Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology
Abstract
:1. Introduction
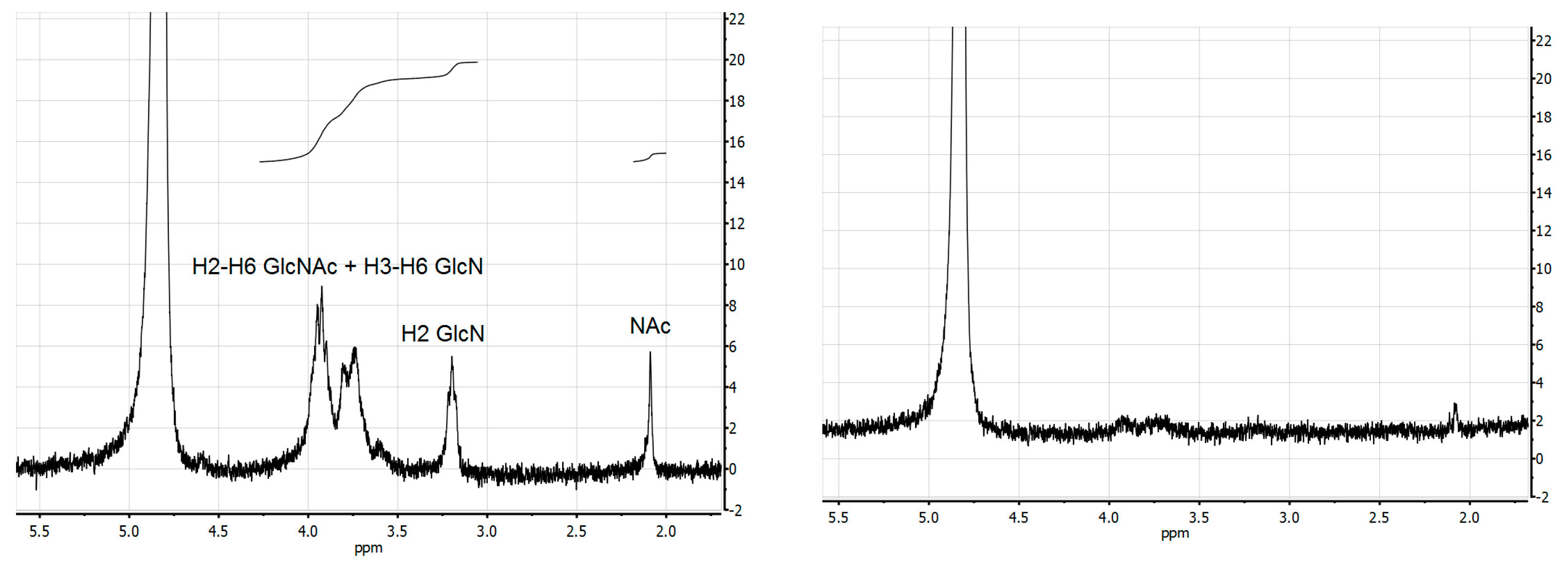
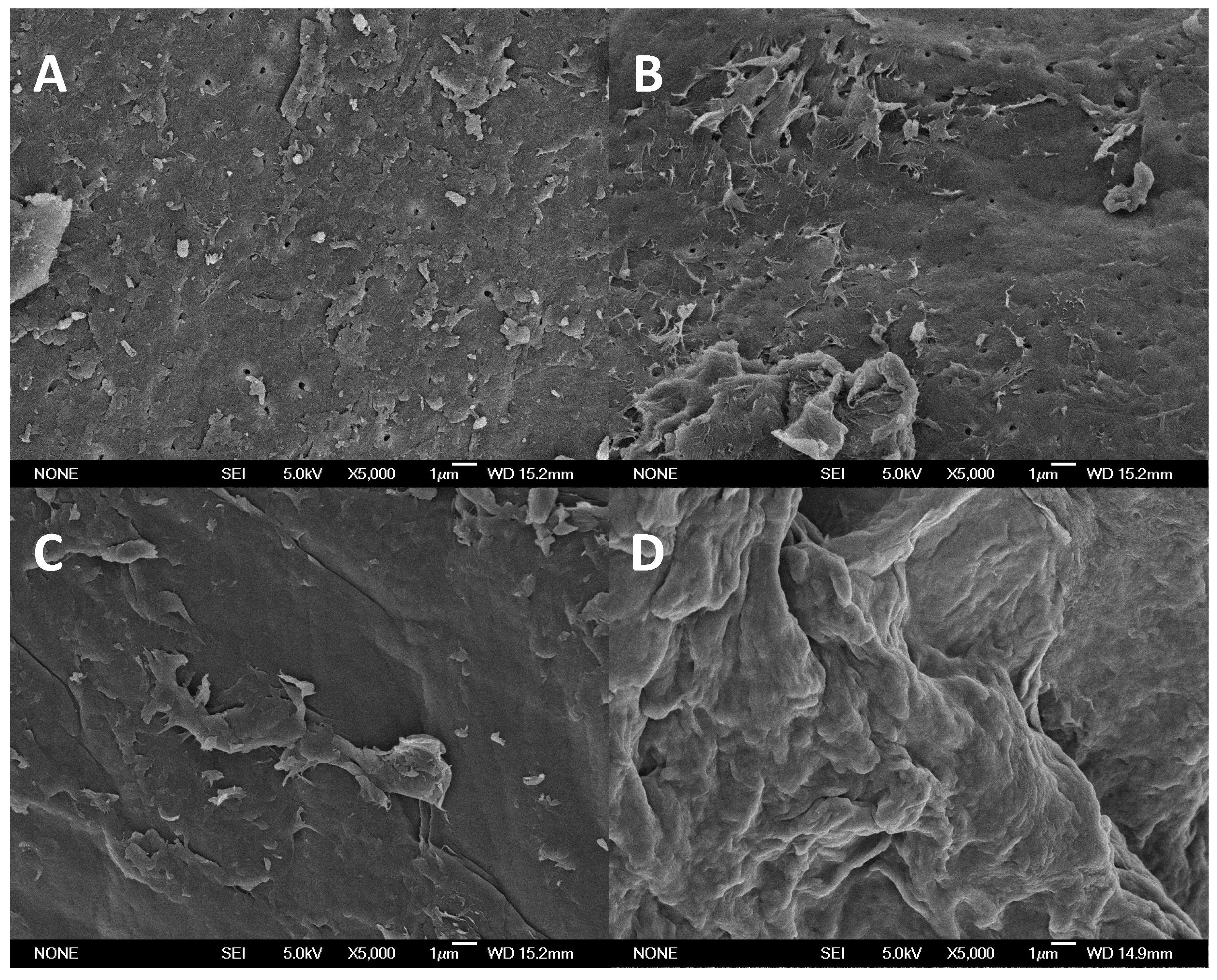
2. Results and Discussion
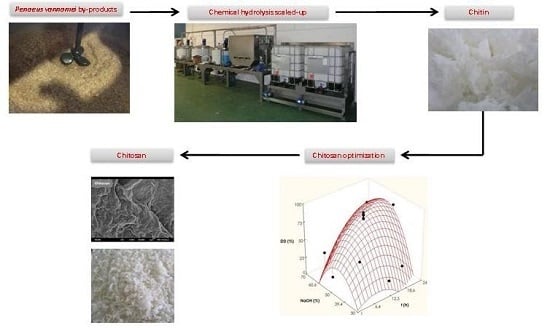
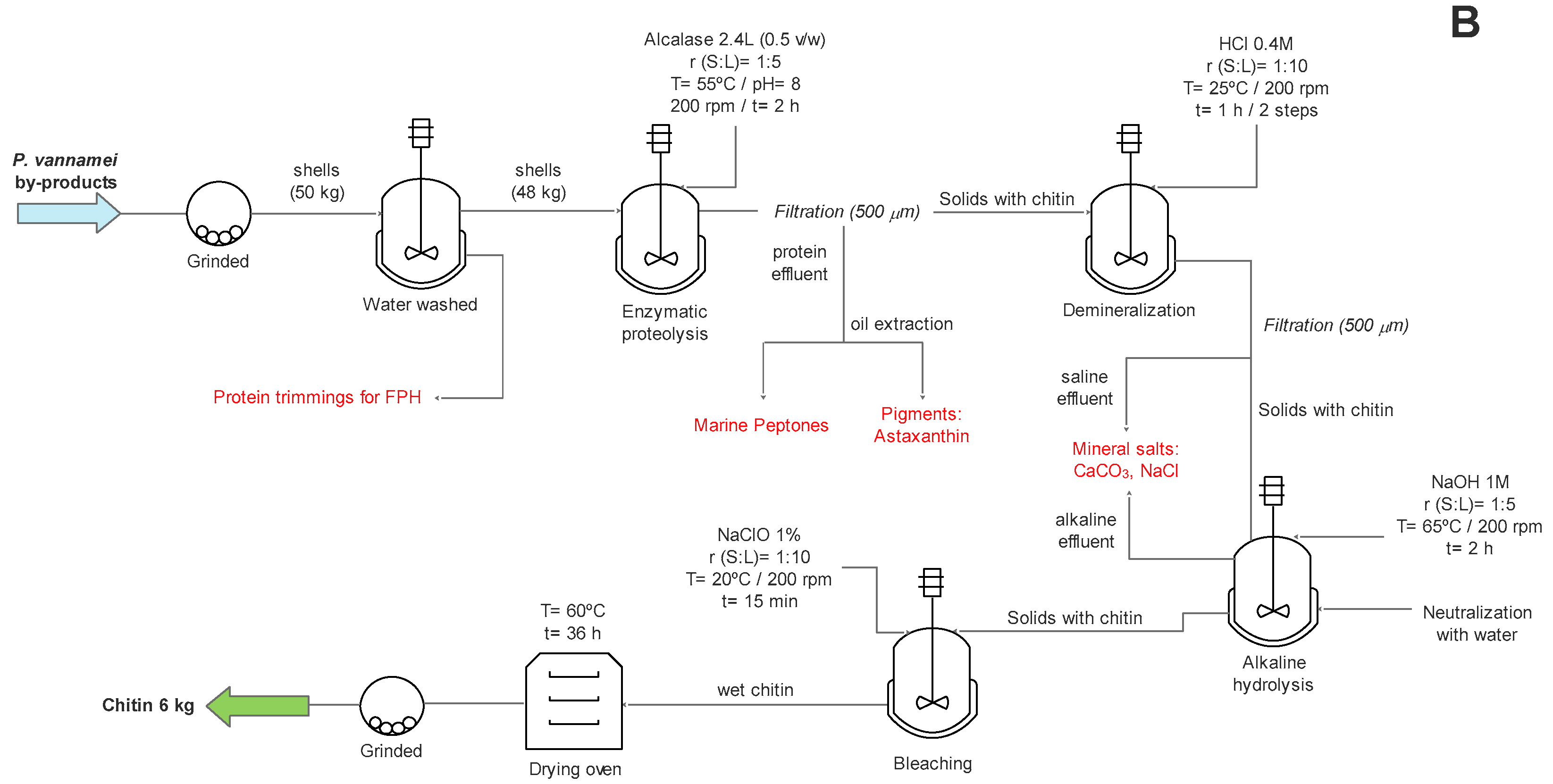
2.1. Pilot Plan Production of Chitin
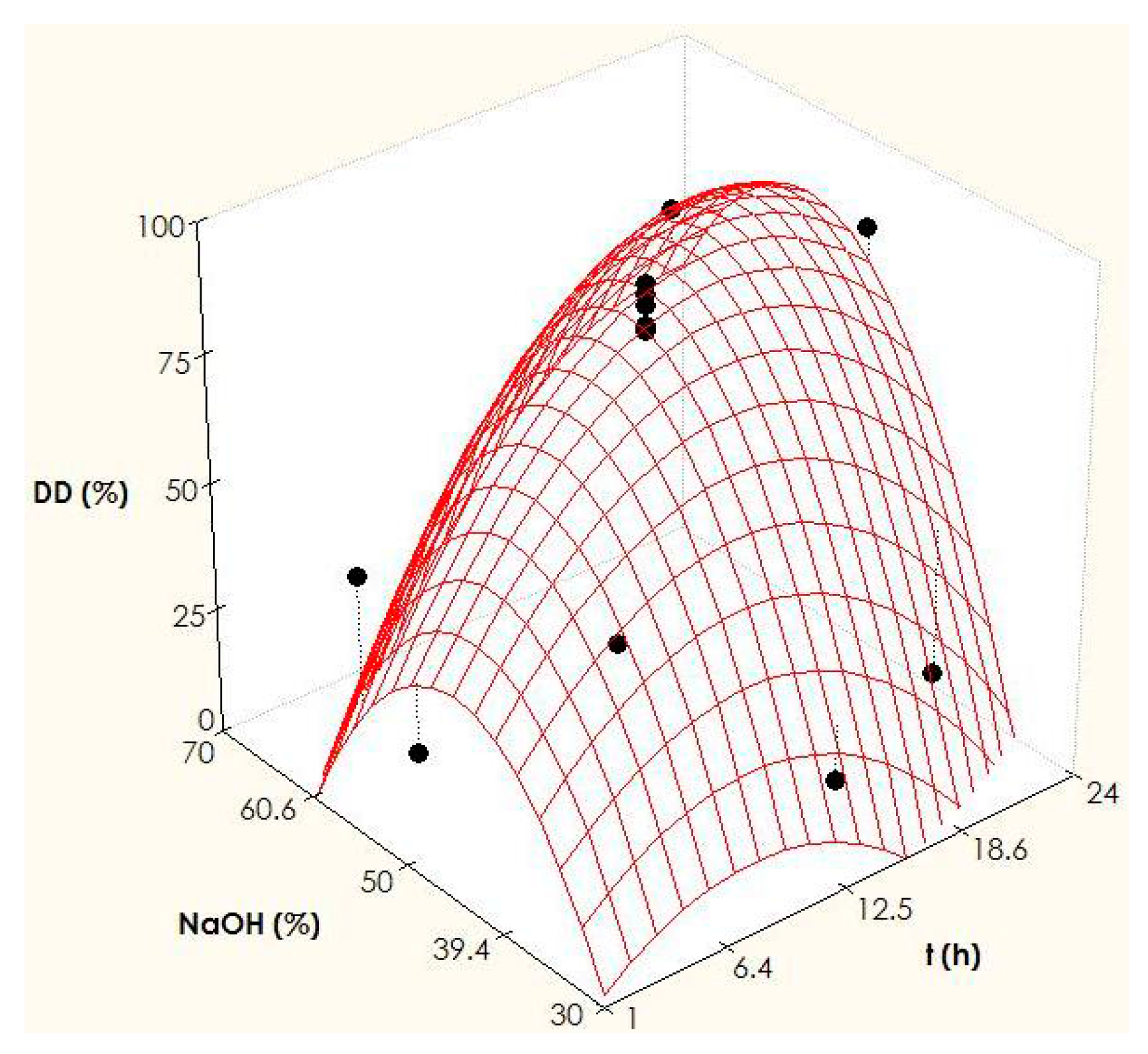
2.2. Optimization of Chitosan Production
3. Experimental Section
3.1. Raw Materials and Extraction of Chitin
3.2. Compositional Characterization of P. vannamei By-Products and Produced Chitin
3.3. Experimental Design for the Production of Chitosan and Statistical Analysis
| Mean Squares Ratios | the Model Is Acceptable When |
| F1 = Model/Total error | |
| F2 = (Model + Lack of fitting)/Model | |
| F3 = Total error/Experimental error | |
| F4 = Lack of fitting/Experimental error |
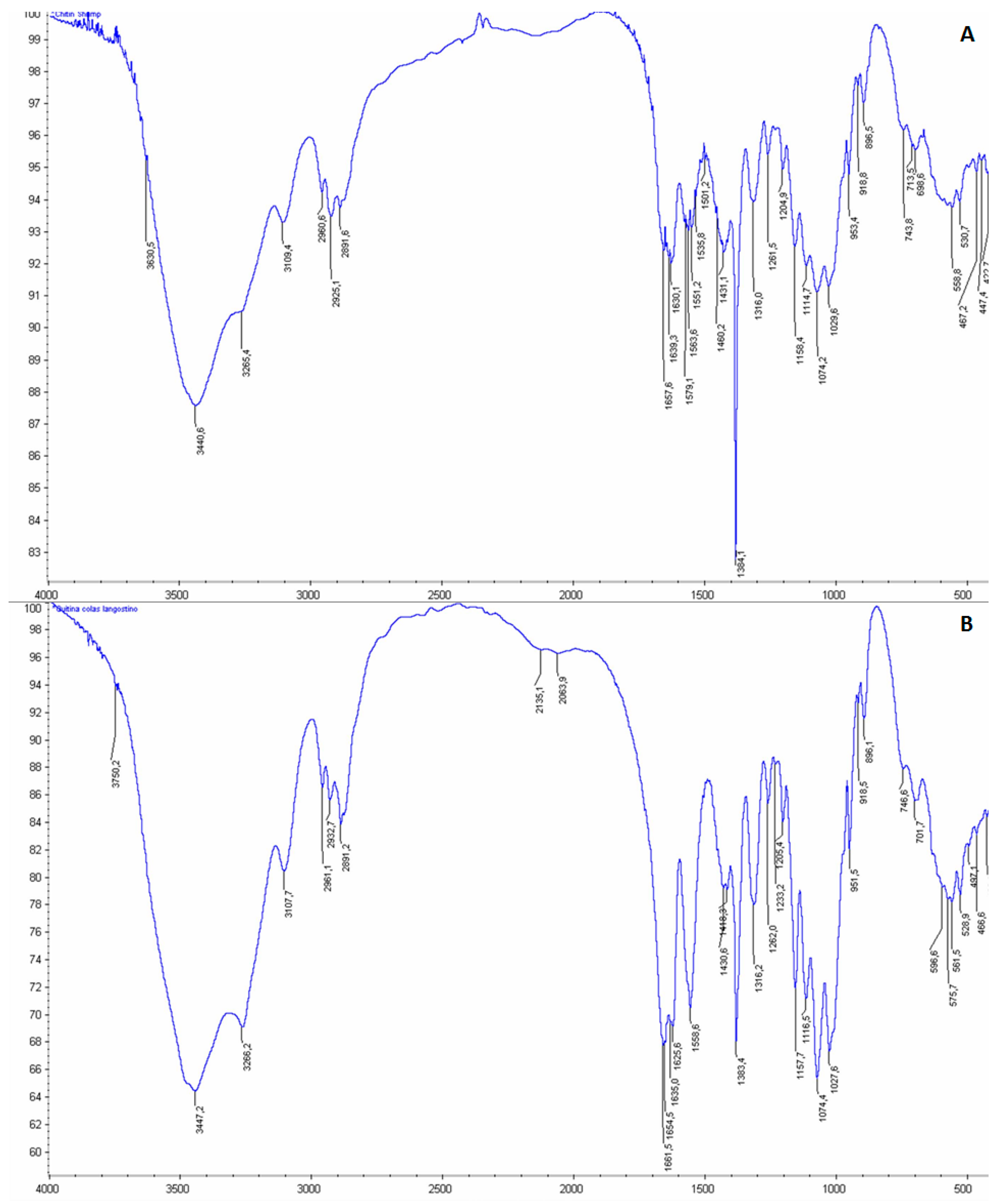
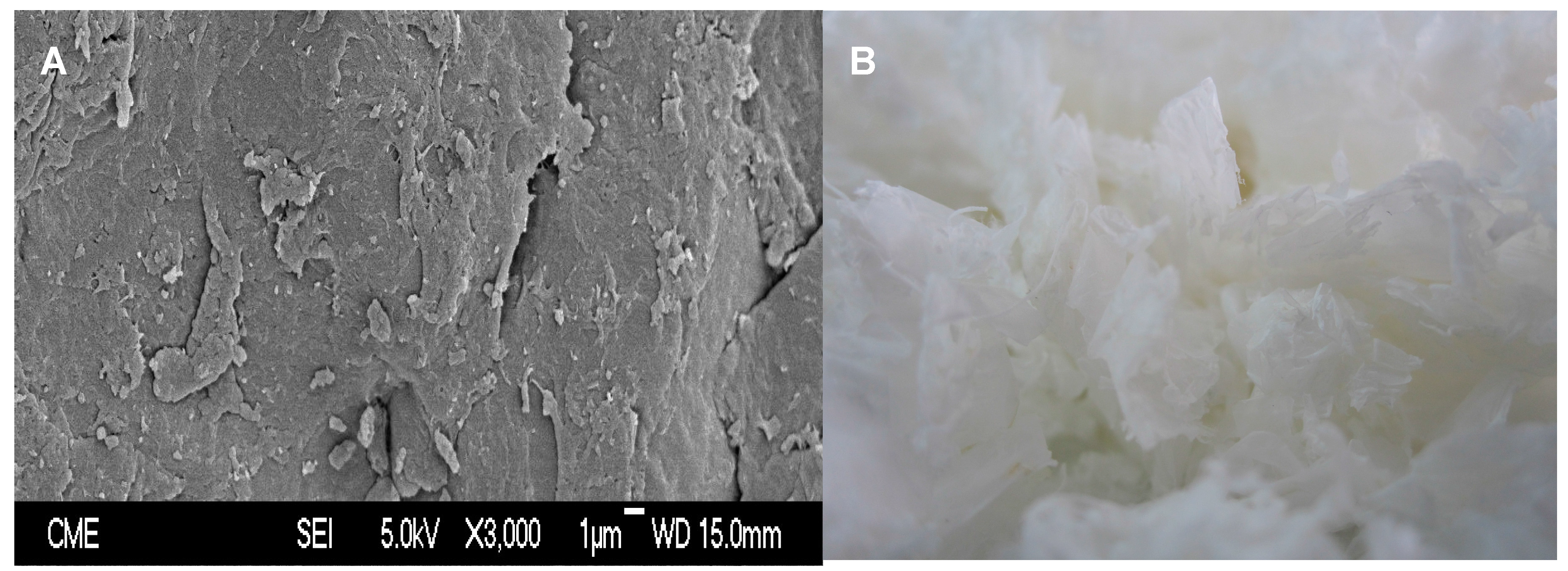
3.4. Chemical and Structural Characterization of Chitin and Chitosan
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture (FAO). The State of World Fisheries and Aquaculture. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; p. 200. ISBN 978-92-5-109185-2. Available online: http://www.fao.org/3/a-i5555e.pdf (accessed on 21 April 2017).
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.; Kadouche, S.; Drouiche, N.; Messaoudi, K.; Messaoudi, B.; Lounici, H. Competitive adsorption of toxic metals on bentonite and use of chitosan as flocculent coagulant to speed up the settling of generated clay suspensions. Chemosphere 2016, 165, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bouhenna, M.; Salah, R.; Bakour, R.; Drouiche, N.; Abdi, N.; Grib, H.; Lounici, H.; Mameri, N. Effects of chitin and its derivatives on human cancer cells lines. Environ. Sci. Pollut. Res. 2015, 22, 15579–15586. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Shahidi, F.; Kim, S.-K. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev. Int. 2000, 16, 159–176. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Benhabiles, M.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Caprioni, R.; Nogueira, M.; Menduiña, A.; Ramos, P.; Pérez-Martín, R.I. Valorisation of effluents obtained from chemical and enzymatic chitin production of Illex argentinus pen by-products as nutrient supplements for various bacterial fermentations. Biochem. Eng. J. 2016, 116, 34–44. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Goosen, M.F.A.; Mameri, N. Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocoll. 2013, 32, 28–34. [Google Scholar] [CrossRef]
- Gildberg, A.; Stenberg, E. A new process for advanced utilisation of shrimp waste. Process Biochem. 2001, 36, 809–812. [Google Scholar] [CrossRef]
- Arancibia, M.Y.; Alemán, A.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Antimicrobial and antioxidant chitosan solutions enriched with active shrimp (Litopenaeus vannamei) waste materials. Food Hydrocoll. 2014, 35, 710–717. [Google Scholar] [CrossRef]
- Kurita, K.; Akao, H.; Yang, J.; Shimojoh, M. Nonnatural branched polysaccharides: Synthesis and properties of chitin and chitosan having disaccharide maltose branches. Biomacromolecules 2003, 4, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem. 2012, 47, 2032–2039. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.d.P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, S.O. Current development of extraction, characterization and evaluation of properties of chitosan and its use in medicine and pharmaceutical industry. Am. J. Polym. Sci. 2016, 6, 86–91. [Google Scholar]
- Duan, S.; Li, L.; Zhuang, Z.; Wu, W.; Hong, S.; Zhou, J. Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydr. Polym. 2012, 89, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Jo, G.; Kuk, J.; Kim, Y.; Oh, K.; Park, R. Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei kctc-3074 and serratia marcescens fs-3. Carbohydr. Polym. 2007, 68, 746–750. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Chaabouni, M.; Rinaudo, M.; Souard, F.; Vanhaverbeke, C.; Jellouli, K.; Nasri, M. Use of a fractional factorial design to study the effects of experimental factors on the chitin deacetylation. Int. J. Biol. Macromol. 2014, 70, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of proteins and minerals removal from shrimp shells to produce highly acetylated chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.-H.; Ye, S.-Q.; Wang, Y. Optimization of the preparation of chitosan from wastes of Penaeus vannamei. Mod. Food Sci. Technol. 2013, 2, 023. [Google Scholar]
- Demim, S.; Drouiche, N.; Aouabed, A.; Benayad, T.; Couderchet, M.; Semsari, S. Study of heavy metal removal from heavy metal mixture using the CCD method. J. Ind. Eng. Chem. 2014, 20, 512–520. [Google Scholar] [CrossRef]
- Demim, S.; Drouiche, N.; Aouabed, A.; Benayad, T.; Dendene-Badachee, O.; Semsari, S. Cadmium and nickel:Assessment of the physiological effects and heavy metal removal using a response surface approach by L. gibba. Ecol. Eng. 2013, 61, 426–435. [Google Scholar] [CrossRef]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3287–3308. [Google Scholar] [CrossRef] [PubMed]
- Gopalakannan, A.; Jasmine, G.I.; Shanmugam, S.; Sugumar, G. Application of proteolytic enzyme, papain for the production of chitin and chitosan from shrimp waste. J. Mar. Biol. Assoc. India 2000, 42, 167–172. [Google Scholar]
- Allwin, S.I.J.; Jeyasanta, K.I.; Patterson, J. Extraction of chitosan from white shrimp (Litopenaeus vannamei) processing waste and examination of its bioactive potentials. Adv. Biol. Res. 2015, 9, 389–396. [Google Scholar]
- Gagne, N.; Simpson, B. Use of proteolytic enzymes to facilitate the recovery of chitin from shrimp wastes. Food Biotechnol. 1993, 7, 253–263. [Google Scholar] [CrossRef]
- Gartner, C.; Peláez, C.A.; López, B.L. Characterization of chitin and chitosan extracted from shrimp shells by two methods. e-Polymers 2010, 10, 748–763. [Google Scholar] [CrossRef]
- Dziril, M.; Grib, H.; Laribi-Habchi, H.; Drouiche, N.; Abdi, N.; Lounici, H.; Pauss, A.; Mameri, N. Chitin oligomers and monomers production by coupling γ radiation and enzymatic hydrolysis. J. Ind. Eng. Chem. 2015, 26, 396–401. [Google Scholar] [CrossRef]
- Xu, J.; McCarthy, S.P.; Gross, R.A.; Kaplan, D.L. Chitosan film acylation and effects on biodegradability. Macromolecules 1996, 29, 3436–3440. [Google Scholar] [CrossRef]
- Kasaai, M. A review of several reported procedures to determinate the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2007, 75, 497–508. [Google Scholar]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2017. [Google Scholar] [CrossRef]
- Commission Regulation (ec) no 629/2008 of 2 July 2008 Amending Regulation (ec) no 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32008R0629 (accessed on 21 April 2017).
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from fish and crustacean by-products hydrolysates stimulate cholecystokinin release in stc-1 cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Goosen, N.; de Wet, L.; Görgens, J. Comparison of hydrolysed proteins from different raw materials in diets for mozambique tilapia Oreochromis mossambicus. Aquac. Int. 2015, 23, 1165–1178. [Google Scholar] [CrossRef]
- Benhabiles, M.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Goosen, M.; Mameri, N. Fish protein hydrolysate production from sardine solid waste by crude pepsin enzymatic hydrolysis in a bioreactor coupled to an ultrafiltration unit. Mater. Sci. Eng. C 2012, 32, 922–928. [Google Scholar] [CrossRef]
- Amado, I.R.; González, M.; Murado, M.A.; Vázquez, J.A. Shrimp wastewater as a source of astaxanthin and bioactive peptides. J. Chem. Technol. Biotechnol. 2016, 91, 793–805. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Murado, M.A.; González, M.P. Recovery of astaxanthin from shrimp cooking wastewater: Optimization of astaxanthin extraction by response surface methodology and kinetic studies. Food Bioprocess Technol. 2015, 8, 371–381. [Google Scholar] [CrossRef]
- García-López, M.; Pérez-Martín, R.I.; Sotelo, C.G. Carotenoid pigments composition of two commonly discarded decapod crustaceans in grand sole and the Galician-Northern Portugal coast fisheries. J. Aquat. Food Prod. Technol. 2016, 25, 114–121. [Google Scholar] [CrossRef]
- Yoon, G.A.; Kim, Y.M.; Chi, G.Y.; Hwang, H.J. Effects of tuna bone and herbal extract on bone metabolism in ovariectomized rats. Nutr. Res. 2005, 25, 1013–1019. [Google Scholar] [CrossRef]
- Herpandi, N.H.; Rosma, A.; Wan Nadiah, W. The tuna fishing industry: A new outlook on fish protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2011, 10, 195–207. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.-D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Raymond, L.; Morin, F.G.; Marchessault, R.H. Degree of deacetylation of chitosan using conductometric titration and solid-state nmr. Carbohydr. Res. 1993, 246, 331–336. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Ottøy, M.H.; Vårum, K.M.; Christensen, B.E.; Anthonsen, M.W.; Smidsrød, O. Preparative and analytical size-exclusion chromatography of chitosans. Carbohydr. Polym. 1996, 31, 253–261. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Vázquez, J.A.; Pandiella, S.S. Optimisation of antioxidants extraction from soybeans fermented by Aspergillus oryzae. Food Chem. 2010, 118, 731–739. [Google Scholar] [CrossRef]
- Weska, R.; Moura, J.M.D.; Batista, L.D.M.; Rizzi, J.; Pinto, L.A.D.A. Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]

| Proximate Composition | Content | |
|---|---|---|
| Raw material | Moisture | 76.0 ± 0.06% dry base |
| Ash | 4.76 ± 0.80% dry base | |
| Protein | 55.0 ± 0.50% dry base | |
| Lipids | 1.6 ± 0.2% dry base | |
| Chitin | Ash | 1.44 ± 0.07% dry base |
| Nitrogen | 6.49 ± 0.03% dry base | |
| Lipids | 0.16 ± 0.03% dry base | |
| C/N | 6.69 | |
| DA | 96.0% dry base | |
| Zn | 21.36 ppm | |
| Na | 883.2 ppm | |
| K | 132.9 ppm | |
| Mn | 80.72 ppm | |
| Mg | 192.3 ppm | |
| P | 1023.3 ppm | |
| Ca | 2892 ppm | |
| Sc | 21.3 ppm | |
| Se | 0.24 ppm | |
| Cu | 2.37 ppm | |
| Fe | 33.24 ppm | |
| Hg | 0.12 ppm | |
| Cd | 0.31 ppm | |
| Pb | 1.13 ppm | |
| t | NaOH | DD (%) | DDp (%) | Coefficients | t-Student | Equation | |
| −1 (4.4 h) | −1 (35.9%) | 54.0 | 44.0 | 87.37 | 50.79 | 87.37 | |
| 1 (20.6 h) | −1 (35.9%) | 19.0 | 28.9 | 12.69 | 9.32 | 12.69 t | |
| −1 (4.4 h) | 1 (64.1%) | 33.0 | 12.6 | 4.54 | 3.33 | 4.54 NaOH | |
| 1 (20.6 h) | 1 (64.1%) | 79.0 | 78.5 | 20.25 | 10.53 | 20.25 t NaOH | |
| −1.41 (1 h) | 0 (50%) | 21.0 | 40.4 | −14.64 | 9.99 | −14.64 t2 | |
| 1.41 (24 h) | 0 (50%) | 85.0 | 76.2 | −31.74 | 21.67 | −31.74 NaOH2 | |
| 0 (12.5 h) | −1.41 (30%) | 20.0 | 17.9 | - | - | - | |
| 0 (12.5 h) | 1.41 (70%) | 18.0 | 30.7 | - | - | - | |
| 0 (12.5 h) | 0 (50%) | 92.0 | 87.4 | - | - | - | |
| 0 (12.5 h) | 0 (50%) | 83.0 | 87.4 | Average value | 58.92 | - | |
| 0 (12.5 h) | 0 (50%) | 84.0 | 87.4 | Expected average value | 87.40 | - | |
| 0 (12.5 h) | 0 (50%) | 88.0 | 87.4 | Var (Ee) | 14.8 | - | |
| 0 (12.5 h) | 0 (50%) | 90.0 | 87.4 | t (α < 0.05; ν = 4) | 2.776 | - | |
| SS | ν | ν | QM | Mean Square Ratios | F-Fisher tests | ||
| Model (M) | 10,822.8 | - | 5 | 2164.6 | QMM/QME = 11.73 | ||
| Error (E) | 1292.2 | - | 7 | 184.6 | QM(M+LF)/QMM = 0.696 | ||
| Exp. Error (Ee) | 59.2 | 4 | - | 14.8 | QME/QMEe = 12.47 | ||
| Lack of Fit (LF) | 1233.0 | 3 | - | 411.0 | QMLF/QMEe = 27.77 | ||
| Total | 12,114.9 | 12 | - | R2 = 0.893 | - | - | |
| - | - | - | - | - | = 0.817 | - | - |
| Coded Values | Natural Values | |
|---|---|---|
| NaOH (%) | t (h) | |
| −1.41 | 30 | 1 |
| −1 | 35.9 | 4.4 |
| 0 | 50 | 12.5 |
| +1 | 64.1 | 20.6 |
| +1.41 | 70 | 24 |
| Codification: Vc = (Vn − V0)/ΔVn | ||
| Decodification: Vn = V0 + (ΔVn × Vc) | ||
| V0 = natural value in the centre of the domain | ||
| Vn = natural value of the variable to codify | ||
| Vc = codified value of the variable | ||
| ΔVn = increment of Vn for unit of Vc | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.A.; Ramos, P.; Mirón, J.; Valcarcel, J.; Sotelo, C.G.; Pérez-Martín, R.I. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Mar. Drugs 2017, 15, 180. https://doi.org/10.3390/md15060180
Vázquez JA, Ramos P, Mirón J, Valcarcel J, Sotelo CG, Pérez-Martín RI. Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology. Marine Drugs. 2017; 15(6):180. https://doi.org/10.3390/md15060180
Chicago/Turabian StyleVázquez, José A., Patrícia Ramos, Jesús Mirón, Jesus Valcarcel, Carmen G. Sotelo, and Ricardo I. Pérez-Martín. 2017. "Production of Chitin from Penaeus vannamei By-Products to Pilot Plant Scale Using a Combination of Enzymatic and Chemical Processes and Subsequent Optimization of the Chemical Production of Chitosan by Response Surface Methodology" Marine Drugs 15, no. 6: 180. https://doi.org/10.3390/md15060180