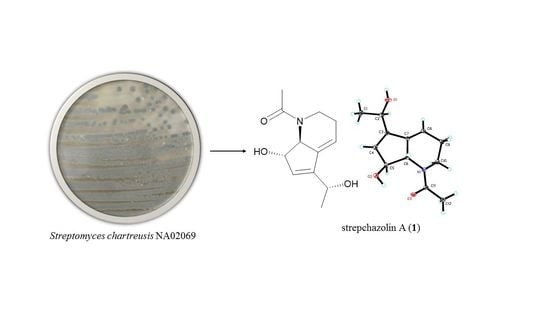
Strepchazolins A and B: Two New Alkaloids from a Marine Streptomyces chartreusis NA02069
Abstract
:1. Introduction
2. Results
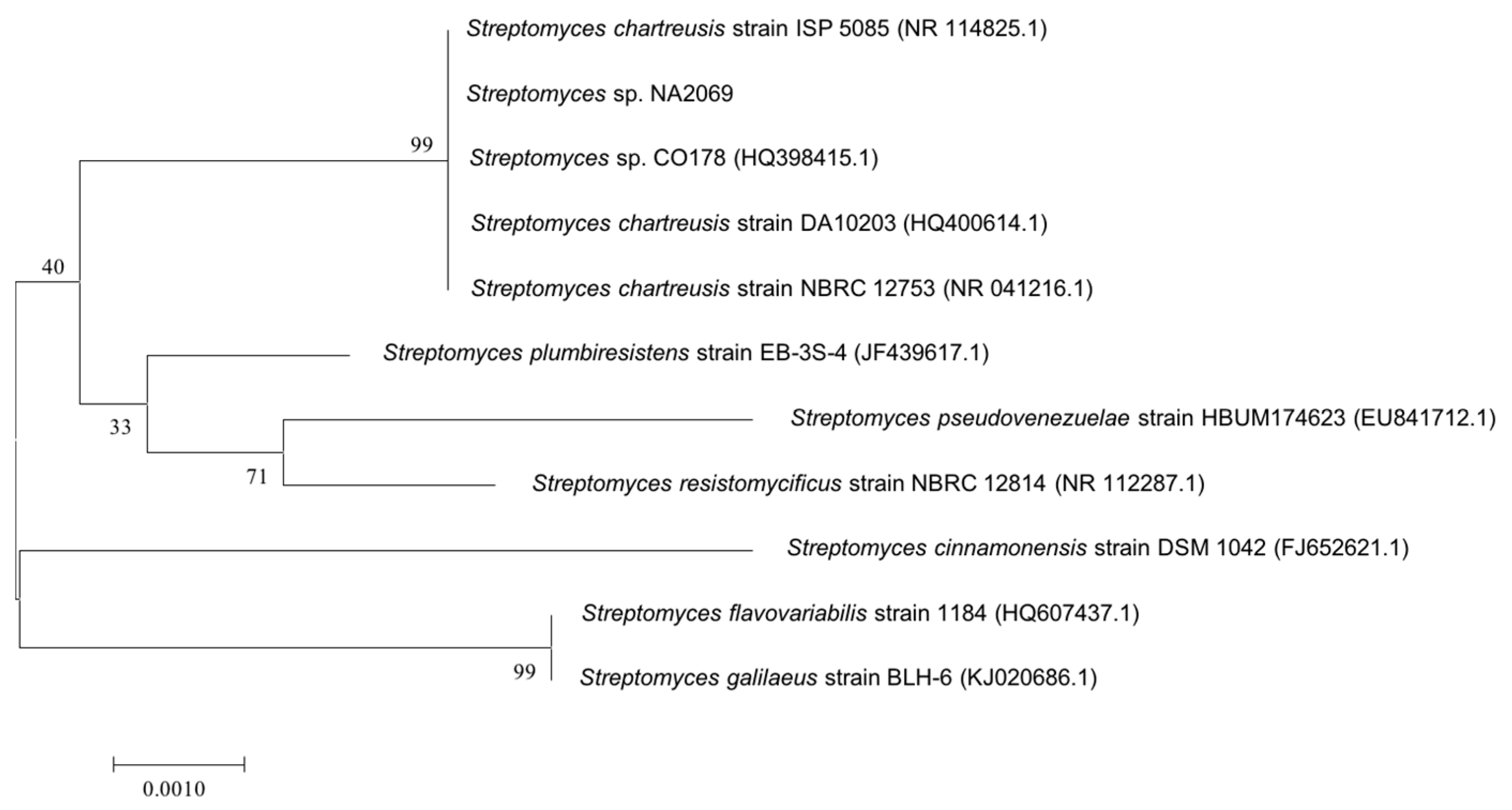
2.1. Taxonomy and Phylogenetic Analysis of the Strain NA02069
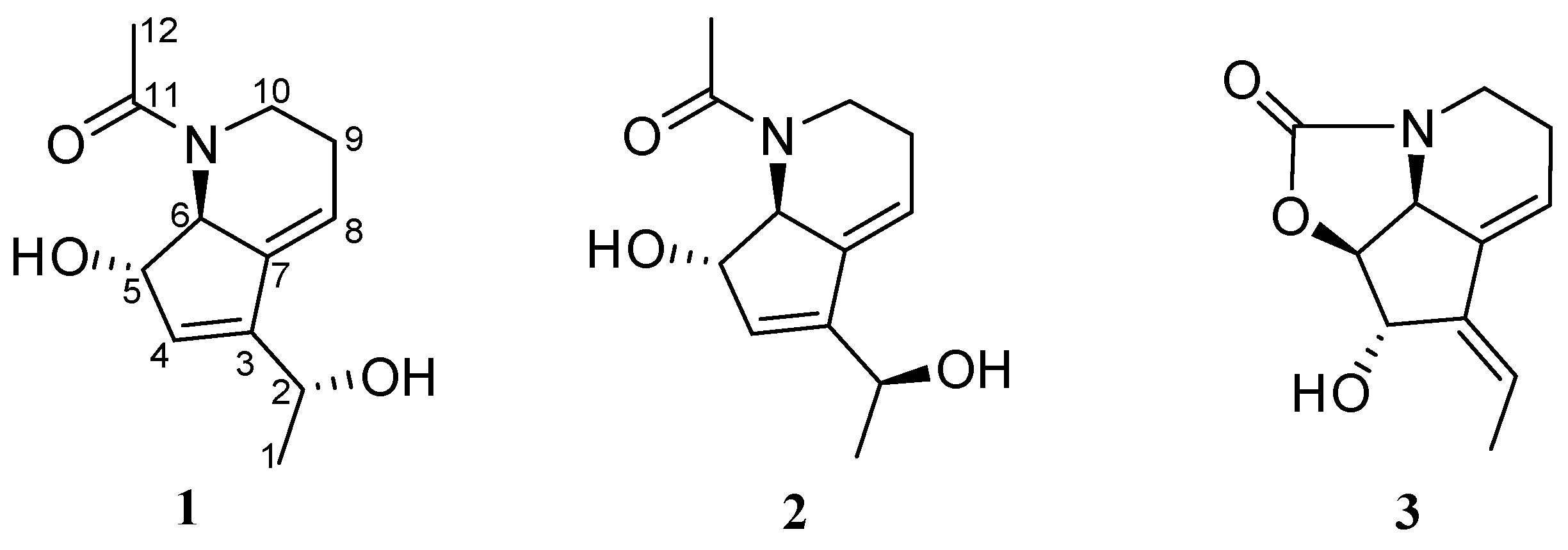
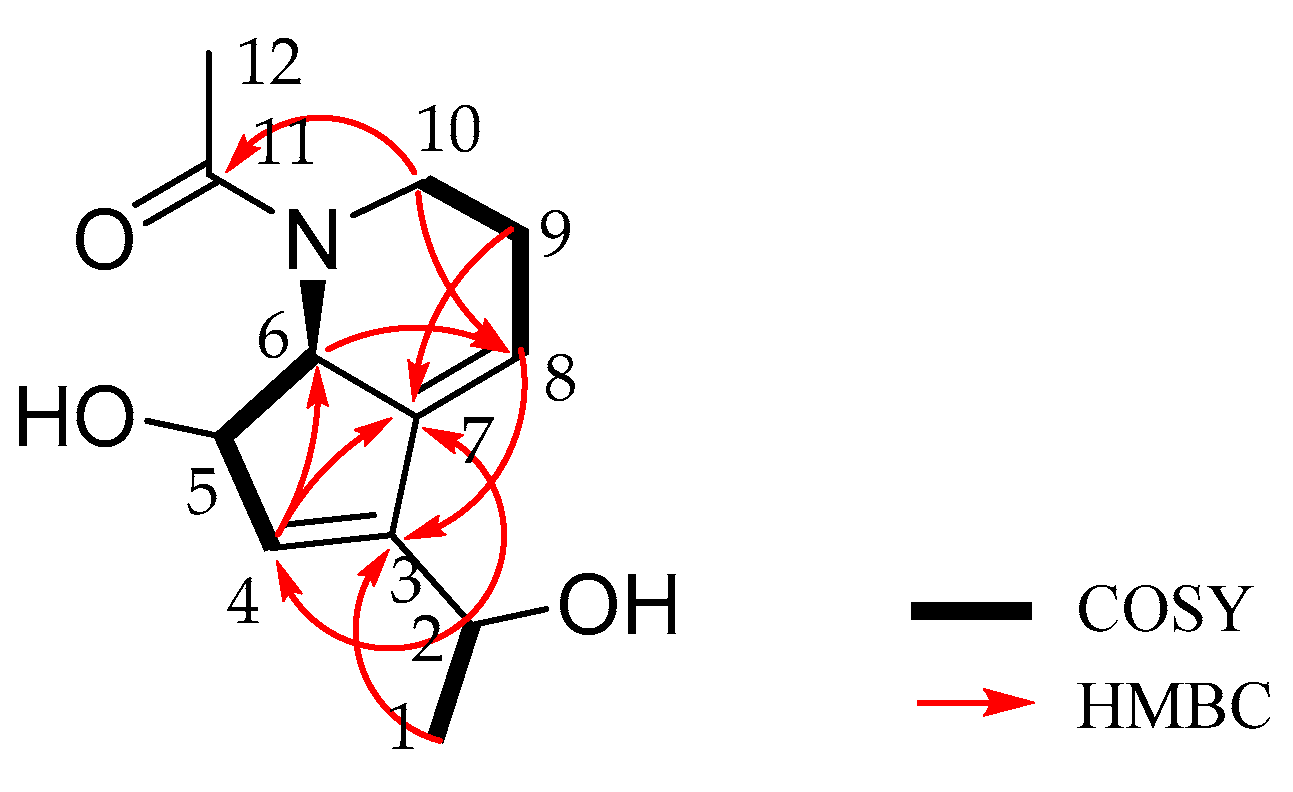
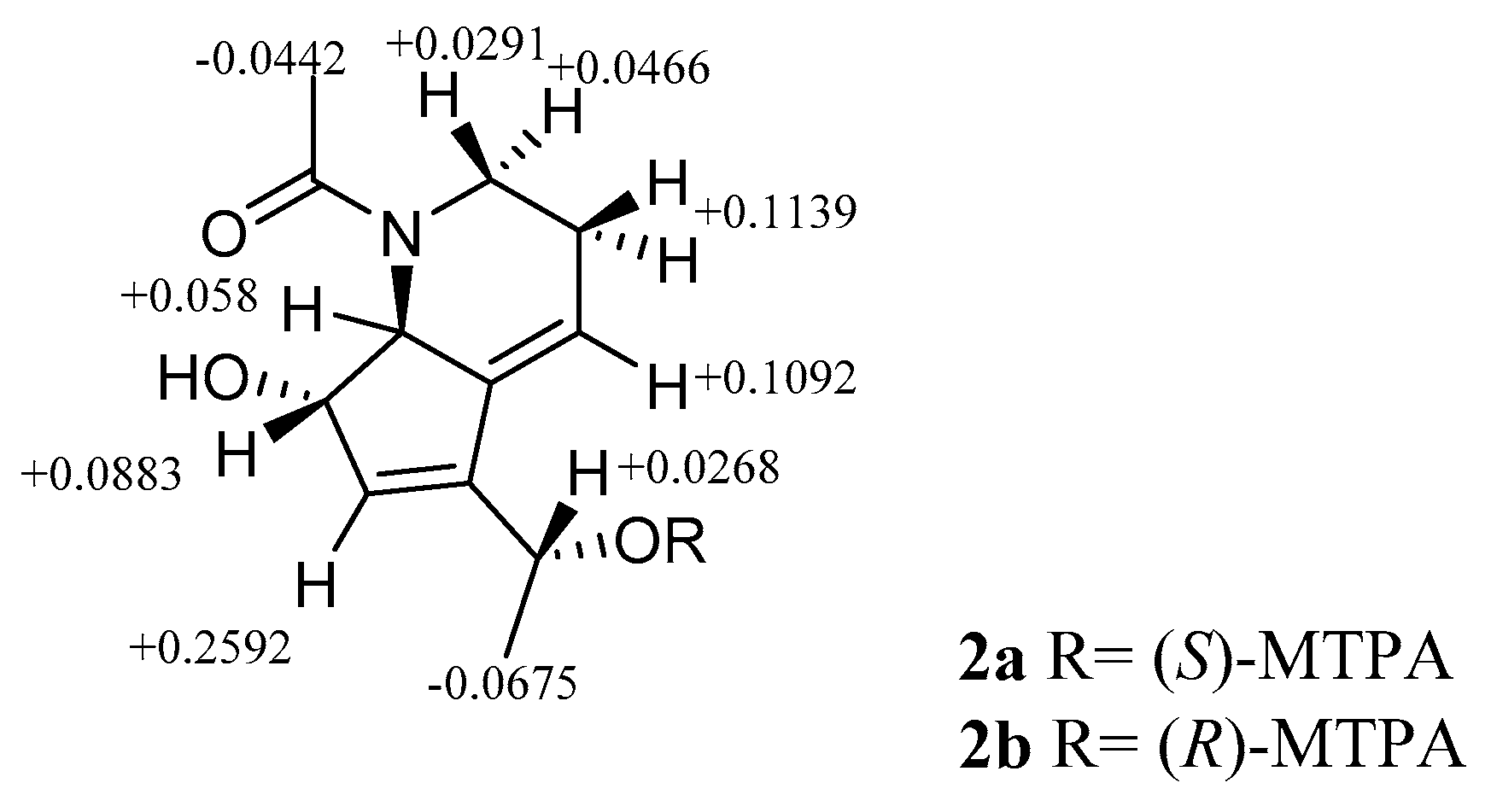
2.2. Isolation and Structure Elucidation
2.3. Bioactivities of Strepchazolins
3. Experimental Section
3.1. General Methods
3.2. Strain Isolation and Cultivation
3.3. Isolation of Compounds 1, 2 and 3
3.4. Crystal Data of 1
3.5. Formation of the (S)- and (R)-MTPA Esters of 2
3.6. Antibacterial Assay
3.7. Bioactivity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Renaissance of marine natural product drug discovery and development. J. Mar. Sci. Res. Dev. 2012, 2, e106. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Marinomycins A–D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora”. J. Am. Chem. Soc. 2006, 128, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Keebeom, K.; SoHyoung, L.; SeongHwan, K. Lajollamycins, Nitro group-bearing spiro-β-lactone-γ-lactams obtained from a marine-derived Streptomyces sp. J. Nat. Prod. 2014, 77, 2099–2104. [Google Scholar]
- McGlinchey, R.P.; Nett, M.; Eustaquio, A.S.; Asolkar, R.N.; Fenical, W.; Moore, B.S. Engineered biosynthesis of antiprotealide and other unnatural salinosporamide proteasome inhibitors. J. Am. Chem. Soc. 2008, 130, 7822–7823. [Google Scholar] [CrossRef] [PubMed]
- Mal, D.; Patra, A.; Roy, H. Convergent and rapid assembly of benzonaphthopyranone cores of chartreusin, chrymutasins and hayumicins. Cheminform 2005, 36, 7895–7898. [Google Scholar] [CrossRef]
- Wu, Q.; Gou, L.; Lin, S. Characterization of the N-methyltransferase CalM involved in calcimycin biosynthesis by Streptomyces chartreusis NRRL 3882. Biochimie 2013, 95, 1487. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.B.; Wang, X.Y.; Wang, T.T. Streptomyces graminifolii sp. nov. isolated from grassland soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 1545. [Google Scholar] [CrossRef] [PubMed]
- Drautz, H.; Zähner, H. Isolation and structure of streptazolin. Helv. Chim. Acta 1981, 64, 1752–1765. [Google Scholar] [CrossRef]
- Felix, F.; Manuel, S.J.; Emilio, Q.; Riguera, R. Determining the absolute stereochemistry of secondary/secondary diols by 1H NMR: Basis and applications. J. Org. Chem. 2005, 70, 3778–3790. [Google Scholar]
- Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C-E, new diterpenes with a fused 6-5-6-6 ring system produced by a marine sponge-derived fungus. Mar. Drugs 2017, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Di Modugno, E.; Erbetti, I.; Ferrari, L.; Galassi, G.; Hammond, S.M.; Xerri, L. In vitro activity of the Tribactam GV104326 against gram-positive, gram-negative, and anaerobic bacteria. Antimicrob. Agents Chemother. 1994, 38, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D., Jr.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Ge, H.M.; Zhu, C.H.; Shi, D.H.; Zhang, L.D.; Xie, D.Q.; Yang, J.; Ng, S.W.; Tan, R.X. Hopeahainol A: An acetylcholinesterase inhibitor from Hopea hainanensis. Chem. Eur. J. 2008, 14, 376–381. [Google Scholar] [CrossRef] [PubMed]

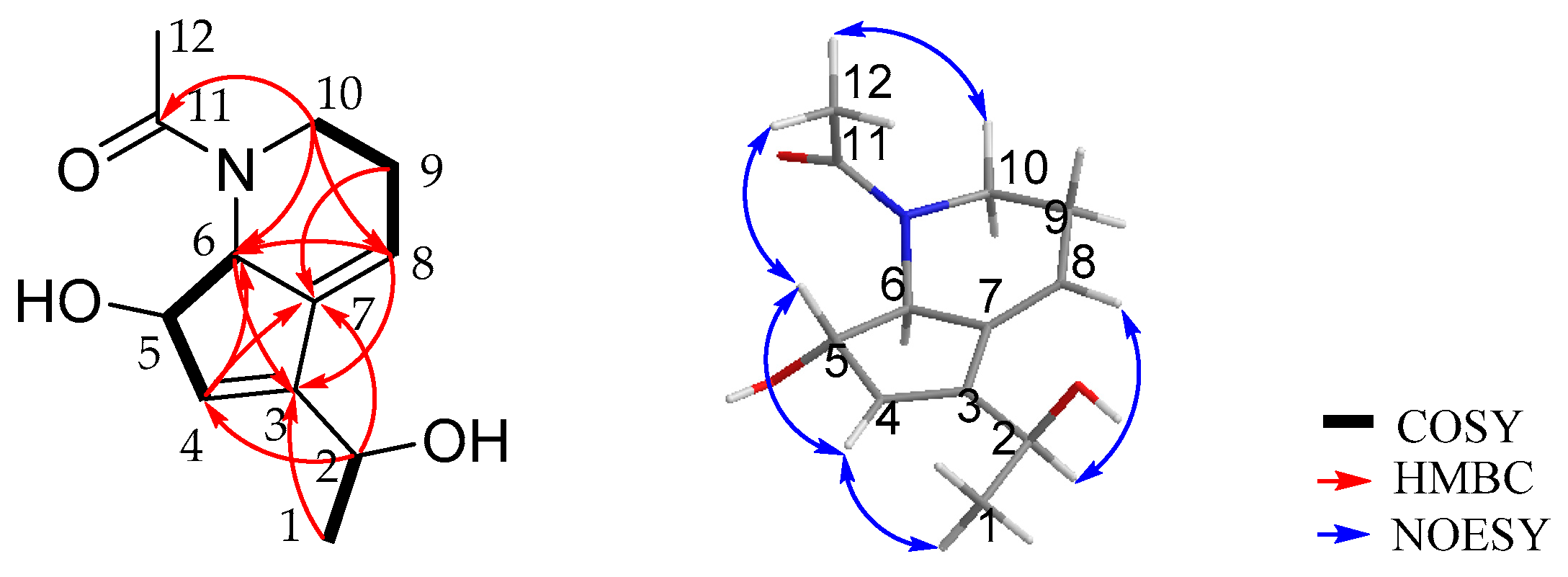
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δc, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 22.6, CH3 | 1.38, d (6.5) | 22.7, CH3 | 1.36, d (6.5) |
| 2 | 64.7, CH | 4.51, q (6.5) | 64.8, CH | 4.55, q (6.5) |
| 3 | 148.5, C | 149.2, C | ||
| 4 | 130.3, CH | 5.85, brs | 129.0, CH | 5.86, brs |
| 5 | 81.0, CH | 4.56, m | 81.0, CH | 4.56, m |
| 6 | 68.4, CH | 4.31, brs | 68.4, CH | 4.30, brs |
| 7 | 140.7, C | 140.9, C | ||
| 8 | 117.8, CH | 6.05, m | 117.2, CH | 5.96, m |
| 9 | 25.8, CH2 | 2.30, m | 25.8, CH2 | 2.30, m |
| 10 | 46.2, CH2 | 3.16, td (12.2,2.9) | 46.2, CH2 | 3.16, td (12.2,2.9) |
| 3.83, dt (12.2,2.9) | 3.83, dt (12.2,2.9) | |||
| 11 | 175.4, C | 175.4, C | ||
| 12 | 22.8, CH3 | 2.19, s | 22.8, CH3 | 2.20, s |
| 1 | 2 | Rifampicin | |
|---|---|---|---|
| Bacillus subtilis | 64.0 | >128.0 | 1.0 |
| Compound | 1 | 2 | Huperzine A |
|---|---|---|---|
| IC50 (μM) | 50.6 | >100.0 | 4.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-L.; Wang, Y.-S.; Liu, C.-L.; Zeng, Y.-J.; Cheng, P.; Jiao, R.-H.; Bao, S.-X.; Huang, H.-Q.; Tan, R.-X.; Ge, H.-M. Strepchazolins A and B: Two New Alkaloids from a Marine Streptomyces chartreusis NA02069. Mar. Drugs 2017, 15, 244. https://doi.org/10.3390/md15080244
Yang C-L, Wang Y-S, Liu C-L, Zeng Y-J, Cheng P, Jiao R-H, Bao S-X, Huang H-Q, Tan R-X, Ge H-M. Strepchazolins A and B: Two New Alkaloids from a Marine Streptomyces chartreusis NA02069. Marine Drugs. 2017; 15(8):244. https://doi.org/10.3390/md15080244
Chicago/Turabian StyleYang, Cheng-Long, Yi-Shuang Wang, Cheng-Li Liu, Ying-Jie Zeng, Ping Cheng, Rui-Hua Jiao, Shi-Xiang Bao, Hui-Qin Huang, Ren-Xiang Tan, and Hui-Ming Ge. 2017. "Strepchazolins A and B: Two New Alkaloids from a Marine Streptomyces chartreusis NA02069" Marine Drugs 15, no. 8: 244. https://doi.org/10.3390/md15080244