Functional Expression and Characterization of the Recombinant N-Acetyl-Glucosamine/N-Acetyl-Galactosamine-Specific Marine Algal Lectin BPL3
Abstract
:1. Introduction
2. Results
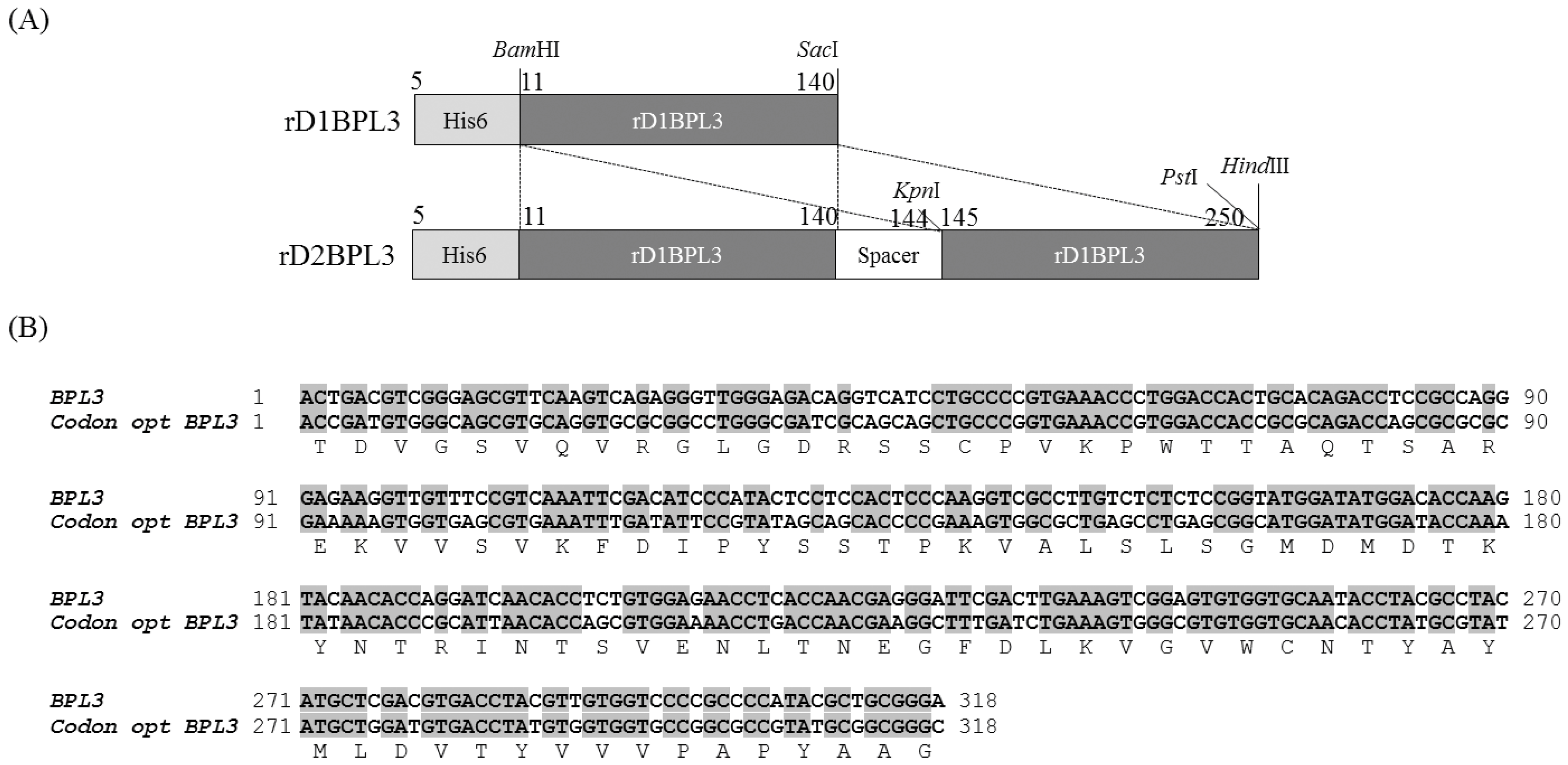
2.1. Cloning of rBPL3
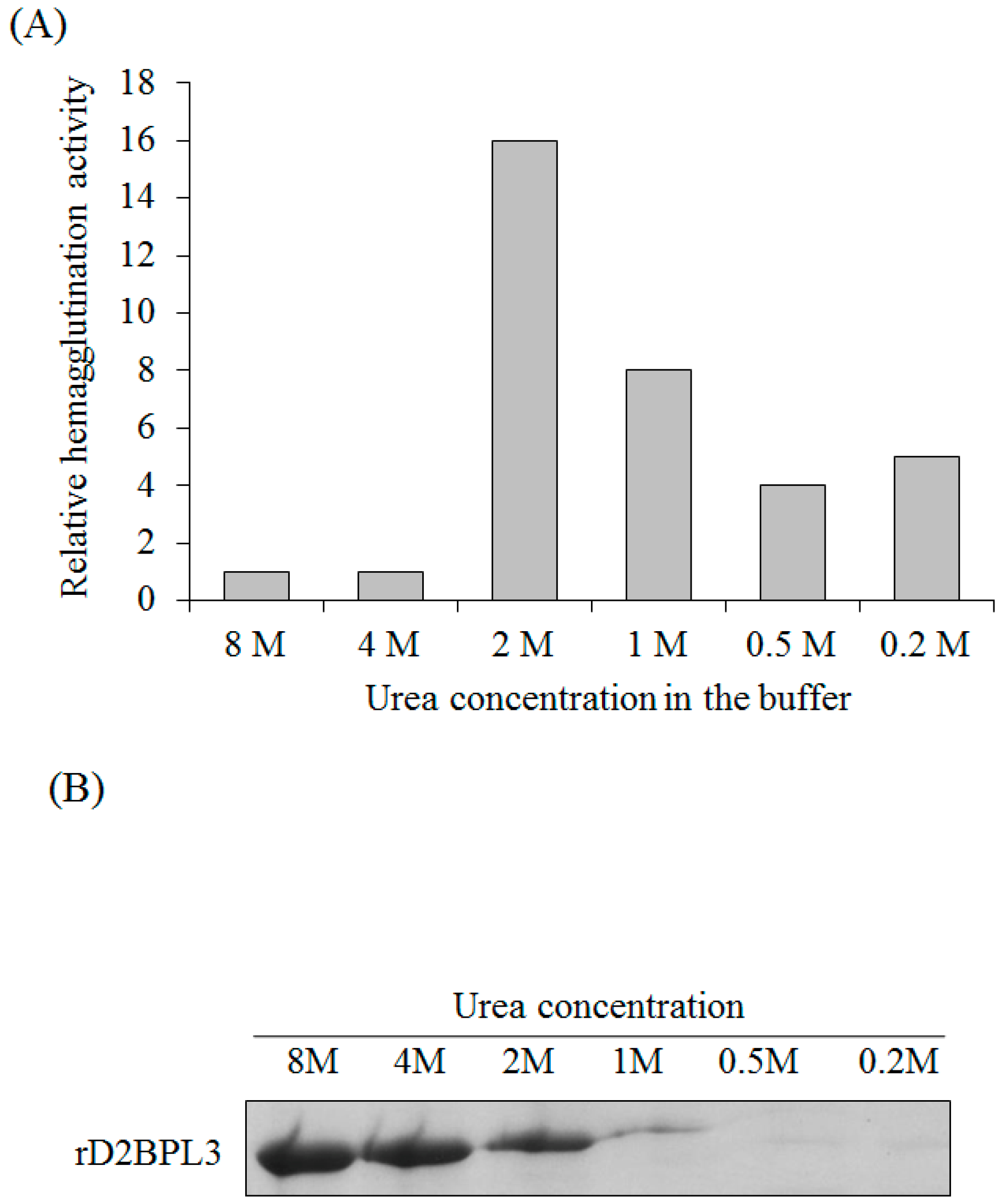
2.2. Selection of an Expression Host and Optimization of Conditions
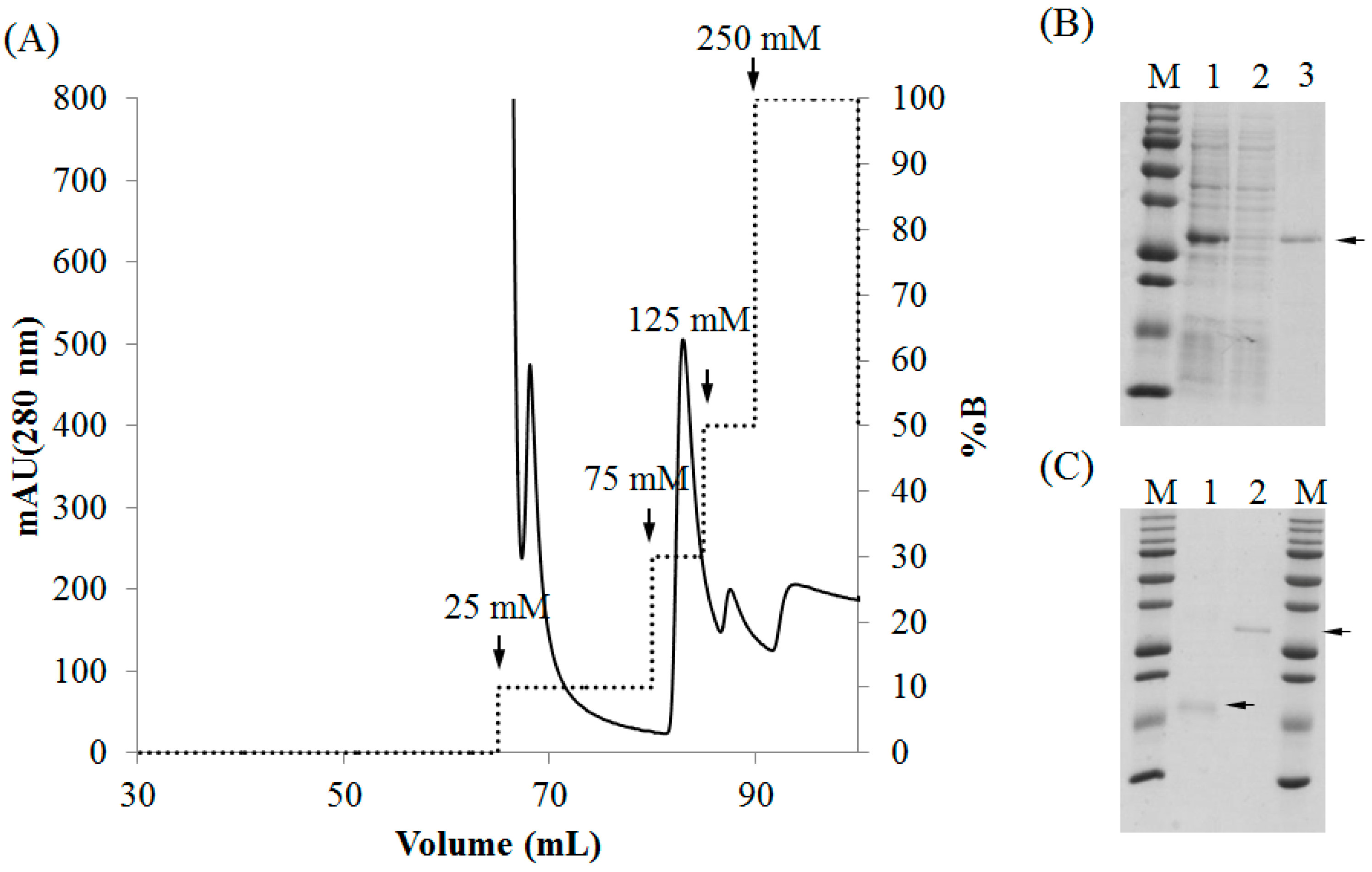
2.3. Purification of Recombinant Lectins
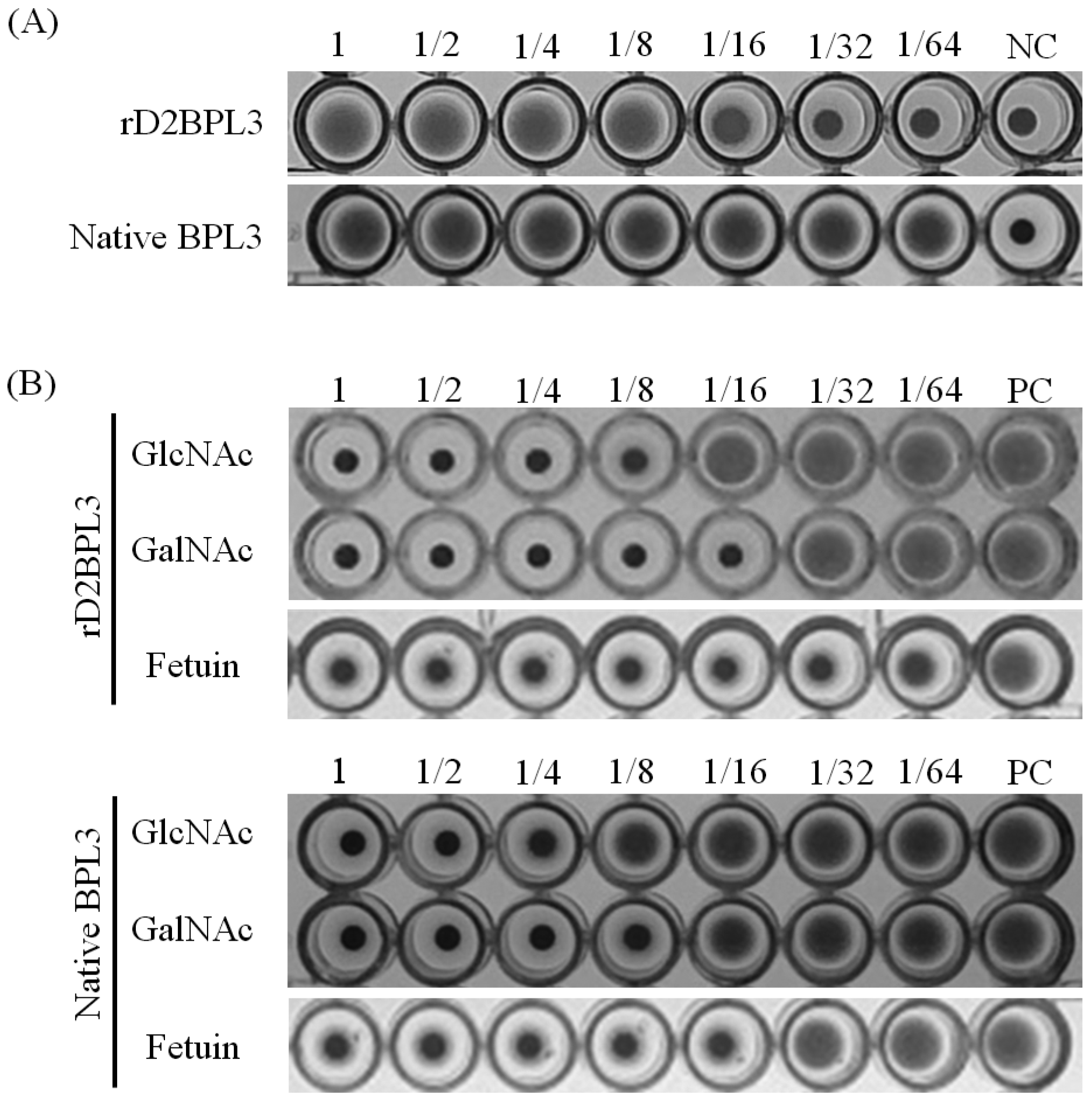
2.4. Carbohydrate Specificity and Heat-Stability of Recombinant Lectin
2.5. Glycan Micro-Array Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of Native BPL3
4.2. Cloning and Construction of the Recombinant Protein
4.3. Optimization of rBPL3 Expression
4.4. Purification of rBPL3.
4.5. Refolding of rBPL3
4.6. Hemagglutination Activity Assay
4.7. Determination of Carbohydrate Specificity
4.8. Glycan Microarray
4.9. Effect of Temperature and Divalent Cations on the Agglutination Activity
4.10. Mass Spectrometry
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dan, X.; Liu, W.; Ng, T.B. Development and applications of lectins as biological tools in biomedical research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Peelen, D.; Smith, L.M. Lectin arrays for profiling cell surface carbohydrate expression. J. Am. Chem. Soc. 2005, 127, 9982–9983. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.; Comunale, M.A.; Herrera, H.; Wang, M.; Houser, J.; Wimmerova, M.; Romano, P.R.; Mehta, A. Development and application of a novel recombinant Aleuria aurantia lectin with enhanced core fucose binding for identification of glycoprotein biomarkers of hepatocellular carcinoma. Proteomics 2016, 16, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, M.; Wingender, J.; Flemming, H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Hortin, G.L.; Trimpe, B.L. Lectin affinity chromatography of proteins bearing O-linked oligosaccharides: Application of jacalin-Sepharose. Anal. Biochem. 1990, 188, 271–277. [Google Scholar] [CrossRef]
- Hovhannisyan, V.A.; Bazukyan, I.L.; Gasparyan, V.K. Application of silver nanoparticles and CdSe quantum dots sensitized with of C-like lectin for detection of St. aureus. Comparison of various approaches. Talanta 2017, 175, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.; Colodel, E.; Schmitz, M.; Gimeno, E.; Driemeier, D. Use of lectin histochemistry to diagnose Sida carpinifolia (Malvaceae) poisoning in sheep. Vet. Rec. 2005, 156, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.L.; Gildersleeve, J.C.; Mahal, L.K. A simple strategy for the creation of a recombinant lectin microarray. Mol. Biosyst. 2008, 4, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K.; Kanwar, J.R.; Kennedy, J.F. Amoebiasis vaccine development: A snapshot on E. histolytica with emphasis on perspectives of Gal/GalNAc lectin. Int. J. Biol. Macromol. 2016, 91, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Haji-Ghassemi, O.; Gilbert, M.; Spence, J.; Schur, M.J.; Parker, M.J.; Jenkins, M.L.; Burke, J.E.; van Faassen, H.; Young, N.M.; Evans, S.V. Molecular basis for recognition of the cancer glycobiomarker, LacdiNAc (GalNAc[beta1→4]GlcNAc), by Wisteria floribunda Agglutinin. J. Biol. Chem. 2016, 291, 24085–24095. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.H.; Arruda, F.V.S.; Nascimento, K.S.; Carneiro, V.A.; Nagano, C.S.; Silva, B.R.; Sampaio, A.H.; Cavada, B.S. Biological applications of plants and algae lectins: An overview. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012; Chapter 23; pp. 533–558. [Google Scholar]
- Han, J.W.; Yoon, K.S.; Klochkova, T.A.; Hwang, M.S.; Kim, G.H. Purification and characterization of a lectin, BPL-3, from the marine green alga Bryopsis plumosa. J. Appl. Phycol. 2011, 23, 745–753. [Google Scholar] [CrossRef]
- Han, J.W.; Yoon, K.S.; Jung, M.G.; Chah, K.H.; Kim, G.H. Molecular characterization of a lectin, BPL-4, from the marine green alga Bryopsis plumosa (Chlorophyta). Algae 2012, 27, 55–62. [Google Scholar] [CrossRef]
- Han, J.W.; Jung, M.G.; Shim, E.Y.; Shim, J.B.; Kim, Y.M.; Kim, G.H. Functional recombinants designed from a fetuin/asialofetuin-specific marine algal lectin, rhodobindin. Mar. Drugs 2015, 13, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.; Teixeira, J.A.; Domingues, L. Recombinant production of plant lectins in microbial systems for biomedical application—The frutalin case study. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosono, M.; Ishikawa, K.; Mineki, R.; Murayama, K.; Numata, C.; Ogawa, Y.; Takayanagi, Y.; Nitta, K. Tandem repeat structure of rhamnose-binding lectin from catfish (Silurus asotus) eggs. Biochim. Biophys. Acta 1999, 1472, 668–675. [Google Scholar] [CrossRef]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High mannose-binding lectin with preference for the cluster of alpha1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Shim, J.; Klochkova, T.A.; Han, J.W.; Kim, G.H. Purification of a sex-specific lectin involved in gamete binding of Aglaothamnion callophyllidicola (Rhodophyta). J. Phycol. 2012, 48, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Jung, M.G.; Kim, M.J.; Yoon, K.S.; Lee, K.P.; Kim, G.H. Purification and characterization of a d-mannose specific lectin from the green marine alga, Bryopsis plumosa. Phycol. Res. 2010, 58, 143–150. [Google Scholar] [CrossRef]
- Yoon, K.S.; Lee, K.P.; Klochkova, T.A.; Kim, G.H. Molecular characterization of the lectin, Bryohealin, involved in protoplast regeneration of the marine alga Bryopsis plumosa (Chlorophyta). J. Phycol. 2008, 44, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.G.; Lee, K.P.; Choi, H.G.; Kang, S.H.; Klochkova, T.A.; Han, J.W.; Kim, G.H. Characterization of carbohydrate combining sites of Bryohealin, an algal lectin from Bryopsis plumosa. J. Appl. Phycol. 2010, 22, 793–802. [Google Scholar] [CrossRef]
- Oliveira, C.; Costa, S.; Teixeira, J.A.; Domingues, L. cDNA cloning and functional expression of the α-d-Galactose-Binding lectin frutalin in Escherichia coli. Mol. Biotechnol. 2009, 43, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liao, X.; Chen, G.; Yap, Y.; Zhang, X. Cloning, expression and purification of Microcystis viridis lectin in Escherichia coli. Mol. Biotechnol. 2011, 47, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Adar, R.; Streicher, H.; Rozenblatt, S.; Sharon, N. Synthesis of soybean agglutinin in bacterial and mammalian cells. Eur. J. Biochem. 1997, 249, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.E.; Carver, J.P.; Dunn, R.J. Production of pea lectin in Escherichia coli. J. Biol. Chem. 1986, 261, 6141–6144. [Google Scholar] [PubMed]
- Upadhyay, S.K.; Saurabh, S.; Rai, P.; Singh, R.; Chandrashekar, K.; Verma, P.C.; Singh, P.K.; Tuli, R. SUMO fusion facilitates expression and purification of garlic leaf lectin but modifies some of its properties. J. Biotechnol. 2010, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Teixeira, J.A.; Domingues, L. Recombinant lectins: An array of tailor-made glycan-interaction biosynthetic tools. Crit. Rev. Biotechnol. 2013, 33, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Berlec, A.; Malovrh, T.; Zadravec, P.; Steyer, A.; Ravnikar, M.; Sabotič, J.; Poljšak-Prijatelj, M.; Štrukelj, B. Expression of a hepatitis A virus antigen in Lactococcus lactis and Escherichia coli and evaluation of its immunogenicity. Appl. Microbiol. Biotechnol. 2013, 97, 4333–4342. [Google Scholar] [CrossRef] [PubMed]
- Arata, Y.; Hirabayashi, J.; Kasai, K. Sugar binding properties of the two lectin domains of the tandem repeat-type galectin LEC-1 (N32) of Caenorhabditis elegans. Detailed analysis by an improved frontal affinity chromatography method. J. Biol. Chem. 2001, 276, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Jeong, S.; Lee, K.H.; Park, Y.K.; Yu, J. Mimicry of tandem repeat peptides against cell surface carbohydrate. J. Am. Chem. Soc. 2002, 124, 13996–13997. [Google Scholar] [CrossRef] [PubMed]
- Skjoedt, M.O.; Roversi, P.; Hummelshoj, T.; Palarasah, Y.; Rosbjerg, A.; Johnson, S.; Lea, S.M.; Garred, P. Crystal structure and functional characterization of the complement regulator mannose-binding lectin (MBL)/ficolin-associated protein-1 (MAP-1). J. Biol. Chem. 2012, 287, 32913–32921. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.F.; Lescar, J.; Chazalet, V.; Audfray, A.; Gagnon, J.; Alvarez, R.; Breton, C.; Imberty, A.; Mitchell, E.P. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J. Biol. Chem. 2006, 281, 20171–20180. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Wu, J.; Gao, S.; Guo, G.; Zhang, Y.; Liu, J. The recombinant expression and activity detection of MAF-1 fusion protein. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Sharma, K.K. Effect of chaotropic agents on the structure-function of recombinant acylpeptide hydrolase. J. Protein Chem. 2002, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K.; Taylor, M.E. Glycan arrays for functional glycomics. Genome Biol. 2002, 3, 1034.1–1034.4. [Google Scholar] [CrossRef]
- Oliveira, C.; Teixeira, J.A.; Schmitt, F.; Domingues, L. A comparative study of recombinant and native frutalin binding to human prostate tissues. BMC Biotechnol. 2009, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.; Felix, W.; Moreira, R.A.; Teixeira, J.A.; Domingues, L. Expression of frutalin, an alpha-d-galactose-binding jacalin-related lectin, in the yeast Pichia pastoris. Protein Expr. Purif. 2008, 60, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tateno, H.; Kaji, H.; Chiba, Y.; Kubota, T.; Hirabayashi, J.; Narimatsu, H. Engineering of recombinant Wisteria floribunda agglutinin specifically binding to GalNAc β 1,4GlcNAc (LacdiNAc). Glycobiology 2017, 27, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, J.; Yennawar, H.P.; Banerjee, S.; Luo, J.; Farber, G.K. The effect of denaturants on protein structure. Protein Sci. 1997, 6, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Klochkova, T.A.; Kwak, M.S.; Kim, G.H. Proteomic profiles and ultrastructure of regenerating protoplast of Bryopsis plumosa (Chlorophyta). Algae 2016, 31, 379–390. [Google Scholar] [CrossRef]
- Hamid, R.; Masood, A.; Wani, I.H.; Rafiq, S. Lectins: Proteins with diverse applications. J. Appl. Pharm. Sci. 2013, 3, S93–S103. [Google Scholar]
- Arenas, M.I.; Romo, E.; De Gaspar, I.; De Bethencourt, F.R.; Sánchez-Chapado, M.; Fraile, B.; Paniagua, R. A lectin histochemistry comparative study in human normal prostate, benign prostatic hyperplasia, and prostatic carcinoma. Glycoconj. J. 1999, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.R. Refolding solubilized inclusion body proteins. Methods Enzymol. 2009, 463, 259–279. [Google Scholar] [PubMed]



| Substance | Minimum Inhibitory Concentration | |
|---|---|---|
| Native BPL3 | rD2BPL3 | |
| Fetuin | 19.53 § | 4.88 § |
| d-Mannose | NI | NI |
| l-Fucose | NI | NI |
| d-Fructose | NI | NI |
| β-Lactose | NI | NI |
| N-acetyl-d-glucosamine | 125 | 62.5 |
| N-acetyl-d-galactosamine | 62.5 | 31.25 |
| d-Galactose | NI | NI |
| d-Glucose | NI | NI |
| d-Maltose | NI | NI |
| No. | Glycan Structure | RFU (Normalized) | |
|---|---|---|---|
| rD2BPL3 | Native BPL3 | ||
| Monosaccharides | |||
| 3 | α-Man-Sp | 1322 | 1025 |
| 4 | α-Fuc-Sp | 1313 | 205 |
| 6 | β-GlcNAc-Sp | 3164 | 348 |
| Disaccharides | |||
| 9 | Gal-β-1,3-GlcNAc-β-Sp | 1646 | 201 |
| 30 | Gal-β-1,3-GalNAc-α-Sp | 64 | 5075 |
| 41 | GalNAc-β-1,4-GlcNAc-β-Sp2 | 993 | 134 |
| 45 | GlcNAc-β-1,2-Man-α-Sp | 1290 | 173 |
| 87 | d-Cellose-β-Sp1 | 1119 | 225 |
| Gangliosides and Sialylated Oligosaccharides | |||
| 24 | Neu5Ac-α-2,6-Gal-β-1,4-Glc-β-Sp | 991 | 178 |
| 69 | Neu5Ac-α-2,6-Gal-β-1,3-(Neu5Ac-α-2,6)-GalNAc-β-Sp | 421 | 2518 |
| 71 | Neu5Ac-α-2,6-(Neu5Ac-α-2,3)-Gal-β-1,3-GalNAc-β-Sp | 993 | 96 |
| Blood Groups, Lewis Antigens and Fucosylated Oligosaccharides | |||
| 34 | Neu5Ac-α-2,3-Gal-β-1,3-(Fuc-α-1,4)-GlcNAc-β-[Sialyl Lewis A]-Sp | 973 | 138 |
| Globo series, Milk Oligosaccharides and GAGs | |||
| 36 | Gal-α-1,4-Gal-β-1,3-GlcNAc-β-Sp | 721 | 1082 |
| O-Glycan, N-Glycans and α-Gal | |||
| 39 | GlcNAc-β-1,6-(Gal-β-1,3)-GalNAc-α-O-Ser-Sp4 | 77 | 1398 |
| Natural Oligosaccharides | |||
| 77 | Glc-α-1,6-Glc-α-1,4-Glc-β-Sp1 | 941 | 190 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-J.; Han, J.-W.; Kim, G.H.; Han, J.W. Functional Expression and Characterization of the Recombinant N-Acetyl-Glucosamine/N-Acetyl-Galactosamine-Specific Marine Algal Lectin BPL3. Mar. Drugs 2018, 16, 13. https://doi.org/10.3390/md16010013
Hwang H-J, Han J-W, Kim GH, Han JW. Functional Expression and Characterization of the Recombinant N-Acetyl-Glucosamine/N-Acetyl-Galactosamine-Specific Marine Algal Lectin BPL3. Marine Drugs. 2018; 16(1):13. https://doi.org/10.3390/md16010013
Chicago/Turabian StyleHwang, Hyun-Ju, Jin-Woo Han, Gwang Hoon Kim, and Jong Won Han. 2018. "Functional Expression and Characterization of the Recombinant N-Acetyl-Glucosamine/N-Acetyl-Galactosamine-Specific Marine Algal Lectin BPL3" Marine Drugs 16, no. 1: 13. https://doi.org/10.3390/md16010013






