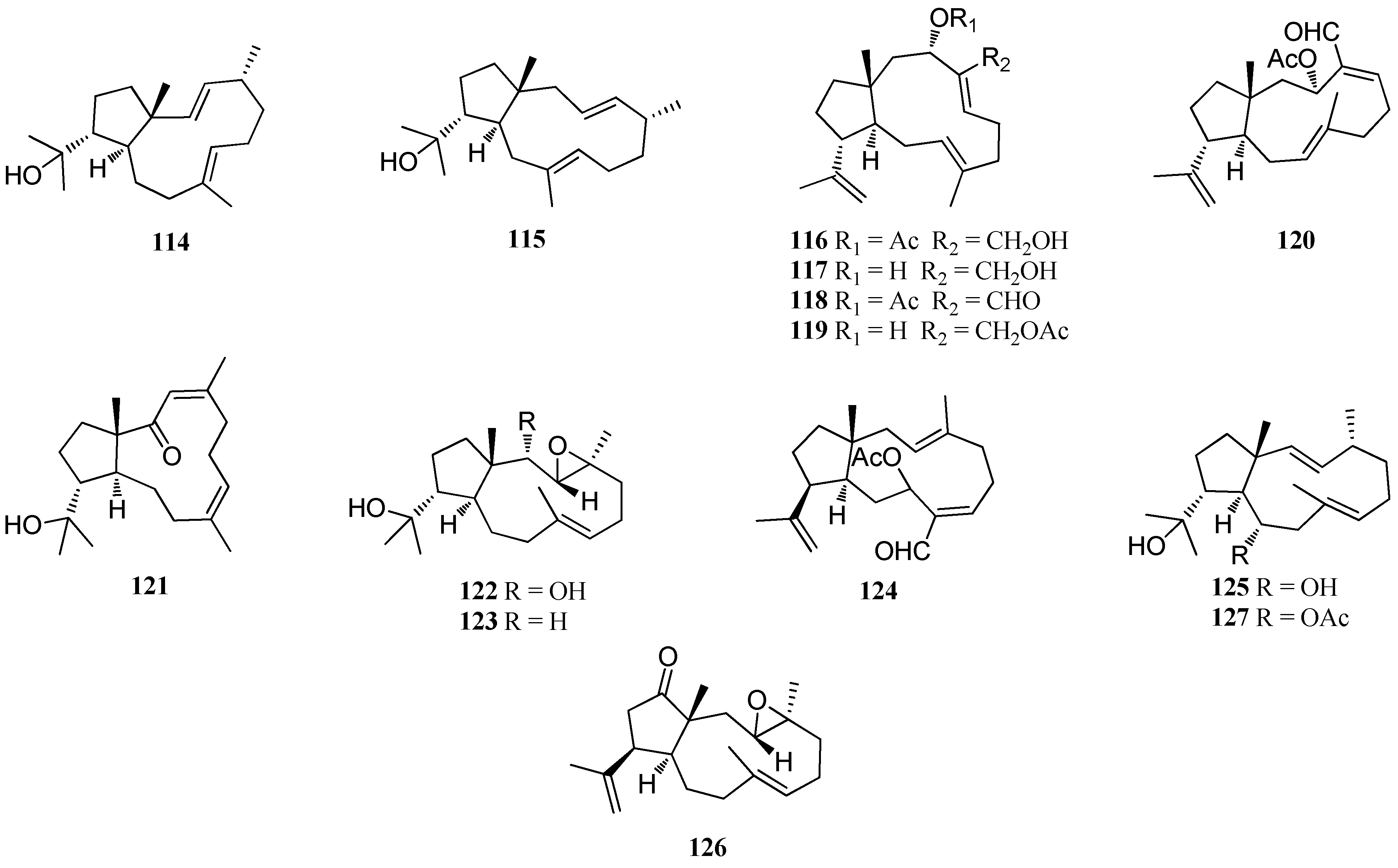
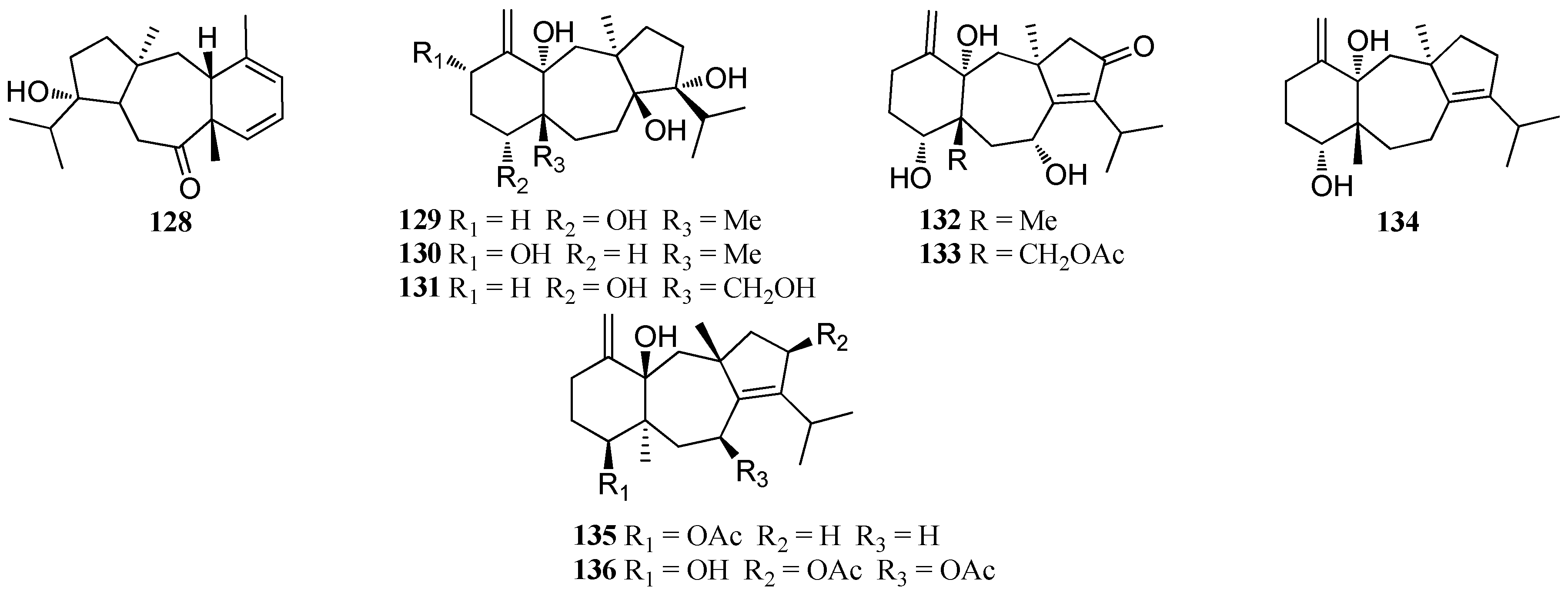





| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Dictyols | Dictyols A and B (1, 2) | D. dichotoma var. implexa, Tyrrhenian sea | nd (not determined) | [18] |
| Dictyol C (3) | D. divaricata, Great Barrier Reef region D. dentata, Boomers Beach Barbados D. dichotoma var. implexa, Tyrrhenian sea D. dichotoma, Patagonia | Protection for DNA damage; Antitumor activity; Antioxidant activity; Antifouling activity | [15,17,18,27,28] | |
| Dictyol D (4) | D. dichotoma var. implexa, Tyrrhenian sea | nd | [18] | |
| Dictyol B acetate (5) | D. dichotoma var. Implexa, Tyrrhenian sea D. caribaea, Dictyota ciliolata, Caribbean coast, Yucatan peninsula | Significant anti-herbivory activity; Selective antialgal activity; Moderate cytotoxicity; Antiproliferative activity | [14,18,19,20] | |
| Dictyol-d-2β-acetate (6) | D. dichotoma, near Puerto Madryn | nd | [21] | |
| Dictyol E (7) | D. dichotoma, Red Sea, Egypt Dictyota spp., Mediterranean Sea | Weak antimicrobial property; Moderate diacylglycerol acyltransferase inhibitory activity | [22,23,24] | |
| Dictyol G acetate (8) | D. volubilis, Geoffrey Bay, Australia D. binghamiae, Barkley Sound, British Columbia | nd | [25,26] | |
| Dictyol H (9) | D. divaricata, Great Barrier Reef region D. dentata, Boomers Beach | Moderate antitumor activity | [27,28] | |
| Dictyol I acetate (10) | D. dichotoma var. Implexa, Northern Adriatic sea | nd | [18] | |
| Dictyol J (11) | D. dichotoma | High algicidal activity | [29] | |
| Pachydictyols | Pachydictyol A (12) | D. dichotoma var. Implexa, Northern Adriatic Sea D. menstrualis, Brazil D. dichotoma, Patagonia D. caribaea D. ciliolata, Caribbean coast, Yucatan peninsula D. dichotoma var. implexa, Red Sea D. volubilis | Potent antithrombotic activity; Moderate cytotoxicity; Potent antifouling activity | [14,15,17,18,20,30,31] |
| Isopachydictyol A (13) | D. menstrualis, Brazil D. caribaea D. dichotoma var. implexa, Red Sea | Potent antithrombotic activity; Strong cytotoxicity | [15,20,30] | |
| Cis-pachydictyol B (14) | D. dichotoma, Red Sea, Egypt | Potent antimicrobial property; Weak cytotoxicity | [22] | |
| Trans-pachydictyol B (15) | D. dichotoma, Red Sea, Egypt | nd | [22] | |
| Pachydictyol C (16) | D. dichotoma, Red Sea, Egypt | Weak cytotoxicity | [22] | |
| 8α,11-Dihydroxy- pachydictyol A (17) | Dictyota sp., Bangsaen Beach, Thailand D. plectens, South China Sea | Strong cytotoxicity; Potent anti-malarial activity; Antiviral activity | [16,32] | |
| 8β-Hydroxy- pachydictyol A (18) | D. dichotoma var. implexa, Red Sea D. bartayresii, Geoffrey Bay, Australia D. plectens, South China Sea | Weak cytotoxicity; Antiviral activity | [15,16,33] | |
| 3,4-Epoxy-13-hydroxy-pachydictyol A (19) | D. dichotoma, Red Sea, Egypt | nd | [34] | |
| Acutilols | Acutilols A and B (20, 21) Acutilol A acetate (22) | D. acutiloba, Tunnels Beach, Hawaii | Potent feeding deterrent | [13,35] |
| Dictyoxides | Dictyoxide (23) | D. dichotoma, Patagonia | Potent antifouling activity | [17] |
| 2-Hydroxydictyoxide (24) | D. divaricata, Great Barrier Reef region | nd | [27] | |
| Dictyoxide A (25) | D. binghamiae, Barkley Sound, British Columbia | nd | [26] | |
| Dictytriols | Dictyotriol A diacetate (26) | D. binghamiae, Barkley Sound, British Columbia | nd | [26] |
| Dictytriol (27) | D. dichotoma, Japan | nd | [26] | |
| Dictyones | Dictyone (28) Dictyone acetate (29) | D. dichotoma, Red Sea, Egypt | Moderate cytotoxicity | [34,36] |
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. volubilis | 30–33 | Magnetic Island, Queensland, Australia | nd | [25] |
| 34–41 | nd | [31] | ||
| D. plectens | 9α-Hydroxydictyol (42) Isodictyol E (43) | South China Sea | Antiviral activity | [16] |
| D. dichotoma | Dictyotadiol (44) | Patagonia | Weak antifouling activity | [17] |
| Dictyohydroperoxide (45) | Troitsa Bay, Russian Far East | Moderate cytotoxicity | [37] | |
| Isopachydictyolal (46) | Saronicos gulf, Greece | Antiviral activity | [38] | |
| Genus Dictyota | 47 | Dictyota spp., Mediterranean Sea | nd | [23] |
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Prenylated-germacrane | Hydroxydilophol (48) | D. masonii Isla Guadalupe, Pacific Mexico | nd | [39] |
| Dilophol (49) | D. divaricata, Great Barrier Reef region | nd | [40] | |
| 3β-Hydroxydilophol (50) | Dictyota sp., Le Brusc Lagoon D. divaricata, Great Barrier Reef region | nd | [40,41] | |
| 3β-Acetoxydilophol (51) Acetoxypachydiol (52) | D. plectens, South China Sea | Weak antiviral activity | [16] | |
| Prenylated-cadinane | Dictyotins A-C (53–55) | D. dichotoma | nd | [42] |
| Ent-erogorgiaene (56) 57 | D. dichotoma, Russian Far-east | nd | [43] | |
| Prenylated-epi-elemane | Dictyoxepin (58) | D. volubilis | nd | [31] |
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. dichotoma | 59–66 | Acicastello, Italy | Antibiotic property | [44] |
| 67 | Acicastello, Italy | Strong cytotoxicity | [44,50] | |
| 68–82 | Indian Ocean | nd | [51] | |
| Dolabellatrienol (83) | D. dichotoma var. Implexa, Red Sea | Moderate cytotoxicity | [15] | |
| D. pardarlis f. pseudohamata | 84–98 | Magnetic Island | nd | [53,54,55] |
| D. bartayresiana | 79–82 98–102 | Hare Island, Indian Ocean | nd | [52] |
| D. pfaffii | 103 | Atol das Rocas, Northeast Brazil | Potent antiviral activity; Significant antimalarial activity | [46,57,58] |
| 104 | Atol das Rocas, Northeast Brazil | Antifeedant activity; Antiviral activity | [13,46,57] | |
| 105 | Atol das Rocas, Northeast Brazil | nd | [46] | |
| Dolabelladienols A - B (106, 107) | Atol das Rocas, Northeast Brazil | Strong antiviral activity | [46] | |
| Dolabelladienol C (108) | Atol das Rocas, Northeast Brazil | nd | [46] | |
| 109 | Atol das Rocas, Northeast Brazil | Strong anti-HSV-1 activity | [57] | |
| D. plectens | 110–113 | South China Sea | Specific antiviral activity | [16] |
| Genus Dictyota | 103 | D. friabilis, Atol das Rocas reef | Potent anti-HIV-1 activity | [56] |
| 114 | D. divaricata, Great Barrier Reef region | nd | [40] | |
| 115 | D. volubilis | nd | [31] | |
| 116 | Dictyota sp., near Portopalo | Significant cytotoxicity | [45] | |
| 117–120 | Dictyota sp., near Portopalo | nd | [45] | |
| 121–123 | Dictyota sp., Le Brusc Lagoon | Antifouling activity | [41] | |
| 124, 125 | Dictyota spp., Mediterranean coasts, Frence and Algeria | nd | [23] | |
| 126 | Dictyota spp., Mediterranean coasts, Frence and Algeria | Anti-adhesion activity; Antibacterial activity | [23,59] | |
| 127 | Dictyota spp., Mediterranean coasts, Frence and Algeria D. menstrualis, Discovery Bay, Jamaica | Anti-adhesion activity; Antibacterial activity; Anti-algal activity | [19,23,41,59] |
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
| D. dichotoma | 128 | Indian Ocean | nd | [51] |
| 129 Dichototeraol (130) Dichotopentaol (131) | Karachi coast, Arabian Sea | nd | [62] | |
| Dichotenones A and B (132, 133) | nd | [47] | ||
| Amijiol (134) Amijiol acetate (135) 136 | D. dichotoma var. Implexa, Red Sea | Antitumor activity; Anti-oxidative activity | [15] | |
| D. cervicornis | 137 | Brazil | Strong antimalarial activity; Antifouling activity; Enzyme inhibitory activity | [64,65,66] |
| 129 138–140 | Baia da Ribeira, Brazil | nd | [61] | |
| 141 | Rio de Janeiro, Brazil | Strong antifeedant activity; Antifouling activity; Antiviral activity | [64,66,67,68] | |
| D. divaricata | 142–145 | Virgin Islands | nd | [69] |
| D. indica | 134 Dictinol (146) Dictindiol (147) Dictintriol (148) | Bulegi, Arabian Sea | nd | [63] |
| D. bartayresiana | 128 149–151 | Hare Island, Indian Ocean | nd | [52] |
| D. linearis | Isoamijiol (152) 14-Deoxyamijiol (153) Amijidictyol (154) | nd | [70,71] | |
| D. plectens | 155–157 | South China Sea | Weak anti-inflammatory activity | [16] |
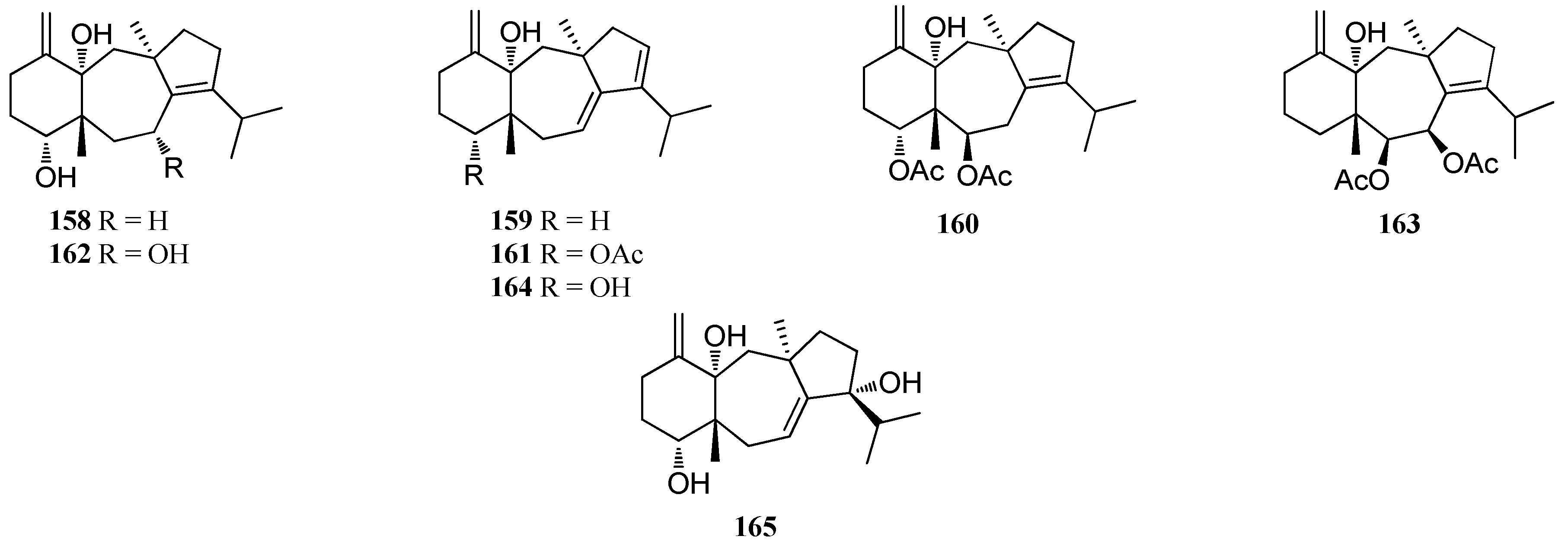
| Genus Dictyota | 137–138 158–160 | Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | nd | [60] |
| 161 | Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | Strong reversible inhibitory activity | [60] | |
| 162 | Mixed collections of D. linearis and D. divaricata, Honduras Bay Islands | Moderate decrease in the twitch height; Weak inhibition of cell division | [60] | |
| 163 | D. furcellata, Cape Peron | nd | [72] | |
| 164, 165 | Dictyota sp., Canary Islands | nd | [73] |
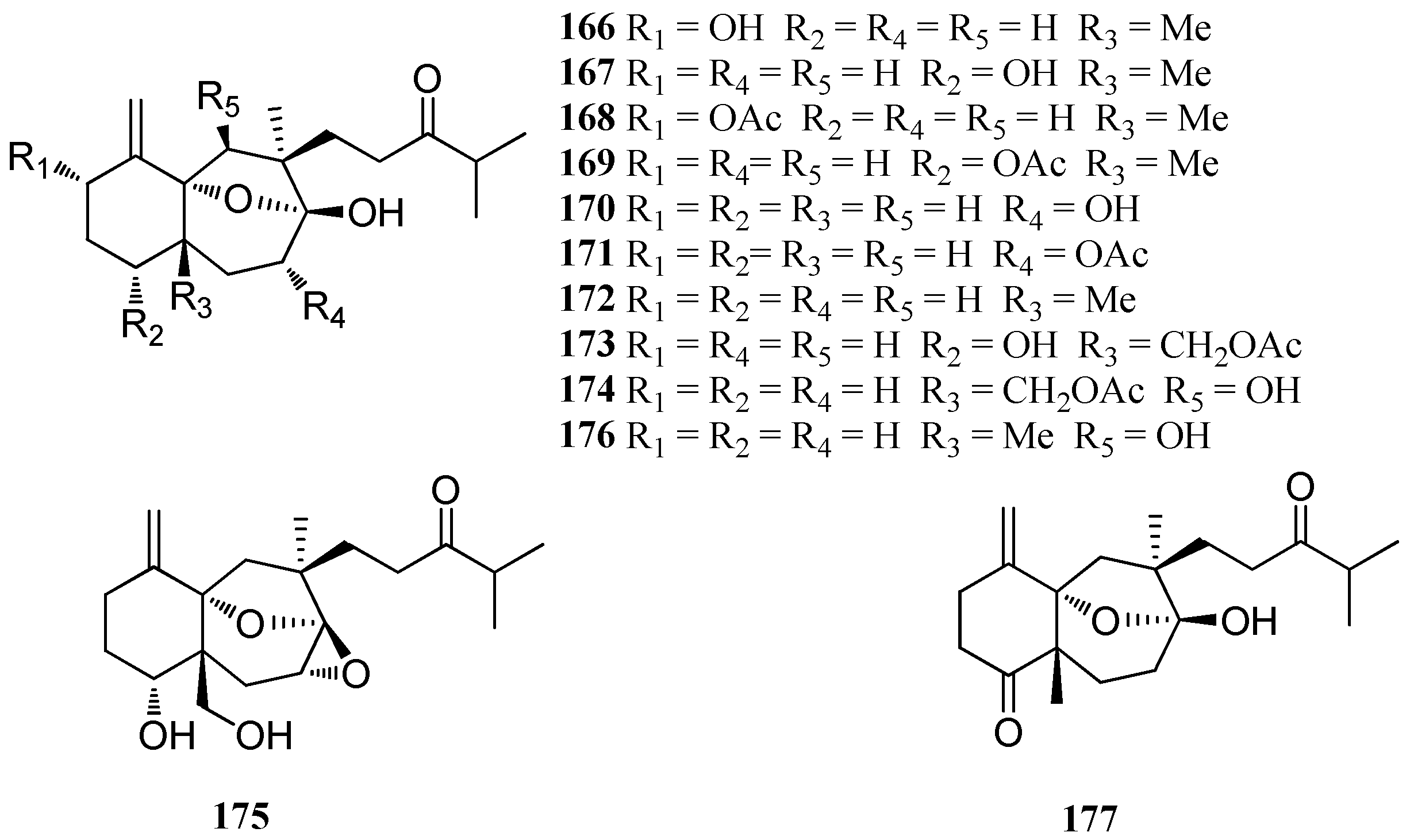
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Linearols | Linearol (166) Isolinearol (167) | D. indica Arabian Sea D. cervicornis Baia da Ribeira Brazil | nd | [74,75] |
| Linearol acetate (168) Isolinearol acetate (169) | D. cervicornis Baia da Ribeira Brazil | nd | [74] | |
| Cervicols | Cervicol (170) Cervicol acetate (171) | D. cervicornis Baia da Ribeira Brazil | nd | [74] |
| Indicols | Indicol (172) Indicarol acetate (173) | D. indica Arabian Sea | nd | [75] |
| Dichotenols | Dichotenol B (174) | D. dichotoma | Significant antibacterial and anti-fungal activity | [47] |
| Dichotenol C (175) | D. dichotoma | nd | [47] | |
| Others | Dichotone (176) | D. dichotoma | Significant antibacterial and anti-fungal activity | [47] |
| Dichotodione (177) | D. dichotoma | nd | [47] |
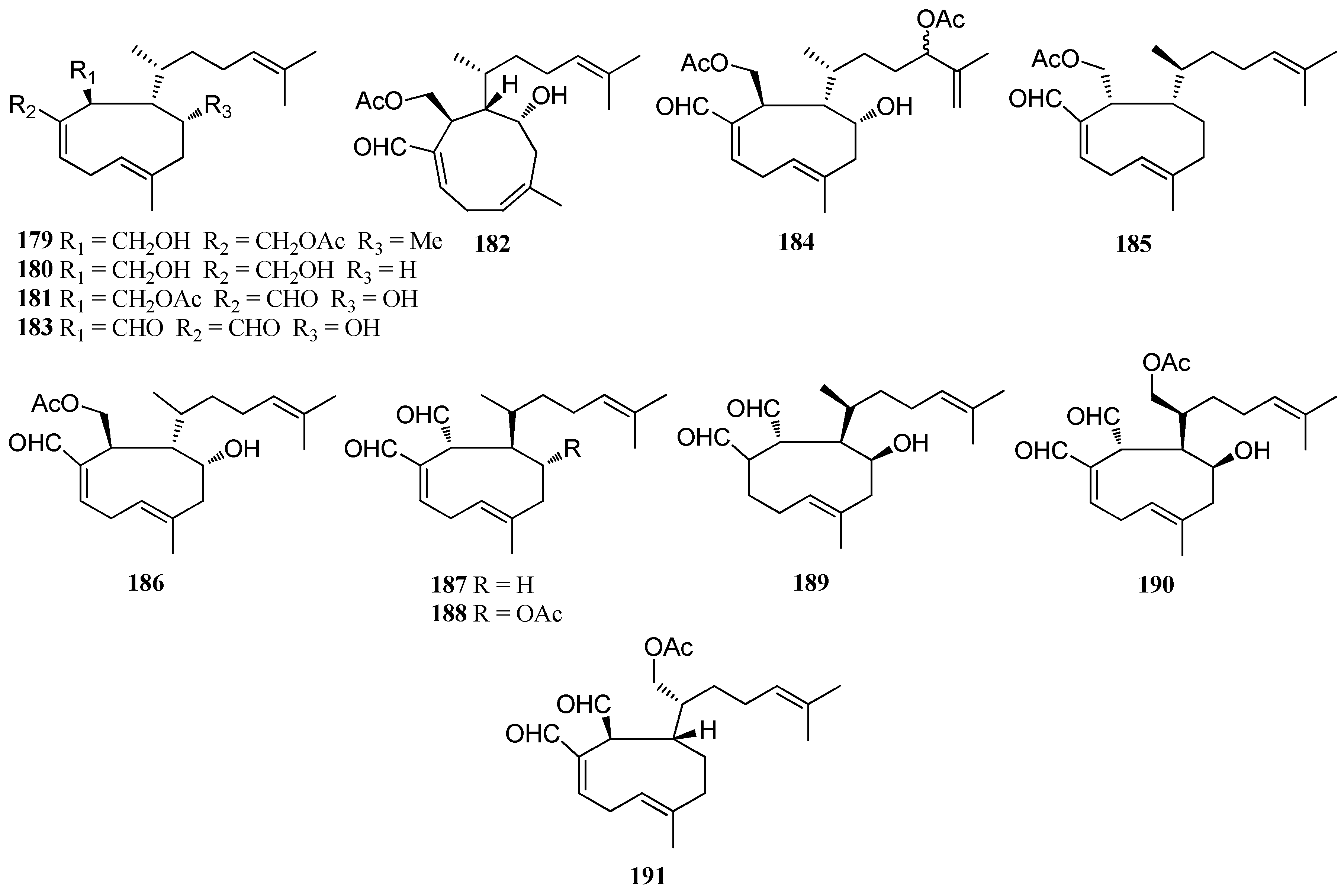
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Monocyclic diterpenes | 179–182 | D. plectens, South China Sea | Antiviral activity | [16] |
| 183 | D. plectens, South China Sea | Specific antiviral activity; Strong anti-inflammatory activity | [16] | |
| 184 | D. plectens, Xuwen coast, China | Weak anti-inflammatory activity | [76] | |
| Acetyldictyolal (185) | D. dichotoma, Oshoro bay, Hokkaido | High cytotoxicity; Weak antifungal activity | [50,78] | |
| Hydroxyacetyldictyolal (186) | Dictyota sp., Le Brusc Lagoon. D. dichotoma, Oshoro bay, Hokkaido | nd | [41,78] | |
| Dictyodial (187) | D. crenulata D. flabellata D. linearis, Chios Island | Good antibiotic activity; Antifungal activity | [38,77] | |
| 4α-Acetyldictyodial (188) | D. linearis, Chios Island | nd | [38] | |
| Hydroxydictyodial (189) | D. spinulosa, Kin Okinawa | Antibiotic activity; Potent antifeedant | [79] | |
| 190 | D. divaricata, Great Barrier Reef region | nd | [27] | |
| 191 | D. ciliolata, Oualidia lagoon | Moderate antifungal activity | [80] | |
| Bicyclic diterpenes | Dictyotalides A-B (192, 193) Nordictyotalide (194) 4-Acetoxydictyolactone (195) | D. dichotoma, Yagachi Okinawa | Significant cytotoxicity | [12] |
| Isodictyohemiacetal (196) Dictyodiacetal (197) | D. dichotoma, Oshoro bay, Hokkaido | nd | [78] | |
| Dictyolactone (198) | D. dichotoma | High algicidal activity; Moderate insecticidal activity; Weak antifungal activity; Significant cytotoxicity | [29,50] | |
| Neodictyolactone (199) | D. linearis, Chios Island | Weak antifungal activity; Cytotoxicity | [38,50] | |
| 200 | D. plectens, Xuwen coast, China | Antiviral activity; Weak anti-inflammatory activity | [76] | |
| 201–203 | D. plectens, Xuwen coast, China | Weak anti-inflammatory activity | [76] | |
| 204 | D. plectens, Xuwen coast, China | Specific antiviral activity; Significant anti-inflammatory activity | [76] | |
| 205 | D. plectens, Xuwen coast, China Dictyota sp., Le Brusc Lagoon | Antiviral activity; Weak anti-inflammatory activity | [41,76] | |
| 206 | D. plectens, South China Sea | Weak antiviral activity | [16] | |
| 207, 208 | Dictyota sp., Bahia de Los Angeles | nd | [81] | |
| 209 | Dictyota sp., Bangsaen Beach, Thailand | Weak anti-tuberculosis activity | [32] | |
| 210 | Dictyota spp., Mediterranean Sea | nd | [23] | |
| 211–213 | D. divaricata, Great Barrier Reef region | nd | [27] | |
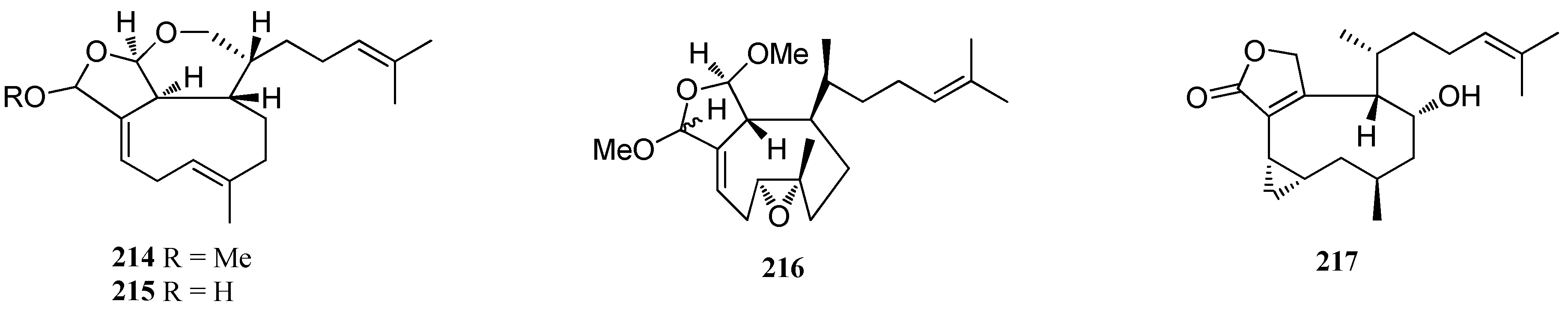
| Tricyclic diterpenes | 214 | D. divaricata, Great Barrier Reef region | nd | [40] |
| Ciliolatale (215) | D. ciliolata, Oualidia lagoon | nd | [80] | |
| Dictyoepoxide (216) | Dictyota sp., Bahia de Los Angeles | High vasopressin receptor antagonist activity | [81] | |
| 4α-Hydroxycrenulatene (217) | Dictyota sp., Bangsaen Beach, Thailand | nd | [32] | |
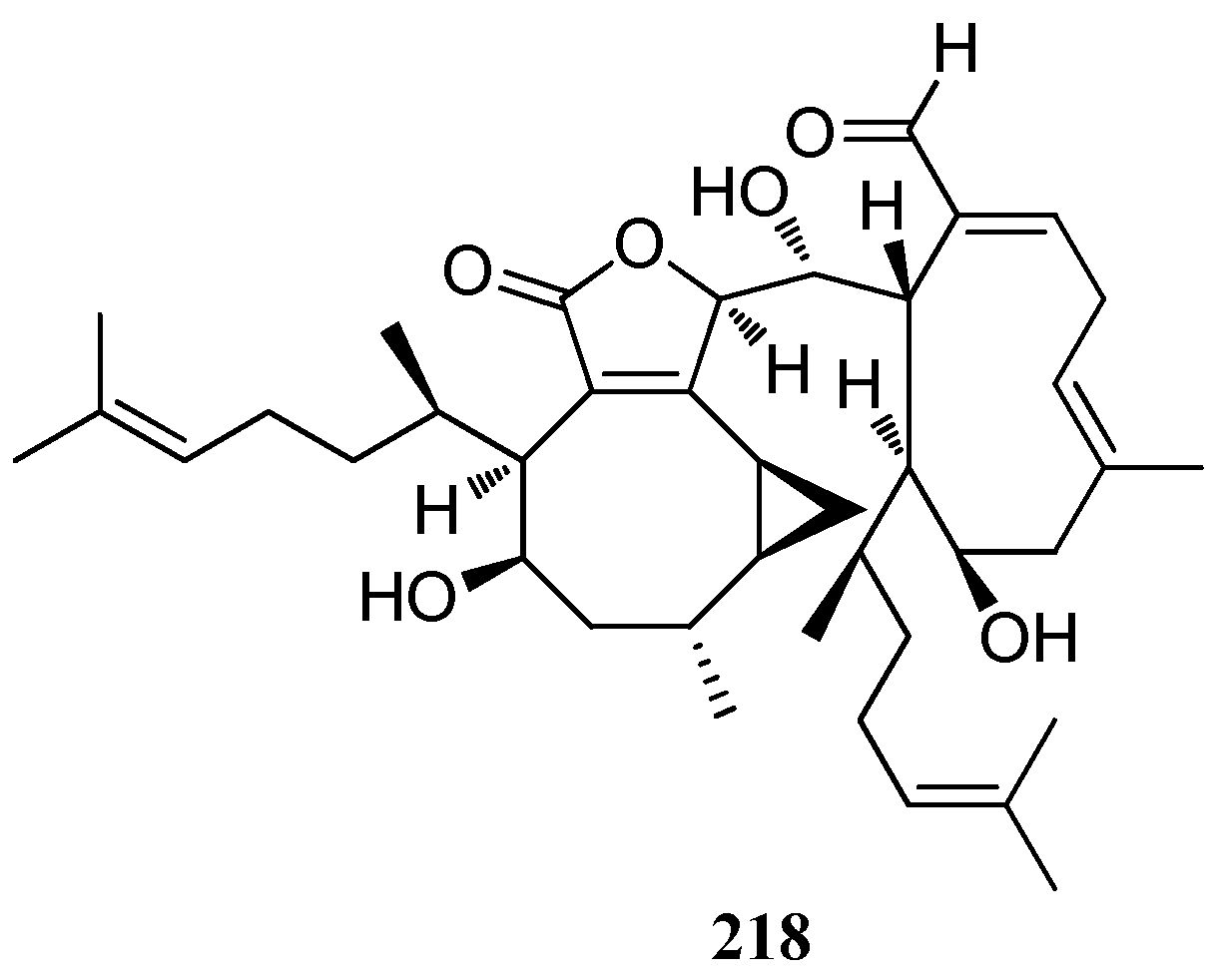
| Bis-diterpene | Dictyotadimer A (218) | Dictyota sp., Mediterranean Sea | nd | [82] |
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Crenulidanes | Crenulacetal A (219) | D. dichotoma D. spinulosa | nd | [83] |
| Crenulacetal B (220) | D. spinulosa, Yagachi Okinawa | nd | [83] | |
| Crenulacetal C (221) | D. dichotoma, Nagahama beach, Ehime | Significant pesticide activity | [84] | |
| Acetoxycrenulide (222) | Dictyota spp., Mediterranean Sea D. dichotoma, Troitsa Bay, Russian Far East | Weak anti-microfouling activity; Strong fish antifeedant activity | [23,37,83,85] | |
| 223 | D. dichotoma, Troitsa Bay, Russian Far East | nd | [37] | |
| 224 | D. divaricata, Great Barrier Reef region | nd | [27] | |
| 225 | D. divaricata | nd | [40] | |
| 226, 227 | D. plectens, South China Sea | Weak antiviral activity | [16] | |
| 4α-Hydroxypachylactone (228) | D. plectens, Xuwen coast, China | Moderate anti-inflammatory activity | [76] | |
| Hydroxycrenulide (229) | Dictyota sp., Mediterranean Sea | Low antifouling activity | [41] | |
| Dichotomanes | Da-1 (230) | D. menstrualis D. pfaffii, Brazil | Significant anti-HIV-1 activity; Thrombin inhibitor; Antifeedant effect; Inhibitory against pasture weeds | [30,86,87,88,89] |
| AcDa-1 (231) | D. menstrualis, Brazil | Significant anti-HIV-1 activity | [85] | |
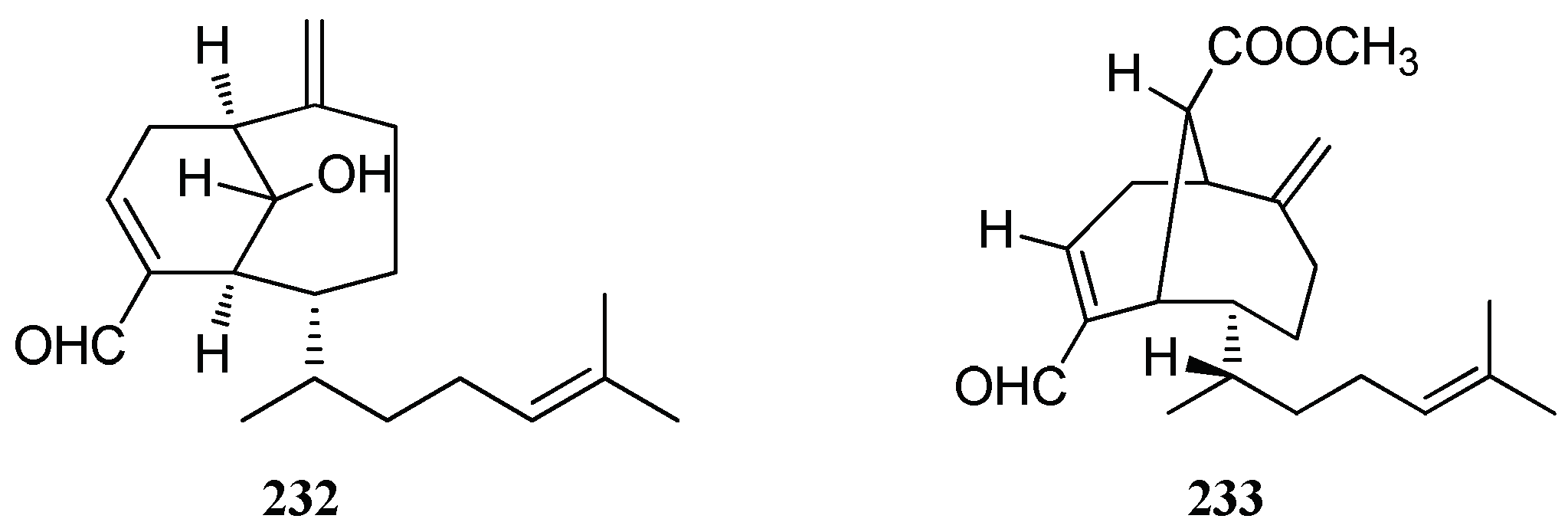
| Crenulanes | Sanadaol (232) | D. dichotoma | High algicidal activity | [29] |
| Acetylsanadanol (233) | D. linearis, Chios Island | nd | [38] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the Marine Algae of the Genus Dictyota. Mar. Drugs 2018, 16, 159. https://doi.org/10.3390/md16050159
Chen J, Li H, Zhao Z, Xia X, Li B, Zhang J, Yan X. Diterpenes from the Marine Algae of the Genus Dictyota. Marine Drugs. 2018; 16(5):159. https://doi.org/10.3390/md16050159
Chicago/Turabian StyleChen, Jiayun, Hong Li, Zishuo Zhao, Xue Xia, Bo Li, Jinrong Zhang, and Xiaojun Yan. 2018. "Diterpenes from the Marine Algae of the Genus Dictyota" Marine Drugs 16, no. 5: 159. https://doi.org/10.3390/md16050159




