Marine and Freshwater Feedstocks as a Precursor for Nitrogen-Containing Carbons: A Review
Abstract
:1. Introduction
1.1. Methods for Manufacturing Nitrogen-Doped Carbons
- Carbonization of a nitrogen-rich natural raw material;
- Carbonization of a nitrogen-rich synthetic material;
- Secondary nitrogen enrichment of a previously produced carbon material in the gaseous phase at a higher temperature;
- Secondary nitrogen enrichment of a previously produced carbon material in the liquid phase by impregnation with nitrogen-rich compounds, then and reheating the material.
1.1.1. Critical Evaluation of Synthetic Precursors as Candidates for Thermal Conversion into Nitrogen-Rich Carbons
1.1.2. Critical Evaluation of Non-Marine Natural Precursors as Candidates for Thermal Conversion into Nitrogen-Rich Carbons
Examples of Plant-Derived Precursors
Examples of Animal-Derived Precursors
Examples of Microorganisms as Precursors
2. Nitrogen-Doped Carbon Materials Obtained from Marine-Derived Feedstock
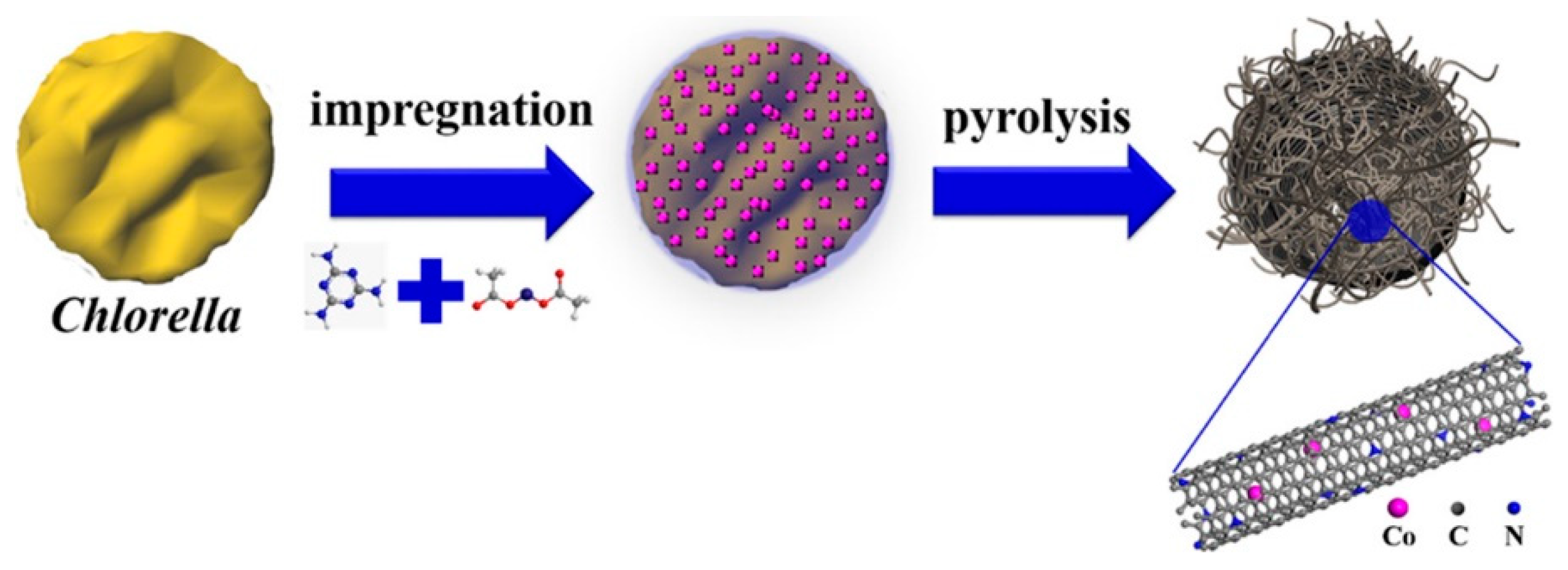
2.1. Nitrogen-Doped Carbon from Algae and Phytoplankton
2.2. Nitrogen-Doped Carbon from the Marine-Derived Precursor: Chitin
2.3. Nitrogen-Doped Carbon from the Marine-Derived Precursor: Chitosan
2.4. Nitrogen-Doped Carbon from Sea Animals
2.5. Nitrogen-Doped Carbon from Parts of Fish
3. Nitrogen-Doped Carbon Materials—Applications
3.1. Nitrogen-Doped Carbon for Oxygen Reduction Reaction
3.2. Nitrogen-Doped Carbon for Supercapacitors and Electric Double Layer Capacitors
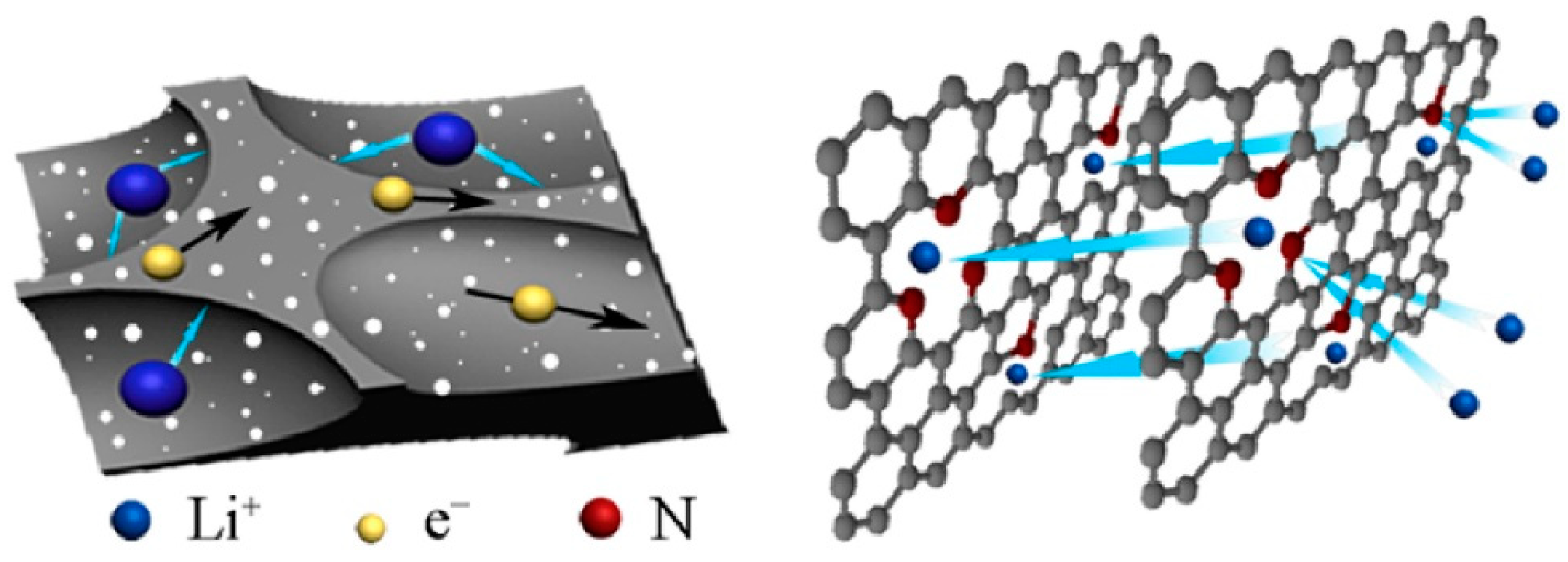
3.3. Nitrogen-Doped Carbon for Batteries
3.3.1. Lithium-Sulfur Batteries
3.3.2. Lithium-O2 Batteries
3.4. Nitrogen-Doped Carbon for Adsorption
3.4.1. Removal of Pollutants from the Gas Phase
3.4.2. Removal of Pollutants from the Liquid Phase
3.5. Biomedical Application of Nitrogen-Doped Carbon Materials Obtained from Marine Resources: Bioimaging
4. Summary
Acknowledgments
Conflicts of Interest
References
- Antolini, E. Nitrogen-doped carbons by sustainable N- and C-containing natural resources as nonprecious catalysts and catalyst supports for low temperature fuel cells. Renew. Sustain. Energy Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R. Węgle aktywne wzbogacone w azot—Otrzymywanie, włąsciwości i potencjalne zastosowania. In Adsorbenty i Katalizatory; Ryczkowki, J., Ed.; Uniwersytet Rzeszowski: Rzeszow, Poland, 2012; Volume 7, pp. 129–144. [Google Scholar]
- Li, J. The effect of surface modification with nitric acid on the mechanical and tribological properties of carbon fiber-reinforced thermoplastic polyimide composite. Surf. Interface Anal. 2009, 41, 759–763. [Google Scholar] [CrossRef]
- Li, W.; Chen, D.; Li, Z.; Shi, Y.; Wan, Y.; Wang, G.; Jiang, Z.; Zhao, D. Nitrogen-containing carbon spheres with very large uniform mesopores: The superior electrode materials for EDLC in organic electrolyte. Carbon 2007, 45, 1757–1763. [Google Scholar] [CrossRef]
- Drage, T.C.; Arenillas, A.; Smith, K.M.; Pevida, C.; Piippo, S.; Snape, C.E. Preparation of carbon dioxide adsorbents from the chemical activation of urea–formaldehyde and melamine–formaldehyde resins. Fuel 2007, 86, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Find, J.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar] [CrossRef]
- Fiset, E.; Rufford, T.E.; Seredych, M.; Bandosz, T.J.; Hulicova-Jurcakova, D. Comparison of melamine resin and melamine network as precursors for carbon electrodes. Carbon 2015, 81, 239–250. [Google Scholar] [CrossRef]
- Duan , J.; Fan , H.; Shen, W. Nitrogen-doped carbon materials prepared from polyurethane foams. ChemistrySelect 2016, 1, 3204–3207. [Google Scholar] [CrossRef]
- Kodama, M.; Yamashita, J.; Soneda, Y.; Hatori, H.; Kamegawa, K. Preparation and electrochemical characteristics of N-enriched carbon foam. Carbon 2007, 45, 1105–1107. [Google Scholar] [CrossRef]
- Machnikowski, J.; Grzyb, B.; Machnikowska, H.; Weber, J.V. Surface chemistry of porous carbons from N-polymers and their blends with pitch. Microporous Mesoporous Mater. 2005, 82, 113–120. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Parent, P.; Suarez-Garcia, F.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascon, J.M.D. Nitrogen in aramid-based activated carbon fibers by TPD, XPS and XANES. Carbon 2006, 44, 2452–2462. [Google Scholar] [CrossRef]
- Pollak, E.; Salitra, G.; Soffer, A.; Aurbach, D. On the reaction of oxygen with nitrogen-containing and nitrogen-free carbons. Carbon 2006, 44, 3302–3307. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Li, J.; Yang, Y.; Shen, H.; Huang, Z.; Xiang, X.; Li, W. A doped activated carbon prepared from polyaniline for high performance supercapacitors. J. Power Sources 2010, 195, 1516–1521. [Google Scholar] [CrossRef]
- Lei, Z.; Zhao, M.; Dang, L.; An, L.; Lu, M.; Lo, A.-Y.; Yu, N.; Liu, S.-B. Structural evolution and electrocatalytic application of nitrogen-doped carbon shells synthesized by pyrolysis of near-monodisperse polyaniline nanospheres. J. Mater. Chem. 2009, 19, 5985–5995. [Google Scholar] [CrossRef]
- Trchová, M.; Konyushenko, E.N.; Stejskal, J.; Kovářová, J.; Ćirić-Marjanović, G. The conversion of polyaniline nanotubes to nitrogen-containing carbon nanotubes and their comparison with multi-walled carbon nanotubes. Polym. Degrad. Stab. 2009, 94, 929–938. [Google Scholar] [CrossRef]
- Rozlívková, Z.; Trchová, M.; Exnerová, M.; Stejskal, J. The carbonization of granular polyaniline to produce nitrogen-containing carbon. Synth. Met. 2011, 161, 1122–1129. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Hromádková, J.i.; Kovárová, J.; Kalendová, A. The carbonization of colloidal polyaniline nanoparticles to nitrogen-containing carbon analogues. Polym. Int. 2010, 59, 875–878. [Google Scholar] [CrossRef]
- Garsuch, A.; Sattler, R.R.; Witt, S.; Klepel, O. Adsorption properties of various carbon materials prepared by template synthesis route. Microporous Mesoporous Mater. 2006, 89, 164–169. [Google Scholar] [CrossRef]
- Cong, K.; Radtke, M.; Stumpf, S.; Schröter, B.; McMillan, D.G.; Rettenmayr, M.; Ignaszak, A. Electrochemical stability of the polymer-derived nitrogen-doped carbon: An elusive goal? Mater. Renew. Sustain. Energy 2015, 4. [Google Scholar] [CrossRef]
- Lezanska, M.; Wloch, J.; Szymański, G.; Szpakowska, I.; Kornatowski, J. Properties of CMK-8 carbon replicas obtained from KIT-6 and pyrrole at various contents of ferric catalyst. Catal. Today 2010, 150, 77–83. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Centeno, T.A. Mesoporous carbons with graphitic structures fabricated by using porous silica materials as templates and iron-impregnated polypyrrole as precursor. J. Mater. Chem. 2005, 15, 1079–1083. [Google Scholar] [CrossRef]
- Yang, C.-M.; Weidenthaler, C.; Spliethoff, B.; Mayanna, M.; Schüth, F. Facile template synthesis of ordered mesoporous carbon with polypyrrole as carbon precursor. Chem. Mater. 2005, 17, 355–358. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Generalized and facile synthesis approach to N-doped highly graphitic mesoporous carbon materials. Chem. Mater. 2005, 17, 1553–1560. [Google Scholar] [CrossRef]
- László, K.; Tombácz, E.; Josepovits, K. Effect of activation on the surface chemistry of carbons from polymer precursors. Carbon 2001, 39, 1217–1228. [Google Scholar] [CrossRef]
- Lu, A.; Kiefer, A.; Schmidt, W.; Schüth, F. Synthesis of polyacrylonitrile-based ordered mesoporous carbon with tunable pore structures. Chem. Mater. 2004, 16, 100–103. [Google Scholar] [CrossRef]
- Kruk, M.; Kohlhaas, K.M.; Dufour, B.; Celer, E.B.; Jaroniec, M.; Matyjaszewski, K.; Ruoff, R.S.; Kowalewski, T. Partially graphitic, high-surface-area mesoporous carbons from polyacrylonitrile templated by ordered and disordered mesoporous silicas. Microporous Mesoporous Mater. 2007, 102, 178–187. [Google Scholar] [CrossRef]
- Xu, B.; Duan, H.; Chu, M.; Cao, G.; Yang, Y. Facile synthesis of nitrogen-doped porous carbon for supercapacitors. J. Mater. Chem. A 2013, 1, 4565–4570. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd(II) and Pb(II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Delmotte, L.; Fioux, P.; Celzard, A. High surface—Highly N-doped carbons from hydrothermally treated tannin. Ind. Crop. Prod. 2015, 66, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hao, Y.; Ma, Y.; Feng, H. Hydrothermal synthesis of highly nitrogen-doped carbon powder. Appl. Surf. Sci. 2012, 258, 2510–2514. [Google Scholar] [CrossRef]
- Thote, J.A.; Iyer, K.S.; Chatti, R.; Labhsetwar, N.K.; Biniwale, R.B.; Rayalu, S.S. In situ nitrogen enriched carbon for carbon dioxide capture. Carbon 2010, 48, 396–402. [Google Scholar] [CrossRef]
- Budaeva, A.D.; Zoltoev, E.V. Porous structure and sorption properties of nitrogen-containing activated carbon. Fuel 2010, 89, 2623–2627. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Kim, Y.J.; Abe, Y.; Yanagiura, T.; Park, K.C.; Shimizu, M.; Iwazaki, T.; Nakagawa, S.; Endo, M.; Dresselhaus, M.S. Easy preparation of nitrogen-enriched carbon materials from peptides of silk fibroins and their use to produce a high volumetric energy density in supercapacitors. Carbon 2007, 45, 2116–2125. [Google Scholar] [CrossRef]
- Lua, A.C.; Guo, J. Preparation and characterization of activated carbons from oil-palm stones for gas-phase adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 151–162. [Google Scholar] [CrossRef]
- Xu, H.; Gao, B.; Cao, H.; Chen, X.; Yu, L.; Wu, K.; Sun, L.; Peng, X.; Fu, J. Nanoporous activated carbon derived from rice husk for high performance supercapacitor. J. Nanomater. 2014, 2014, 714010. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Zhang, Z.; Yang, Q.; Lei, Z. Nitrogen-doped interconnected carbon nanosheets from pomelo mesocarps for high performance supercapacitors. Electrochim. Acta 2016, 190, 862–871. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Cao, G.; Wu, F.; Yang, Y. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Olejniczak, A.; Leżańska, M.; Pacuła, A.; Nowak, P.; Włoch, J.; Łukaszewicz, J.P. Nitrogen-containing mesoporous carbons with high capacitive properties derived from a gelatin biomolecule. Carbon 2015, 91, 200–214. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Lai, Y.; Liu, Y.; Li, J. Highly ordered nitrogen-rich mesoporous carbon derived from biomass waste for high-performance lithium–sulfur batteries. Carbon 2015, 84, 399–408. [Google Scholar] [CrossRef]
- Schnepp, Z.; Zhang, Y.; Hollamby, M.J.; Pauw, B.R.; Tanaka, M.; Matsushita, Y.; Sakka, Y. Doped-carbon electrocatalysts with trimodal porosity from a homogeneous polypeptide gel. J. Mater. Chem. A 2013, 1, 13576–13581. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Kim, S.T.; Shin, D.-B.; Park, N.; Kim, Y.; Lee, J.-S.; Cho, J. Metal-free ketjenblack incorporated nitrogen-doped carbon sheets derived from gelatin as oxygen reduction catalysts. Nano Lett. 2014, 14, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yin, J.; Wang, X.; Wang, H.; Yang, X. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors. Adv. Funct. Mater. 2013, 23, 1305–1312. [Google Scholar] [CrossRef]
- Hau, H.H.; Gralnick, J.A. Ecology and biotechnology of the genus shewanella. Ann. Rev. Microbiol. 2007, 61, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ren, G.; Jiang, C.; Lu, X.; Zhu, Y.; Jiang, L.; Dai, L. High performance heteroatoms quaternary-doped carbon catalysts derived from shewanella bacteria for oxygen reduction. Sci. Rep. 2015, 5, 17064. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Matsuda, S.; Kato, S.; Hashimoto, K.; Nakanishi, S. Redox-responsive switching in bacterial respiratory pathways involving extracellular electron transfer. ChemSusChem 2010, 3, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Liu, H.; Kato, S.; Hashimoto, K.; Nakanishi, S. Negative faradaic resistance in extracellular electron transfer by anode-respiring geobacter sulfurreducens cells. Enviro. Sci. Technol. 2011, 45, 10163–10169. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Xiong, T.; Shi, C.; Zhou, J.; Zhou, K.; Zhu, N.; Li, L.; Tang, Z.; Chen, S. Bioreduction of precious metals by microorganism: Efficient gold@N-doped carbon electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2016, 55, 8416–8420. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, C.; Guo, X.; Paul Chen, J. Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride. J. Colloid Interface Sci. 2015, 441, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Escobar, B.; Pérez-Salcedo, K.Y.; Alonso-Lemus, I.L.; Pacheco, D.; Barbosa, R. N-doped porous carbon from Sargassum spp. As metal-free electrocatalysts for oxygen reduction reaction in alkaline media. Int. J. Hydrog. Energy 2017, 42, 30274–30283. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J. Phycol. 1998, 34, 798–811. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Liu, F.; Peng, H.; You, C.; Fu, Z.; Huang, P.; Song, H.; Liao, S. High-performance doped carbon catalyst derived from nori biomass with melamine promoter. Electrochim. Acta 2014, 138, 353–359. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N,O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Li, X.; Zeng, J.; Du, L.; Liao, S. Nitrogen self-doped carbon nanoparticles derived from spiral seaweeds for oxygen reduction reaction. RSC Adv. 2016, 6, 27535–27541. [Google Scholar] [CrossRef]
- Fan, Z.; Li, J.; Zhou, Y.; Fu, Q.; Yang, W.; Zhu, X.; Liao, Q. A green, cheap, high-performance carbonaceous catalyst derived from Chlorella pyrenoidosa for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrog. Energy 2017, 42, 27657–27665. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, W.; Yang, B. Thermal characteristics and surface morphology of char during co-pyrolysis of low-rank coal blended with microalgal biomass: Effects of nannochloropsis and Chlorella. Bioresour. Technol. 2018, 249, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Deng, Y.; Yu, J.; Zheng, L.; Du, L.; Song, H.; Liao, S. From Chlorella to nestlike framework constructed with doped carbon nanotubes: A biomass-derived, high-performance, bifunctional oxygen reduction/evolution catalyst. ACS Appl. Mater. Interfaces 2017, 9, 32168–32178. [Google Scholar] [CrossRef] [PubMed]
- Hencz, L.; Gu, X.; Zhou, X.; Martens, W.; Zhang, S. Highly porous nitrogen-doped seaweed carbon for high-performance lithium–sulfur batteries. J. Mater. Sci. 2017, 52, 12336–12347. [Google Scholar] [CrossRef]
- Godavarthi, S.; Mohan Kumar, K.; Vázquez Vélez, E.; Hernandez-Eligio, A.; Mahendhiran, M.; Hernandez-Como, N.; Aleman, M.; Martinez Gomez, L. Nitrogen doped carbon dots derived from sargassum fluitans as fluorophore for DNA detection. J. Photochem. Photobiol. B Biol. 2017, 172, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Park, H.Y.; Yang, D.-S.; Bhattacharjya, D.; Yu, J.-S. Seaweed-derived heteroatom-doped highly porous carbon as an electrocatalyst for the oxygen reduction reaction. ChemSusChem 2014, 7, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, Y.; Ma, Y.; Li, B.; Liu, Z.; Lu, C.; Liu, X.; Wei, Y.; Zhu, Z.; Zhang, Y. Algae biomass as a precursor for synthesis of nitrogen-and sulfur-co-doped carbon dots: A better probe in arabidopsis guard cells and root tissues. J. Photochem. Photobiol. B Biol. 2017, 174, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.; Sevilla, M.; White, R.J.; Rothe, R.; Titirici, M.-M. Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem 2012, 5, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass derived nitrogen-doped highly porous carbon material with a hierarchical porous structure for high-performance lithium/sulfur batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Kamedulski, P.; Lukaszewicz, J.P. Pyrolysis of Chlorella vulgaris as a green chemistry method for manufacturing of nitrogen doped porous carbon materials of high application potential. Mater. Express 2017, 7, 25–34. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yang, S.; Li, D.; Xie, Z.; Wang, B.; Xia, Y.; Quan, F. Facile strategy to produce N-doped carbon aerogels derived from seaweed for lithium-ion battery anode. J. Alloys Compd. 2017, 701, 256–261. [Google Scholar] [CrossRef]
- Shen, L.; Liu, X.; Ou, L.; Wang, Z.; Tan, S.; Mai, W. Phytoplankton derived and KOH activated mesoporous carbon materials for supercapacitors. Mater. Lett. 2017, 205, 98–101. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, M.; Park, C.W.; Kim, J.-D. Sustainable fabrication of nitrogen activated carbon from Chlorella vulgaris for energy storage devices. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 102–106. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, M.S.; Kim, J.-D. Nitrogen doped activated carbon with nickel oxide for high specific capacitance as supercapacitor electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 323–329. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Wang, X.; Fan, Q.; Gibbons, W.; Johnson, T.; Luo, B.; Gu, Z. Pyrolytic cyanobacteria derived activated carbon as high performance electrode in symmetric supercapacitor. Energy 2016, 94, 666–671. [Google Scholar] [CrossRef]
- Gao, J.; Xie, J.; Liu, X.; Hu, H. Preparation and evaluation of modified cyanobacteria-derived activated carbon for H2 adsorption. RSC Adv. 2017, 7, 20412–20421. [Google Scholar] [CrossRef]
- Li, D.; Lv, C.; Liu, L.; Xia, Y.; She, X.; Guo, S.; Yang, D. Egg-box structure in cobalt alginate: A new approach to multifunctional hierarchical mesoporous N-doped carbon nanofibers for efficient catalysis and energy storage. ACS Cent. Sci. 2015, 1, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, X.; Lv, C.; Zhu, A.; Zhu, X.; Guo, S.; Chen, C.; Yang, D. Seaweed-derived route to Fe2O3 hollow nanoparticles/N-doped graphene aerogels with high lithium ion storage performance. ACS Appl. Mater. Interfaces 2016, 8, 7047–7053. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Zhu, J. Binder-free nitrogen-doped carbon paper electrodes derived from polypyrrole/cellulose composite for Li–O2 batteries. J. Power Sources 2016, 306, 559–566. [Google Scholar] [CrossRef]
- Nowak, A.P.; Lisowska-Oleksiak, A. Red algae—An alternative source of carbon material for energy storage application. Int. J. Electrochem. Sci. 2014, 9, 3715–3724. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Szosland, L.; Szumilewicz, J.; Steplewski, W. Dibutyrylchitin: Preparation, Characteristic and Application; Struszczyk, H., Pospieszny, H., Gamzazade, A., Eds.; Polish Chitin Society Series; Polish-Russian Monograph Chitin and Chitosan; Polish Chitin Society: Lodz, Poland, 1999; Volume 1, pp. 77–92. [Google Scholar]
- Romano, P.; Fabritius, H.; Raabe, D. The exoskeleton of the lobster homarus americanus as an example of a smart anisotropic biological material. Acta Biomater. 2007, 3, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friák, M.; Sachs, C.; Fabritius, H.-O.; Raabe, D.; Neugebauer, J. Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, S.; Fabritius, H.; Petrov, M.; Friák, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and optimal use of design principles of arthropod exoskeletons studied by ab initio-based multiscale simulations. J. Mech. Behav. Biomed. Mater. 2011, 4, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G. Chemiczna Funkcjonalizacja Powierzchni dla Potrzeb Nanotechnologii; Cursiva: Poznań, Poland, 2011. [Google Scholar]
- Zarzycki, R.; Modrzejewsk, Z.; Sujka, W. Sorpcja Jonów Metali na Hydrożelu Chitozanowym; Polska Akademia Nauk: Łódź, Poland, 2008. [Google Scholar]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (hexactinellida: Porifera). J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Szatkowski, T.; Jesionowski, T. Hydrothermal synthesis of spongin-based materials. In Extreme Biomimetics; Springer International Publishing: Cham, Switzerland, 2017; pp. 251–274. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Stocker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F. Extreme biomimetics: A carbonized 3D spongin scaffoldas a novel support for nanostructured manganese oxide (IV) and its electrochemical applications. Nano Res. 2018, 1–16. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Walter, J.; Lota, G.; Wojciechowski, J.; Stöcker, H.; Galli, R.; Stelling, A.L.; Himcinschi, C.; Niederschlag, E. Synthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions. RSC Adv. 2014, 4, 61743–61752. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Motylenko, M.; Langer, E.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Kljajić, Z.; Szatkowski, T.; Kutsova, V.Z. Renewable chitin from marine sponge as a thermostable biological template for hydrothermal synthesis of hematite nanospheres using principles of extreme biomimetics. Bioinspired Mater. 2015, 1, 12–22. [Google Scholar] [CrossRef]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stöcker, H.; Himcinschi, C. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Stepniak, I.; Galinski, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisegang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors—Preparation and characterization. RSC Adv. 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Kierzek, K. Materiały Węglowe Aktywowane Wodorotlenkiem Potasu. Ph.D. Thesis, Uniwersytet Wrocławski, Wrocław, Poland, 2006. [Google Scholar]
- Stolarek, P.; Ledakowicz, S. Pyrolysis kinetics of chitin by non-isothermal thermogravimetry. Thermochim. Acta 2005, 433, 200–208. [Google Scholar] [CrossRef]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stab. 2005, 87, 389–394. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, S.; Liu, Y.; Sun, H.; Jia, S.; Shi, J.; Pedersen, C.M.; Wang, Y.; Hou, X. Pyrolysis of chitin biomass: TG–MS analysis and solid char residue characterization. Carbohydr. Polym. 2015, 133, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H.; Zawadzki, J. Chitosan pyrolysis and adsorption properties of chitosan and its carbonizate. Carbohydr. Res. 2010, 345, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Gauden, P.A.; Terzyk, A.P.; Lukaszewicz, J.P. Nano-structured carbon matrixes obtained from chitin and chitosan by a novel method. J. Nanosci. Nanotechnol. 2015, 16, 2623–2631. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P. Discussion remarks on the role of wood and chitin constituents during carbonization. Front. Mater. 2015, 2, 20. [Google Scholar] [CrossRef]
- Ilnicka, A.; Łukaszewicz, J.P. Nitrogen-doped chitin carbon materials. Inżynieria i Ochrona Środowiska 2016, 19, 205–2015. [Google Scholar] [CrossRef]
- White, R.J.; Antonietti, M.; Titirici, M.-M. Naturally inspired nitrogen doped porous carbon. J. Mater. Chem. 2009, 19, 8645–8650. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Shopsowitz, K.E.; MacLachlan, M.J. Mesoporous nitrogen-doped carbon from nanocrystalline chitin assemblies. J. Mater. Chem. A 2014, 2, 5915–5921. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Liu, S.; Zhang, X.; Wu, T.; Ge, X.; Zang, Y.; Zhao, H.; Wang, G. Shrimp-shell derived carbon nanodots as carbon and nitrogen sources to fabricate three-dimensional N-doped porous carbon electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, H.; Zhang, X.; Liu, R.; Liu, S.; Wang, G.; Zhang, Y.; Zhao, H. Fe/Fe2O3 nanoparticles anchored on Fe-N-doped carbon nanosheets as bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Nano Res. 2016, 9, 2123–2137. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, R.; Zang, Y.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/CoO nanoparticles immobilized on Co-N-doped carbon as trifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Commun. 2016, 52, 5946–5949. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Ledesma, B.; Álvarez-Murillo, A.; Sabio, E.; González, J.F.; González, C.M. Production of cost-effective mesoporous materials from prawn shell hydrocarbonization. Nanoscale Res. Lett. 2016, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Lan, H.; Kuang, C.; Wang, H.; Guo, L. Superior potassium storage in chitin-derived natural nitrogen-doped carbon nanofibers. Carbon 2018, 128, 224–230. [Google Scholar] [CrossRef]
- Kucinska, A.; Cyganiuk, A.; Lukaszewicz, J.P. A microporous and high surface area active carbon obtained by the heat-treatment of chitosan. Carbon 2012, 50, 3098–3101. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Bengisu, M.; Yilmaz, E. Oxidation and pyrolysis of chitosan as a route for carbon fiber derivation. Carbohydr. Polym. 2002, 50, 165–175. [Google Scholar] [CrossRef]
- Zeng, L.; Qin, C.; Wang, L.; Li, W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr. Polym. 2011, 83, 1553–1557. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Ilnicka, A; Łukaszewicz, J.P. Method for Producing Nanoporous Activated Carbons with a High Nitrogen Content. Poland Patent No. 222353, 31 August 2016.
- Ilnicka, A.; Łukaszewicz, J.P. Activated Carbons with High Nitrogen Content and High Electric Conductivity and Method for Producing Activated Carbons, Preferably for Manufacturing of Electrodes. Poland Patent No. P.411926, 9 April 2015. [Google Scholar]
- Ilnicka, A.; Łukaszewicz, J.P. Method for Producing a Biocidal Product. Poland Patent No. 227337, 11 November 2017. [Google Scholar]
- Kucinska, A.; Golembiewski, R.; Lukaszewicz, J.P. Synthesis of N-rich activated carbons from chitosan by chemical activation. Sci. Adv. Mater. 2014, 6, 290–297. [Google Scholar] [CrossRef]
- Kucińska, A.; Łukaszewicz, J.P. Nano-CaCO3 as template to preparation from chitosan of nitrogen-rich mesoporous carbon materials. Inżynieria i Ochrona Środowiska 2013, 16, 191–202. [Google Scholar]
- Ilnicka, A.; Lukaszewicz, J.P. Synthesis of N-rich microporous carbon materials from chitosan by alkali activation using Na2CO3. Mater. Sci. Eng. B 2015, 201, 66–71. [Google Scholar] [CrossRef]
- Ilnicka, A.; Walczyk, M.; Lukaszewicz, J.P. The fungicidal properties of the carbon materials obtained from chitin and chitosan promoted by copper salts. Mater. Sci. Eng. C 2015, 52, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Walczyk, M.; Lukaszewicz, J.P.; Janczak, K.; Malinowski, R. Antimicrobial carbon materials incorporating copper nano-crystallites and their PLA composites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kumar, A.; Ganguly, A.; Papakonstantinou, P. Thermal stability study of nitrogen functionalities in a graphene network. J. Phys. Condens. Matter 2012, 24, 235503. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Yang, L.; Liu, X.; Zhang, B.; Liu, Y.; Xiao, Y.; Zheng, M.; Lei, B.; Zhang, H. Preparation of chitosan-based activated carbon and its electrochemical performance for EDLC. J. Electrochem. Soc. 2013, 160, H321–H326. [Google Scholar] [CrossRef]
- Olejniczak, A.; Lezanska, M.; Wloch, J.; Kucinska, A.; Lukaszewicz, J.P. Novel nitrogen-containing mesoporous carbons prepared from chitosan. J. Mater. Chem. A 2013, 1, 8961–8967. [Google Scholar] [CrossRef]
- Leżańska, M.; Olejniczak, A.; Pacuła, A.; Szymański, G.; Włoch, J. The influence of microporosity creation in highly mesoporous N-containing carbons obtained from chitosan on their catalytic and electrochemical properties. Catal. Today 2014, 227, 223–232. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, H.; Meng, Y.; Xie, K.; Wei, Z.; Guo, Z. High-performance all-carbon yarn micro-supercapacitor for an integrated energy system. Adv. Mater. 2014, 26, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ma, J.; Chen, J.; Shen, C.; Li, H.; Liu, W. Carbonaceous sulfur-containing chitosan–Fe(III): A novel adsorbent for efficient removal of copper (II) from water. Chem. Eng. J. 2015, 259, 372–380. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, G.; Zhang, M.; Fan, X.; Yu, C.; Yang, J.; Xiao, N.; Qiu, J. Boric acid-mediated B,N-codoped chitosan-derived porous carbons with a high surface area and greatly improved supercapacitor performance. Nanoscale 2015, 7, 5120–5125. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Wróbel-Iwaniec, I.; Díez, N.; Gryglewicz, G. Chitosan-based highly activated carbons for hydrogen storage. Int. J. Hydrog. Energy 2015, 40, 5788–5796. [Google Scholar] [CrossRef]
- Qu, J.; Geng, C.; Lv, S.; Shao, G.; Ma, S.; Wu, M. Nitrogen, oxygen and phosphorus decorated porous carbons derived from shrimp shells for supercapacitors. Electrochim. Acta 2015, 176, 982–988. [Google Scholar] [CrossRef]
- Lota, K.; Acznik, I.; Sierczynska, A.; Lota, G. The capacitance properties of activated carbon obtained from chitosan as the electrode material for electrochemical capacitors. Mater. Lett. 2016, 173, 72–75. [Google Scholar] [CrossRef]
- Śliwak, A.; Díez, N.; Miniach, E.; Gryglewicz, G. Nitrogen-containing chitosan-based carbon as an electrode material for high-performance supercapacitors. J. Appl. Electrochem. 2016, 46, 667–677. [Google Scholar] [CrossRef]
- Guo, D.; Wei, H.; Chen, X.; Liu, M.; Ding, F.; Yang, Z.; Yang, Y.; Wang, S.; Yang, K.; Huang, S. 3D hierarchical nitrogen-doped carbon nanoflower derived from chitosan for efficient electrocatalytic oxygen reduction and high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 18193–18206. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Hassan, M.A.; Fetyan, A.; Kayarkatte, M.K.; Lauermann, I.; Roth, C. A promising N-doped carbon-metal oxide hybrid electrocatalyst derived from crustacean’s shells: Oxygen reduction and oxygen evolution. Appl. Catal. B Environ. 2017, 214, 137–147. [Google Scholar] [CrossRef]
- Walve, J.; Larsson, U. Carbon, nitrogen and phosphorus stoichiometry of crustacean zooplankton in the baltic sea: Implications for nutrient recycling. J. Plankton Res. 1999, 21, 2309–2321. [Google Scholar] [CrossRef]
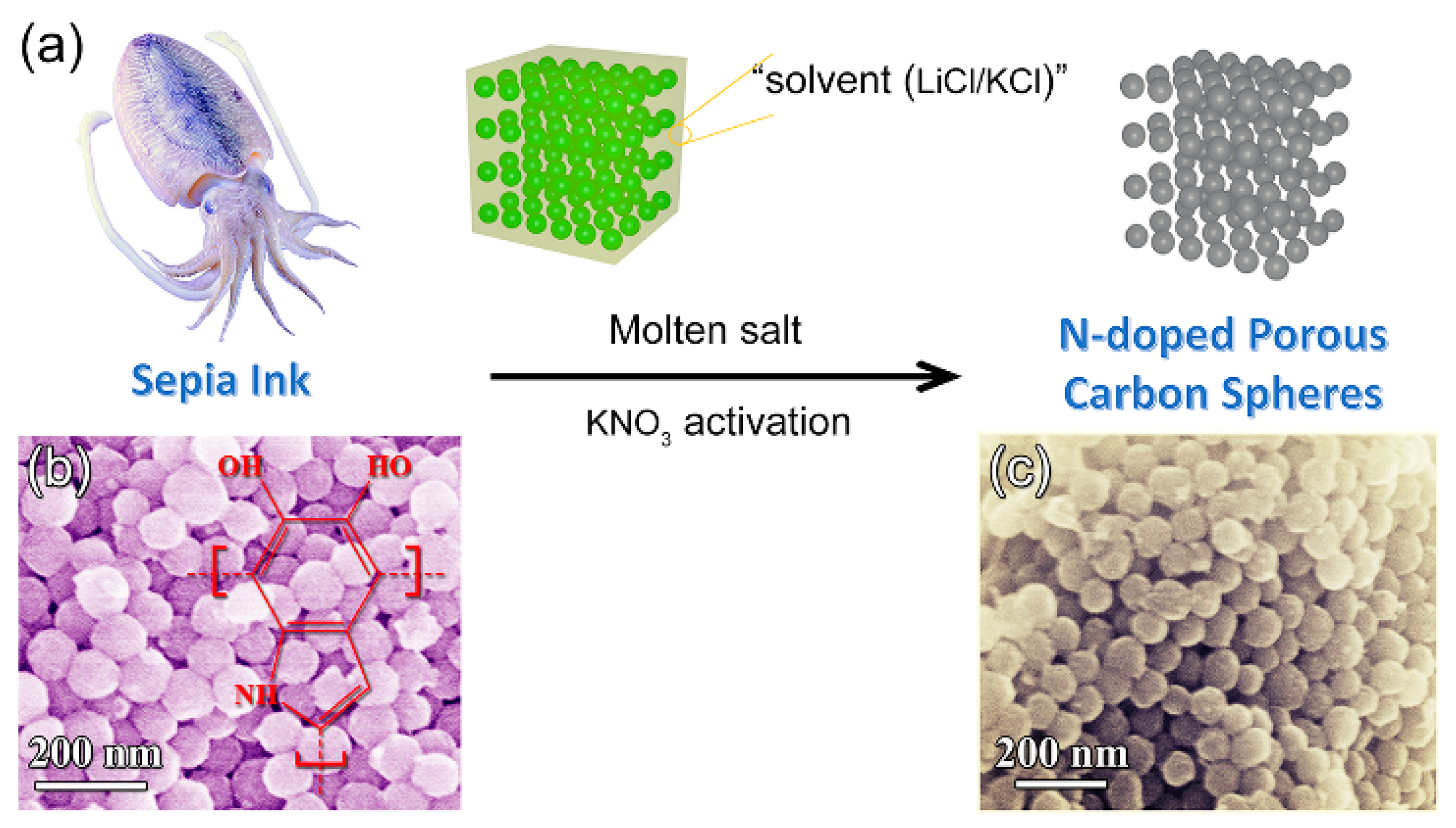
- Liu, Y.; Hong, L.; Kempf, V.R.; Wakamatsu, K.; Ito, S.; Simon, J.D. Ion-exchange and adsorption of Fe(III) by sepia melanin. Pigment Cell Res. 2004, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Wang, J.; Ding, B.; Chang, Z.; Wang, Y.; Dou, H.; Zhang, X. Nitrogen-doped porous carbon nanospheres from natural sepia ink: Easy preparation and extraordinary capacitive performance. ChemNanoMat 2017, 3, 895–901. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Fish scale derived nitrogen doped hierarchical porous carbon—A high rate performing anode for lithium ion cell. Electrochim. Acta 2015, 182, 1–10. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Microstructure, mechanical, and biomimetic properties of fish scales from pagrus major. J. Struct. Biol. 2003, 142, 327–333. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, Y.; Wang, W. A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J. Mater. Chem. 2010, 20, 4773–4775. [Google Scholar] [CrossRef]
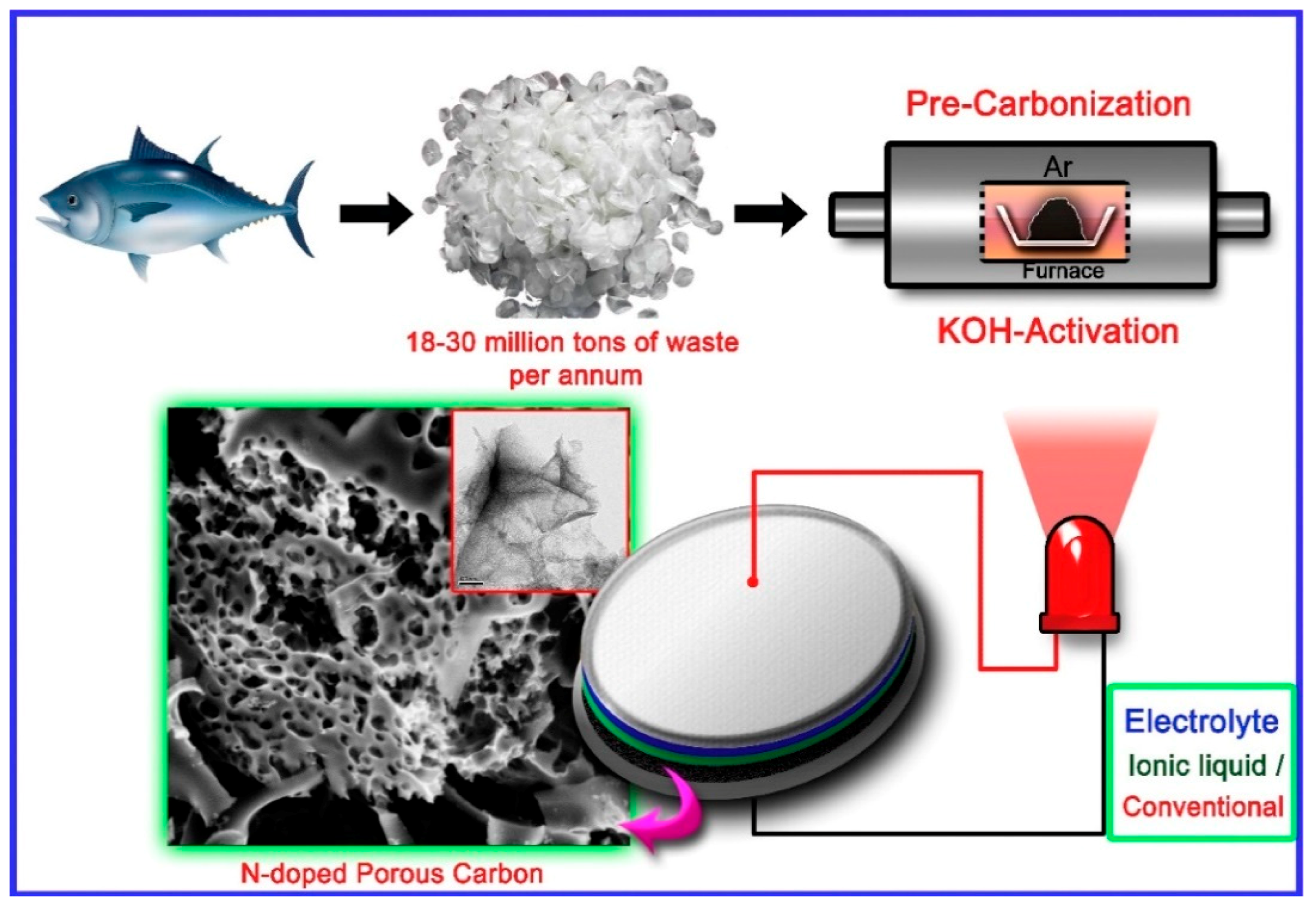
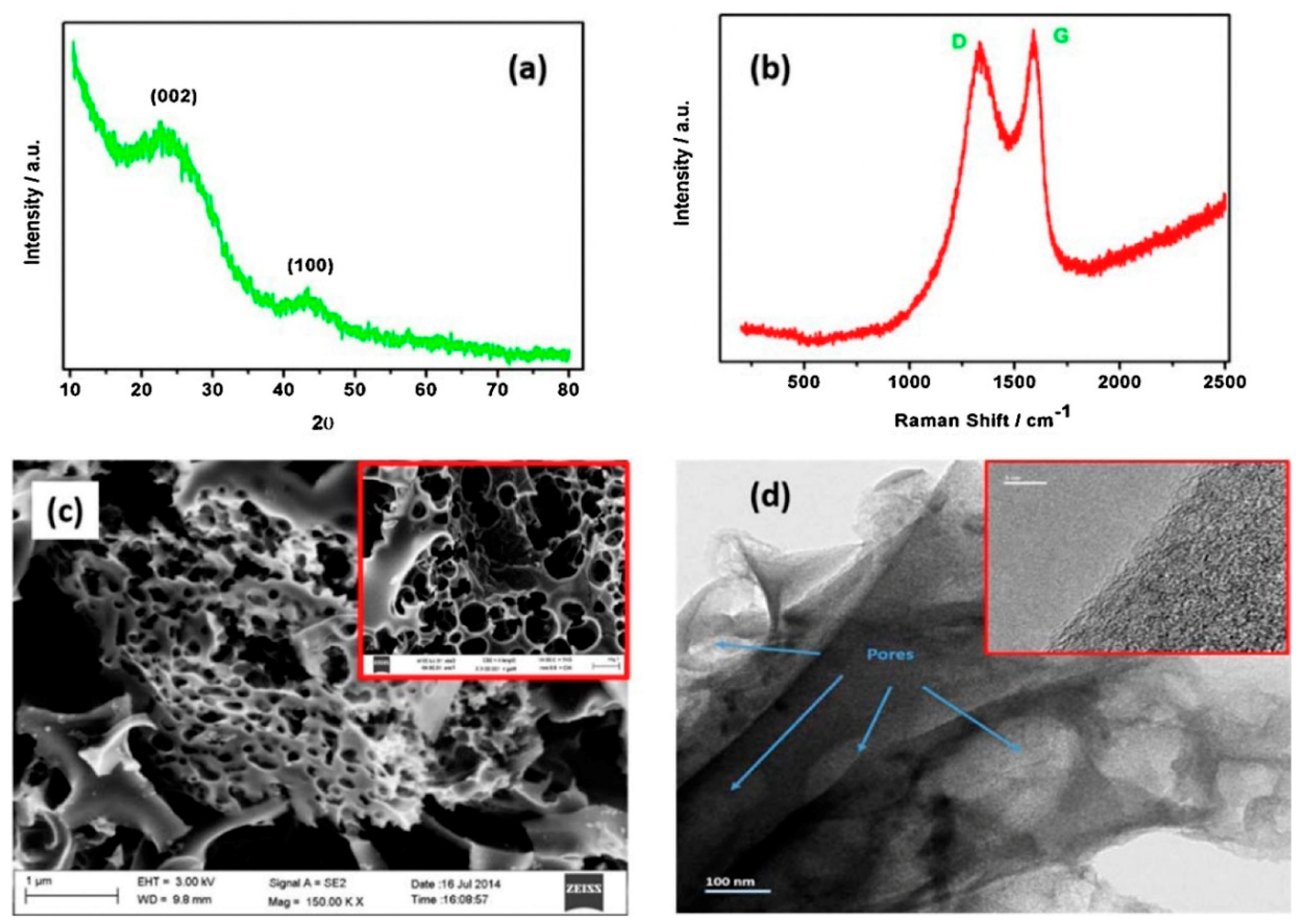
- Wang, H.; Wang, K.; Song, H.; Li, H.; Ji, S.; Wang, Z.; Li, S.; Wang, R. N-doped porous carbon material made from fish-bones and its highly electrocatalytic performance in the oxygen reduction reaction. RSC Adv. 2015, 5, 48965–48970. [Google Scholar] [CrossRef]
- Wu, G.; Feng, M.; Zhan, H. Generation of nitrogen-doped photoluminescent carbonaceous nanodots via the hydrothermal treatment of fish scales for the detection of hypochlorite. RSC Adv. 2015, 5, 44636–44641. [Google Scholar] [CrossRef]
- Yu, F.; Wang, M.; Huang, B.; Peng, Q.; Huang, Y. Acid-treatment effect on the N-doped porous carbon obtained from fish scales for Cr(VI) removal. Chem. Pap. 2017, 71, 2261–2269. [Google Scholar] [CrossRef]
- Huang, B.; Shao, H.; Liu, N.; Xu, Z.J.; Huang, Y. From fish scales to highly porous N-doped carbon: A low cost material solution for CO2 capture. RSC Adv. 2015, 5, 88171–88175. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Wang, F.; Huang, Y. Nitrogen-doped hierarchical lamellar porous carbon synthesized from the fish scale as support material for platinum nanoparticle electrocatalyst toward the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, C.; Wang, W.; Zhang, H.; Gao, M.; Xiong, X.; Wang, A.; Yuan, K.; Huang, Y.; Wang, F. A novel porous nanocomposite of sulfur/carbon obtained from fish scales for lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 3334–3339. [Google Scholar] [CrossRef]
- Guo, C.; Hu, R.; Liao, W.; Li, Z.; Sun, L.; Shi, D.; Li, Y.; Chen, C. Protein-enriched fish “biowaste” converted to three-dimensional porous carbon nano-network for advanced oxygen reduction electrocatalysis. Electrochim. Acta 2017, 236, 228–238. [Google Scholar] [CrossRef]
- Praveen, W.; Sujith, V.; Prabhakaran, K. Nitrogen doped microporous carbon by ZnCl2 activation of protein. Mater. Res. Express 2017, 4, 095602. [Google Scholar]
- Wang, R.; Song, H.; Li, H.; Wang, H.; Mao, X.; Ji, S. Mesoporous nitrogen-doped carbon derived from carp with high electrocatalytic performance for oxygen reduction reaction. J. Power Sources 2015, 278, 213–217. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.-M.; Saito, N.; Lee, M.-H. A simple synthesis method for nanostructured Co-WC/carbon composites with enhanced oxygen reduction reaction activity. Sci. Technol. Adv. Mater. 2016, 17, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wu, W. Nanotechnology-enabled energy harvesting for self-powered micro-/nanosystems. Angew. Chem. Int. Ed. 2012, 51, 11700–11721. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, K.I. Nanostructured platinum-free electrocatalysts in alkaline direct alcohol fuel cells: Catalyst design, principles and applications. RSC Adv. 2016, 6, 89523–89550. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.B.; Dai, L. Bcn graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, C. Fabrication of nitrogen-doped nano-onions and their electrocatalytic activity toward the oxygen reduction reaction. Sci. Rep. 2017, 7, 4178. [Google Scholar] [CrossRef] [PubMed]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Nitrogen-doped carbon nanoparticle–carbon nanofiber composite as an efficient metal-free cathode catalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 6962–6971. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mayoral, E.; Calvino-Casilda, V.; Soriano, E. Metal-supported carbon-based materials: Opportunities and challenges in the synthesis of valuable products. Catal. Sci. Technol. 2016, 6, 1265–1291. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, H. A cheap and non-destructive approach to increase coverage/loading of hydrophilic hydroxide on hydrophobic carbon for lightweight and high-performance supercapacitors. Sci. Rep. 2015, 5, 18108. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Ganesan, P.; Popov, B.N. Development of non-precious metal oxygen-reduction catalysts for pem fuel cells based on N-doped ordered porous carbon. Appl. Catal. B Environ. 2009, 93, 156–165. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; MacDonald, B.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Soft-templating synthesis of N-doped mesoporous carbon nanospheres for enhanced oxygen reduction reaction. Chem. Asian J. 2015, 10, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, T.; Sun, F.; Li, C.; Lin, Y. Fe/N co-doped mesoporous carbon nanomaterial as an efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2017, 231, 549–556. [Google Scholar] [CrossRef]
- Yan, X.-H.; Xu, B.-Q. Mesoporous carbon material co-doped with nitrogen and iron (Fe-N-C): High-performance cathode catalyst for oxygen reduction reaction in alkaline electrolyte. J. Mater. Chem. A 2014, 2, 8617–8622. [Google Scholar] [CrossRef]
- Lu, J.; Bo, X.; Wang, H.; Guo, L. Nitrogen-doped ordered mesoporous carbons synthesized from honey as metal-free catalyst for oxygen reduction reaction. Electrochim. Acta 2013, 108, 10–16. [Google Scholar] [CrossRef]
- Schaetzle, O.; Barriere, F.; Schroder, U. An improved microbial fuel cell with laccase as the oxygen reduction catalyst. Energy Environ. Sci. 2009, 2, 96–99. [Google Scholar] [CrossRef]
- You, S.; Gong, X.; Wang, W.; Qi, D.; Wang, X.; Chen, X.; Ren, N. Enhanced cathodic oxygen reduction and power production of microbial fuel cell based on noble-metal-free electrocatalyst derived from metal-organic frameworks. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef]
- Alabadi, A.; Yang, X.; Dong, Z.; Li, Z.; Tan, B. Nitrogen-doped activated carbons derived from a co-polymer for high supercapacitor performance. J. Mater. Chem. A 2014, 2, 11697–11705. [Google Scholar] [CrossRef]
- Garcia, B.; Candelaria, S.; Cao, G. Nitrogenated porous carbon electrodes for supercapacitors. J. Mater. Sci. 2012, 47, 5996–6004. [Google Scholar] [CrossRef]
- Lota, G.; Grzyb, B.; Machnikowska, H.; Machnikowski, J.; Frackowiak, E. Effect of nitrogen in carbon electrode on the supercapacitor performance. Chem. Phys. Lett. 2005, 404, 53–58. [Google Scholar] [CrossRef]
- Ma, C.; Shi, J.; Song, Y.; Zhang, D.; Zhai, X.; Zhong, M.; Guo, Q.; Liu, L. Preparation and capacitive properties of nitrogen-enriched hierarchical porous carbon. Int. J. Electrochem. Sci 2012, 7, 7587–7599. [Google Scholar]
- Shi, Q.; Zhang, R.; Lv, Y.; Deng, Y.; Elzatahrya, A.A.; Zhao, D. Nitrogen-doped ordered mesoporous carbons based on cyanamide as the dopant for supercapacitor. Carbon 2015, 84, 335–346. [Google Scholar] [CrossRef]
- Wan, M.M.; Sun, X.D.; Li, Y.Y.; Zhou, J.; Wang, Y.; Zhu, J.H. Facilely fabricating multifunctional N-enriched carbon. ACS Appl. Mater. Interfaces 2016, 8, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Jurewicz, K.; Babeł, K.; Ziółkowski, A.; Wachowska, H. Capacitance behaviour of the ammoxidised coal. J. Phys. Chem. Solids 2004, 65, 269–273. [Google Scholar] [CrossRef]
- Hulicova, D.; Kodama, M.; Hatori, H. Electrochemical performance of nitrogen-enriched carbons in aqueous and non-aqueous supercapacitors. Chem. Mater. 2006, 18, 2318–2326. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Frackowiak, E.; Lota, G.; Machnikowski, J.; Vix-Guterl, C.; Béguin, F. Optimisation of supercapacitors using carbons with controlled nanotexture and nitrogen content. Electrochim. Acta 2006, 51, 2209–2214. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, H.H.; Jin, H.-J. Pseudocapacitive effects of N-doped carbon nanotube electrodes in supercapacitors. Materials 2012, 5, 1258. [Google Scholar] [CrossRef]
- Qin, C.; Lu, X.; Yin, G.; Jin, Z.; Tan, Q.; Bai, X. Study of activated nitrogen-enriched carbon and nitrogen-enriched carbon/carbon aerogel composite as cathode materials for supercapacitors. Mater. Chem. Phys. 2011, 126, 453–458. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Nitrogen doped superporous carbon prepared by a mild method. Enhancement of supercapacitor performance. Int. J. Hydrogen Energy 2016, 41, 19691–19701. [Google Scholar] [CrossRef]
- Song, Y.; Hu, S.; Dong, X.; Wang, Y.; Wang, C.; Xia, Y. A nitrogen-doped hierarchical mesoporous/microporous carbon for supercapacitors. Electrochim. Acta 2014, 146, 485–494. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Ke, Q.; Wang, J. Effects of nitrogen doping on supercapacitor performance of a mesoporous carbon electrode produced by a hydrothermal soft-templating process. J. Mater. Chem. A 2014, 2, 11753–11758. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Zhao, L.; Tian, C.; Zhao, D.; Zhou, W.; Yin, J.; Wang, R.; Fu, H. B and N isolate-doped graphitic carbon nanosheets from nitrogen-containing ion-exchanged resins for enhanced oxygen reduction. Sci. Rep. 2014, 4, 5184. [Google Scholar] [CrossRef] [PubMed]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Kodiweera, N.; Stallworth, P.E.; Greenbaum, S.; Bandosz, T.J. Effect of surface phosphorus functionalities of activated carbons containing oxygen and nitrogen on electrochemical capacitance. Carbon 2009, 47, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.-M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. 2008, 120, 379–382. [Google Scholar] [CrossRef]
- Chen, L.-F.; Zhang, X.-D.; Liang, H.-W.; Kong, M.; Guan, Q.-F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Hulicova-Jurcakova, D.; Lu, G.Q.; Bandosz, T.J. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon 2008, 46, 1475–1488. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Chen, Z.-G.; Lu, G.Q.; Cheng, H.-M. Synthesis and electrochemical property of boron-doped mesoporous carbon in supercapacitor. Chem. Mater. 2008, 20, 7195–7200. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Ravikumar, R.; Gopukumar, S. High quality nmp exfoliated graphene nanosheet-SnO2 composite anode material for lithium ion battery. Phys. Chem. Chem. Phys. 2013, 15, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, G.; Wang, C. General scalable strategy toward heterogeneously doped hierarchical porous graphitic carbon bubbles for lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 21661–21668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; Huang, Z.-H.; Liang, H.-W.; Gao, H.-L.; Yu, S.-H. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Huang, X.; Zhou, L. Encapsulation of α-Fe2O3 nanoparticles in graphitic carbon microspheres as high-performance anode materials for lithium-ion batteries. Nanoscale 2015, 7, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire electrodes for electrochemical energy storage devices. Chem. Rev. 2014, 114, 11828–11862. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Zhang, X. Encapsulating sulfur into hierarchically ordered porous carbon as a high-performance cathode for lithium–sulfur batteries. Chem. A Eur. J. 2013, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Wang, X.; Lai, Y.; Liu, Y.; Li, J. Synthesis of hierarchical porous honeycomb carbon for lithium-sulfur battery cathode with high rate capability and long cycling stability. Electrochim. Acta 2014, 137, 439–446. [Google Scholar] [CrossRef]
- Yu, L.; Brun, N.; Sakaushi, K.; Eckert, J.; Titirici, M.M. Hydrothermal nanocasting: Synthesis of hierarchically porous carbon monoliths and their application in lithium–sulfur batteries. Carbon 2013, 61, 245–253. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Glans, P.-A.; Zhang, Y.; Zhu, J.; Guo, J. Electronic structure and chemical bonding of a graphene oxide-sulfur nanocomposite for use in superior performance lithium-sulfur cells. Phys. Chem. Chem. Phys. 2012, 14, 13670–13675. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, Q.; Zhao, M.-Q.; Huang, J.-Q.; Cheng, X.-B.; Tian, G.-L.; Peng, H.-J.; Wei, F. Nitrogen-doped aligned carbon nanotube/graphene sandwiches: Facile catalytic growth on bifunctional natural catalysts and their applications as scaffolds for high-rate lithium-sulfur batteries. Adv. Mater. 2014, 26, 6100–6105. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium−sulfur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Qian, W.; Zhu, L.; Yang, C. Biomass-derived porous carbon with micropores and small mesopores for high-performance lithium–sulfur batteries. Chem.A Eur. J. 2016, 22, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shen, Y.; Zhang, W.; Yu, L.; Yi, Z.; Yin, W.; Wang, D.; Huang, Y.; Wang, J.; Wang, D.; et al. A solution-phase bifunctional catalyst for lithium–oxygen batteries. J. Am. Chem. Soc. 2014, 136, 8941–8946. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, D.; Yu, L.; Zhang, W.; Shang, Y.; Tang, H.; Wu, J.; Cao, A.; Huang, Y. A high-capacity lithium–air battery with pd modified carbon nanotube sponge cathode working in regular air. Carbon 2013, 62, 288–295. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Rychlicki, G.; Biniak, S.; Łukaszewicz, J.P. New correlations between the composition of the surface layer of carbon and its physicochemical properties exposed while paracetamol is adsorbed at different temperatures and pH. J. Colloid Interface Sci. 2003, 257, 13–30. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.-S. Efficient carbon dioxide capture over a nitrogen-rich carbon having a hierarchical micro-mesopore structure. Fuel 2012, 95, 360–364. [Google Scholar] [CrossRef]
- Mangun, C.L.; DeBarr, J.A.; Economy, J. Adsorption of sulfur dioxide on ammonia-treated activated carbon fibers. Carbon 2001, 39, 1689–1696. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A. The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 2003, 41, 1925–1932. [Google Scholar] [CrossRef]
- Boudou, J.-P.; Chehimi, M.; Broniek, E.; Siemieniewska, T.; Bimer, J. Adsorption of H2S or SO2 on an activated carbon cloth modified by ammonia treatment. Carbon 2003, 41, 1999–2007. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated carbon catalyst for selective oxidation of hydrogen sulphide: On the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Adib, F.; Bagreev, A.; Bandosz, T.J. Adsorption/oxidation of hydrogen sulfide on nitrogen-containing activated carbons. Langmuir 2000, 16, 1980–1986. [Google Scholar] [CrossRef]
- Li, P.-Z.; Zhao, Y. Nitrogen-rich porous adsorbents for CO2 capture and storage. Chem. An Asian J. 2013, 8, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Bagreev, A.; Menendez, J.A.; Dukhno, I.; Tarasenko, Y.; Bandosz, T.J. Oxidative adsorption of methyl mercaptan on nitrogen-enriched bituminous coal-based activated carbon. Carbon 2005, 43, 208–210. [Google Scholar] [CrossRef]
- Abe, M.; Kawashima, K.; Kozawa, K.; Sakai, H.; Kaneko, K. Amination of activated carbon and adsorption characteristics of its aminated surface. Langmuir 2000, 16, 5059–5063. [Google Scholar] [CrossRef]
- Bashkova, S.; Bandosz, T.J. The effects of urea modification and heat treatment on the process of NO2 removal by wood-based activated carbon. J. Colloid Interface Sci. 2009, 333, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Burg, P.; Fydrych, P.; Cagniant, D.; Nanse, G.; Bimer, J.; Jankowska, A. The characterization of nitrogen-enriched activated carbons by IR, XPS and lser methods. Carbon 2002, 40, 1521–1531. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Meisner, G.P. Preparation of nanoporous carbon particles and their cryogenic hydrogen storage capacities. J. Phys. Chem. C 2008, 112, 1516–1523. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Mokaya, R. Enhanced hydrogen storage capacity of high surface area zeolite-like carbon materials. J. Am. Chem. Soc. 2007, 129, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, W.B.; Shi, J.S.; Xin, F.W. Influence of doping nitrogen, sulfur, and phosphorus on activated carbons for gas adsorption of H2, CH4 and CO2. RSC Adv. 2016, 6, 50138–50143. [Google Scholar] [CrossRef]
- Giraudet, S.; Zhu, Z. Hydrogen adsorption in nitrogen enriched ordered mesoporous carbons doped with nickel nanoparticles. Carbon 2011, 49, 398–405. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Hydrogen storage in high surface area carbons with identical surface areas but different pore sizes: Direct demonstration of the effects of pore size. J. Phys. Chem. C 2012, 116, 25734–25740. [Google Scholar] [CrossRef]
- Pacuła, A.; Mokaya, R. Synthesis and high hydrogen storage capacity of zeolite-like carbons nanocast using as-synthesized zeolite templates. J. Phys. Chem. C 2008, 112, 2764–2769. [Google Scholar] [CrossRef]
- Biniak, S.; Pakuła, M.; Szymański, G.S.; Światkowski, A. Effect of activated carbon surface oxygen- and/or nitrogen-containing groups on adsorption of copper(II) ions from aqueous solution. Langmuir 1999, 15, 6117–6122. [Google Scholar] [CrossRef]
- Walczyk, M.; Świątkowski, A.; Pakuła, M.; Biniak, S. Electrochemical studies of the interaction between a modified activated carbon surface and heavy metal ions. J. Appl. Electrochem. 2005, 35, 123–130. [Google Scholar] [CrossRef]
- Xiao, B.; Thomas, K.M. Adsorption of aqueous metal ions on oxygen and nitrogen functionalized nanoporous activated carbons. Langmuir 2005, 21, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.; Nakajima, T. Adsorption of aromatic compounds on CxN-coated activated carbon. Carbon 2000, 38, 1947–1954. [Google Scholar] [CrossRef]








| Synthetic Polymer | SBET (m2/g) | CN% | References |
|---|---|---|---|
| Melamine–formaldehyde resin | 1460 | 7.0 wt % | [4] |
| Melamine–formaldehyde resin | 12–56 | 30.3–31.7 wt % | [5] |
| Urea–formaldehyde resin | 5–2479 | 3.1–12.3 wt % | [5] |
| Melamine resin | 900 | 1.7–2.4 wt % | [6] |
| Melamine resin | 1–496 | 3.03–4.04 at % | [7] |
| Polyurethane | 13–112 | 0.71–6.31 wt % | [8] |
| Polyurethane | <1 | 7–10 wt % | [9] |
| Polyvinylpyridine | 519–1420 | 1.4–2.6 wt % | [10] |
| Polyamide | 1–1329 | 4.18–8.02 wt % | [11] |
| Polyimide | 137 | 3.67 at % | [12] |
| Polyaniline | 325–514 | 6.7–10.89 at % | [13] |
| Polyaniline | 115–670 | 6.5–12.6 wt % | [14] |
| Polyaniline | 24–94 | 8.7–13.3 wt % | [15] |
| Polyaniline | n.d. 1 | 10.3–14.9 wt % | [16] |
| Polyaniline | 200–205 | 2.1–10.8 wt % | [17] |
| Pyrrole | 900–1200 | 5.8–9.9 at % | [18] |
| Polypyrrole | 2086 | 3.05 at % | [19] |
| Polypyrrole | 781–1243 | 4.31–7.26 wt % | [20] |
| Polypyrrole | 1060–1170 | 4.24–4.72 wt % | [21] |
| Polypyrrole | 1560 | 5.5 at % | [22] |
| Acetonitrile | 286–1034 | 7.0–8.8 wt % | [23] |
| Polyacetonitrile | 620–832 | 1.7–7.2 wt % | [10] |
| Polyacetonitrile | 544 | 5.3 at % | [24] |
| Polyacetonitrile | 644–800 | C/N 5.75–22.26 at % | [25] |
| Polyacetonitrile | 520–840 | n.d. | [26] |
| Stage | Agent | Aim | Result |
|---|---|---|---|
| 1. | Chitosan powder and water premixed mechanically | Softening of chitosan and its transformations into a homogenous paste | Chitosan–water viscose paste |
| 2. | Addition of aqueous HCl solution with a pH of about 7 | Protonation of –NH2 to –NH3+ groups and testing of hydrophobic properties | Absorption of water and the formation of a low-viscosity homogeneous paste |
| 3. | Optional addition of an aqueous urea solutionFor alternative treatments B–F, see Table 3 | Optional introduction of additional amounts of nitrogenFor alternative aims B–F, see Table 3 | Increased amount of nitrogen in the carbon matrixFor alternative results B–F, see Table 3 |
| 4. | Addition of an aqueous solution of Na2CO3 | Introducing a substance capable to form nanocrystallites of a pore-genic hard template | Chitosan gel supplemented with a pore-genic (template) substance (Na2CO3) uniformly distributed in the gel |
| 5. | Drying | Removal of excess water and crystallization of Na2CO3 nanocrystallites (pore-genic template) in the dry chitosan mass | Removal of excess water and crystallization of Na2CO3 nanocrystallites (pore-genic template) in the dry chitosan mass |
| 6. | Oxygen-free carbonization | Obtaining a carbon matrix with embedded Na2CO3 nanocrystallites (pore-genic hard template) | Non-porous carbon matrix enriched with nitrogen |
| 7. | Etching with concentrated HCl | Removal of pore-genic/template Na2CO3 nanocrystals from carbon matrix | Raw porous carbon matrix |
| 8. | Washing with distilled water | Removal of water-soluble pollutants and Na2CO3 residues | Carbon matrix with a developed specific surface area, pore structure and a significant nitrogen content without pollutants and template residues |
| Method | Description of the Method | References |
|---|---|---|
| B | Forgo on Na2CO3 solution. Addition of an aqueous solution of ZnCl2. Then, steps 7 and 8 are combined and rely on long-term rinsing with hot distilled water until the chloride ions disappear. | [120] |
| C | Forgo on Na2CO3 solution. Addition of aqueous H3PO4 solution. Then, steps 7 and 8 are combined and rely on long-term rinsing with hot distilled water until the phosphate ions have disappeared. | [102] |
| D | Forgo on Na2CO3 solution. Addition of a solid and insoluble template, e.g., CaCO3. | [121] |
| U | In all variants of the synthesis method, i.e., A, B, C, or D, the addition of a water-soluble, nitrogen-containing, low molecular weight substance to increase the nitrogen content is optionally used. | [122] |
| E | In all variants of the synthesis method, i.e., A, B, C, D, or U, the addition of soluble metal salts is used to give new properties, e.g., catalytic or biocidal properties. | [123,124] |
| Precursor | Modification/T (°C)/t (h) | SBET (m2/g) | N (wt %) | Vt (cm3/g) | Vme (cm3/g) | References |
|---|---|---|---|---|---|---|
| CH 1 + Na2CO3 | A/600/1 | 10–441 | n.d.2 | n.d. | n.d. | [112] |
| CH + ZnCl2 | B/600–800/1 | 583–1932 | 4.5–7.5 | 0.31–1.33 | 0.008–0.507 | [120] |
| CH + Na2CO3 | A/600–900/1 | 441–1148 | 2.8–6.5 | 0.18–0.70 | >1% | [122] |
| CH + Na2CO3 + CH4N2O | A + U/600/1 | 121–430 | 9.4–13.1 | 0.11–0.21 | >1% | [122] |
| CH + H3PO4 | C/600/1 | 970–1484 | 4.7–6.1 | 0.439–1.543 | 0.408–1.515 | [102] |
| CH + ZnCl2 + Cu(NO3)2 | B + E | 102–1159 | 5.0–7.8 | n.d. | n.d. | [123] |
| CH + Cu(NO3)2 | Without template/700/1 | 102–123 | 7.8–7.9 | n.d. | n.d. | [124] |
| Year | Precursor | Synthesis: T (°C)/t (h) | Obtained Carbon Material | Applications | References |
|---|---|---|---|---|---|
| 2013 | CH + K2CO3 | 600–800/1 | Microporous SBET = 1.4–2469 m2/g, CN% = 1.29–7.60 wt % | Accumulation of CO2 | [126] |
| 2013 | CH + secondary activation of NaOH | 400–600/1 later calcination with NaOH/400–600/1 | Microporous SBET = do 3500 m2/g, CN% = do 5.4 wt % | Supercapacitor | [127] |
| 2013 | CH + SiO2 | 900/4 | Mesoporous SBET = 608–1337 m2/g, CN% = 2.17–8.83 wt % | n.d. 2 | [128] |
| 2014 | CH + ZnCl2 or KOH or CO2 | 600–850/3–5 | Mesoporous SBET = 912–1770 m2/g, CN% = 3.92–5.37 wt % | Electrode material | [129] |
| 2014 | CH + SWNT 1 | 600/2 | Mesoporous, conductive composite SBET = 628 m2/g, CN% = n.d. | Supercapacitor | [130] |
| 2015 | CH + FeCl3 | 450/1 | Nonporous SBET = 4.7–62.8 m2/g, CN% = n.d. | Removal of Cu2+ ions from water | [131] |
| 2015 | CH + H3BO3 | 800/1 | Micro/mesoporous, conductive carbon matrix SBET = 3–710 m2/g, CN% = 8.19 wt % | Supercapacitor | [132] |
| 2015 | CH, secondary activation of KOH | 800/3 later calcination with KOH/700–1000/2 | Micro/mesoporous containing graphene nanostructures SBET = do 2435 m2/g, CN% = n.d. | Supercapacitor | [133] |
| 2015 | CH + secondary activation of KOH | 700–1000/30 min, later calcination with KOH/700–800/1 | Microporous SBET = 922–3066 m2/g, CN% = n.d. | Accumulation of hydrogen | [134] |
| 2015 | Shrimp shell + H3PO4 | 400–600/1 | Mesoporous SBET = 38–774 m2/g, CN% = 2.9–3.9 wt % | Supercapacitor | [135] |
| 2016 | CH, secondary activation of KOH | 650/1 later calcination with KOH/750–850/ | Mesoporous SBET = 2397–2807 m2/g, CN% = 0.2–0.4 Carbonised with CH SBET = 1.3 m2/g, CN% = n.d. | Supercapacitor | [136] |
| 2016 | CH + activation of CO2 | 900/15 min, later activation CO2/900/n.d. | Microporous SBET = 1101 m2/g, CN% = do 5.4 wt % | Supercapacitor | [137] |
| 2017 | CH + SiO2 | 155/12 | Mesoporous SBET = 907 m2/g, CN% = n.d. | Electrode material | [138] |
| 2018 | CH + Nd2O3 | 500–900/2 | Mesoporous SBET = n.d., CN% = 7.41 at % | Energy storage | [139] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilnicka, A.; Lukaszewicz, J.P. Marine and Freshwater Feedstocks as a Precursor for Nitrogen-Containing Carbons: A Review. Mar. Drugs 2018, 16, 142. https://doi.org/10.3390/md16050142
Ilnicka A, Lukaszewicz JP. Marine and Freshwater Feedstocks as a Precursor for Nitrogen-Containing Carbons: A Review. Marine Drugs. 2018; 16(5):142. https://doi.org/10.3390/md16050142
Chicago/Turabian StyleIlnicka, Anna, and Jerzy P. Lukaszewicz. 2018. "Marine and Freshwater Feedstocks as a Precursor for Nitrogen-Containing Carbons: A Review" Marine Drugs 16, no. 5: 142. https://doi.org/10.3390/md16050142





