The Understanding of the Metazoan Skeletal System, Based on the Initial Discoveries with Siliceous and Calcareous Sponges
Abstract
:1. Introduction: Evolution of the Metazoan Skeleton
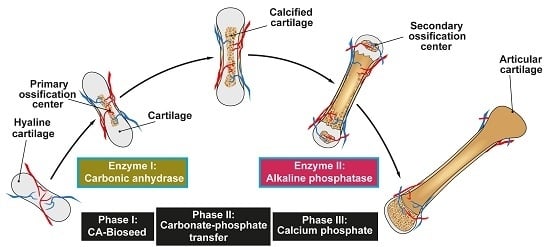
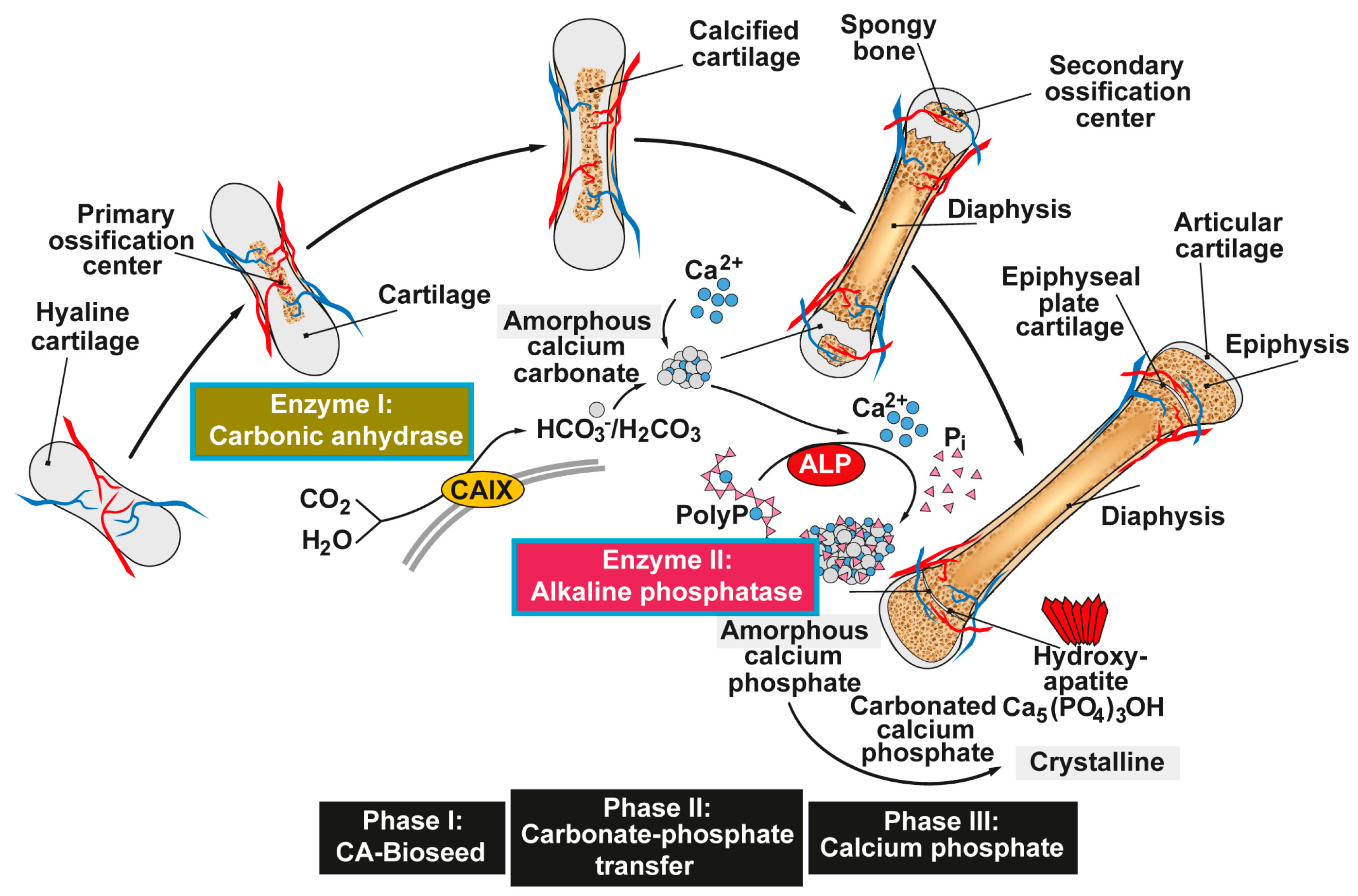
2. Composition of Vertebrate Bone: Ca-Carbonate and Ca-Phosphate Deposits
3. Ca-Carbonate Bio-Seeds Initiate Ca-Phosphate Deposits: Carbonic Anhydrase
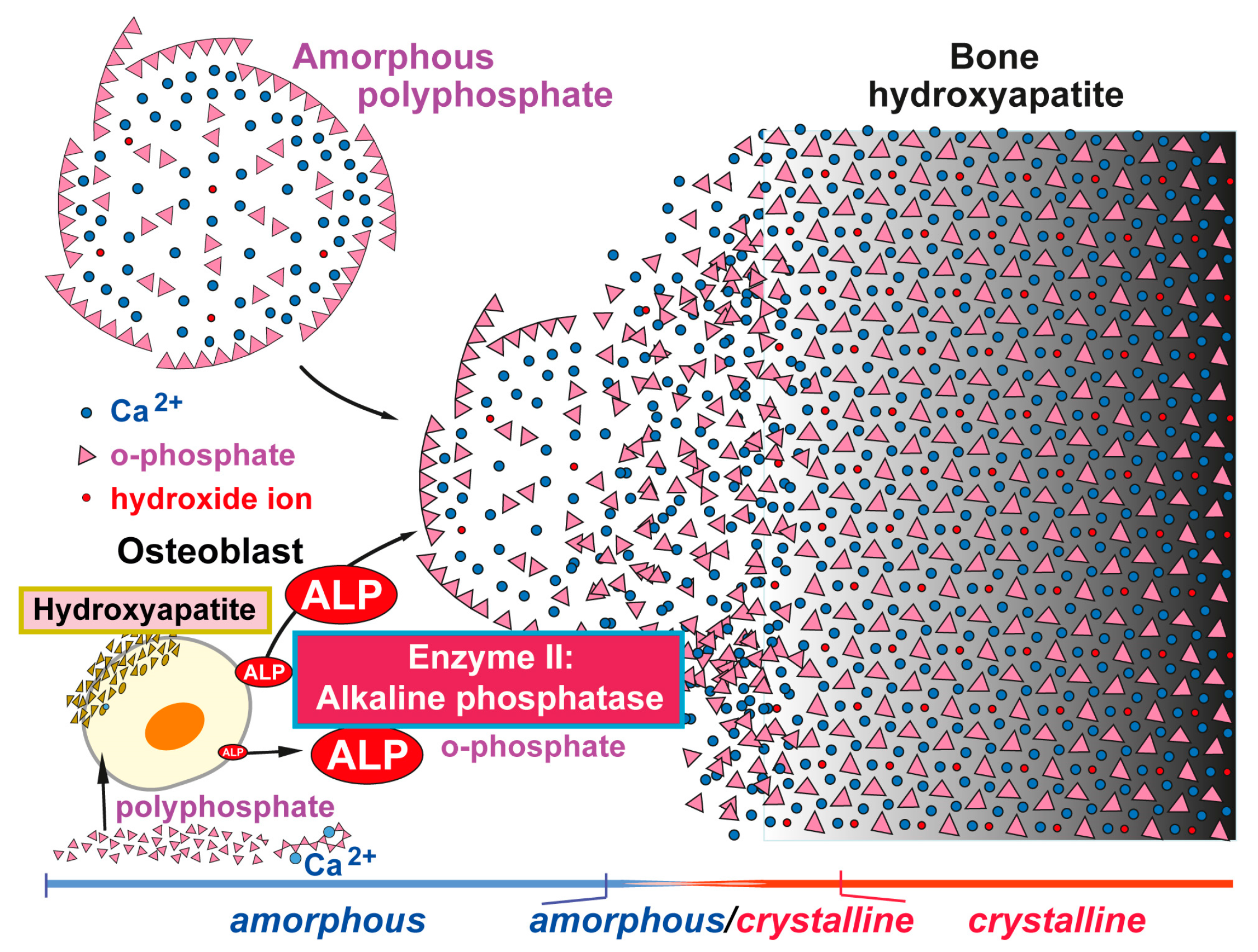
4. Transformation of Ca-Carbonate to Ca-Phosphate Deposits: Non-Enzymatic Step
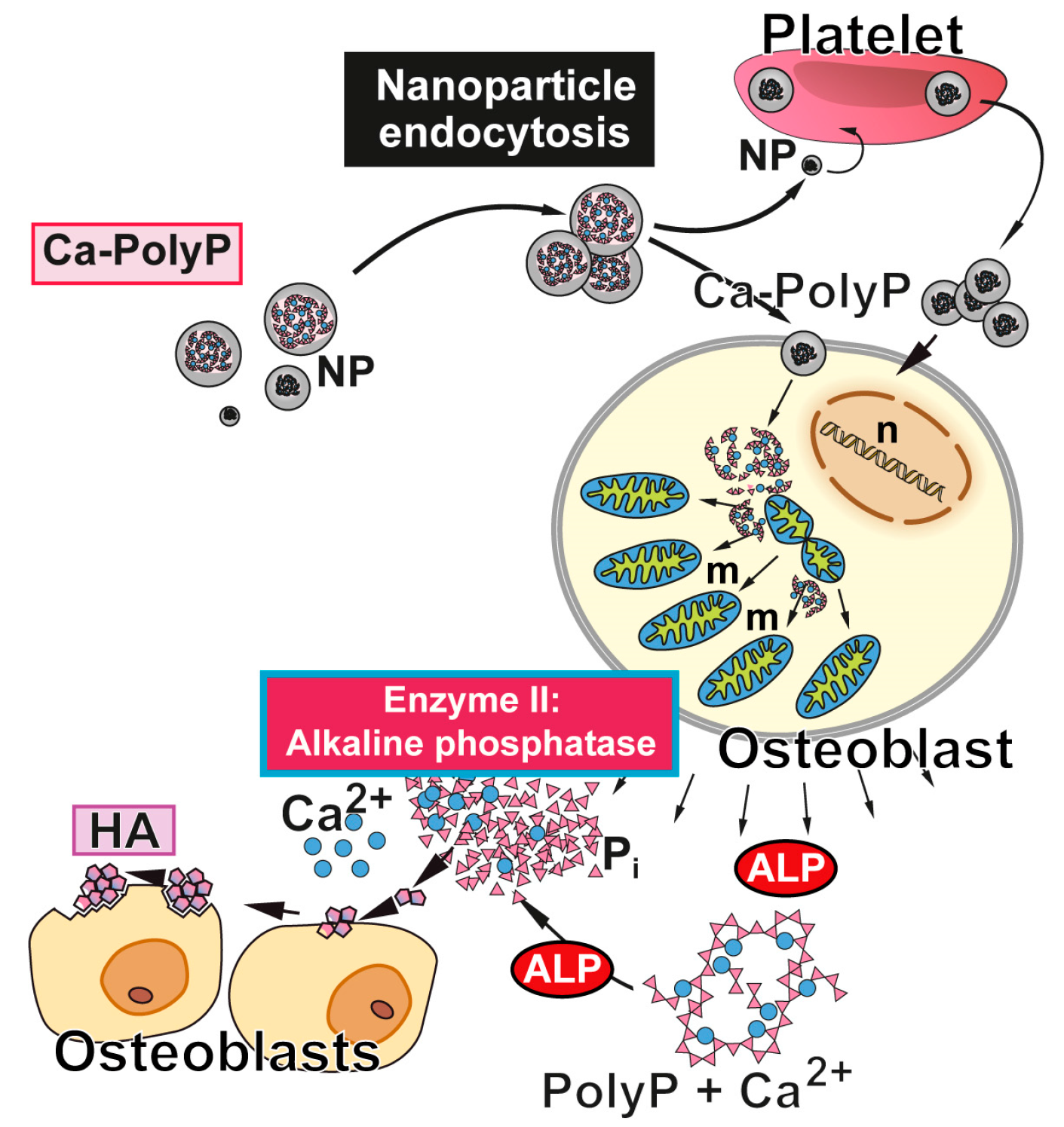
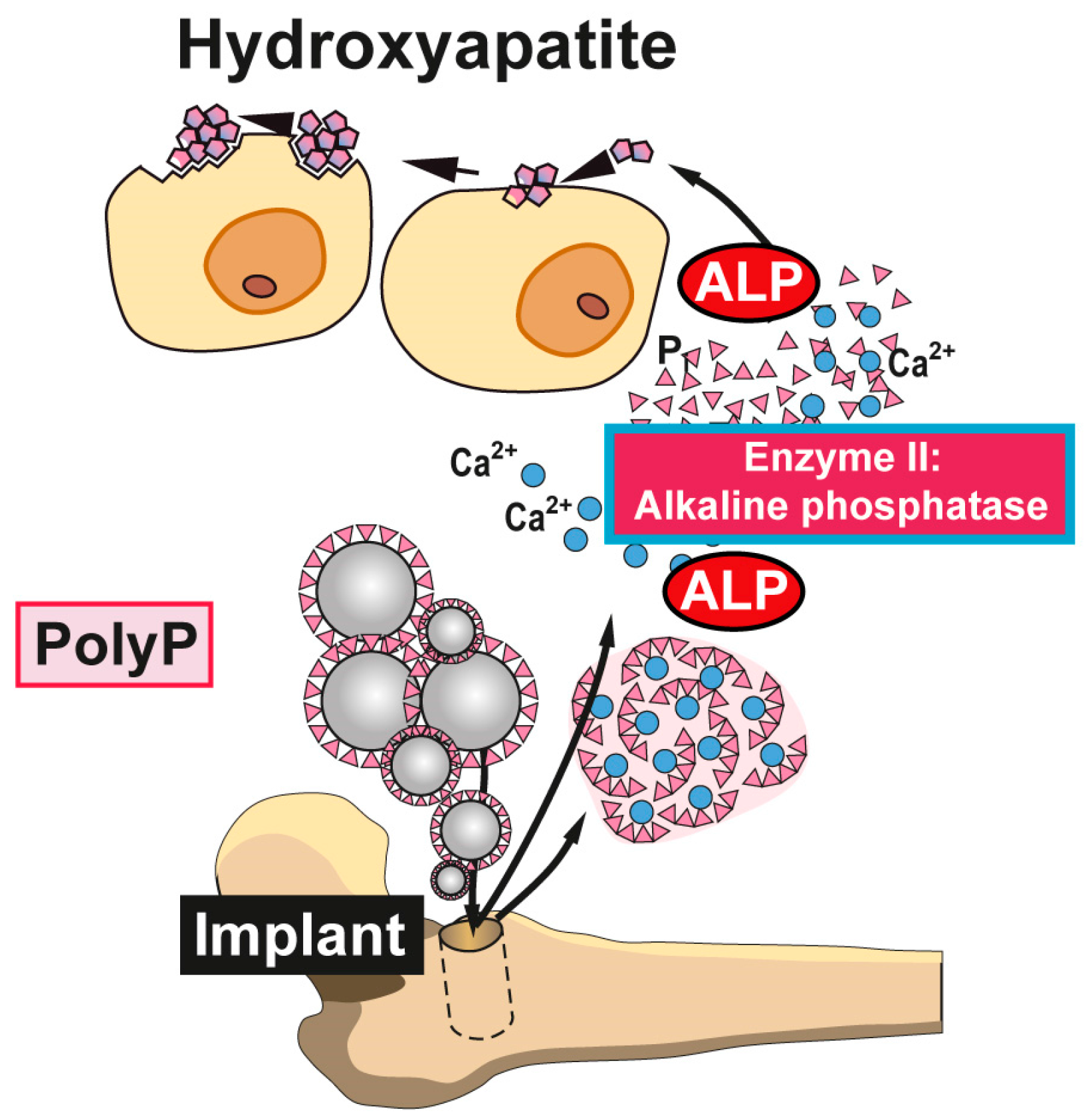
5. Origin of the Phosphate in Bone Mineral: Inorganic Polyphosphate
6. Reaction Chain from Ca-Carbonate Bio-Seeds to Ca-Phosphate-Based Hydroxyapatite
7. Contribution of the Knowledge on Sponge Skeleton for Biomedicine
Acknowledgments
Conflicts of Interest
References
- Müller, W.E.G. How was the metazoan threshold crossed? The hypothetical Urmetazoa. Comp. Biochem. Physiol. A 2001, 129, 433–460. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wiens, M.; Adell, T.; Gamulin, V.; Schröder, H.C.; Müller, I.M. Bauplan of Urmetazoa: Basis for genetic complexity of Metazoa. Int. Rev. Cytol. 2004, 235, 53–92. [Google Scholar] [PubMed]
- Simion, P.; Philippe, H.; Baurain, D.; Jager, M.; Richter, D.J.; Di Franco, A.; Roure, B.; Satoh, N.; Quéinnec, É.; Ereskovsky, A.; et al. A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals. Curr. Biol. 2017, 27, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Jékely, G.; Paps, J.; Nielsen, C. The phylogenetic position of ctenophores and the origin(s) of nervous systems. EvoDevo 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.X.; Hittinger, C.T.; Rokas, A. Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nat. Ecol. Evol. 2017, 1, 0126. [Google Scholar] [CrossRef]
- Nosenko, T.; Schreiber, F.; Adamska, M.; Adamski, M.; Eitel, M.; Hammel, J.; Maldonado, M.; Müller, W.E.G.; Nickel, M.; Schierwater, B.; et al. Deep metazoan phylogeny: When different genes tell different stories. Mol. Phylogenet. Evol. 2013, 67, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, W.; Perović, S.; Kruse, M.; Krasko, A.; Batel, R.; Müller, W.E.G. Origin of the integrin-mediated signal transduction: Functional studies with cell cultures from the sponge Suberites domuncula. Eur. J. Biochem. 1999, 260, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Müller, I.M.; Müller, W.E.G. Early evolution of Metazoan serine/threonine- and tyrosine kinases: Identification of selected kinases in marine sponges. Mol. Biol. Evol. 1997, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Leys, S.P.; Müller, I.M.; Müller, W.E.G. Phylogenetic position of the Hexactinellida within the phylum Porifera based on amino acid sequence of the protein kinase C from Rhabdocalyptus dawsoni. J. Mol. Evol. 1998, 46, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Schütze, J.; Custodio, M.R.; Efremova, S.M.; Müller, I.M.; Müller, W.E.G. Evolutionary relationship of Metazoa within the eukaryotes based on molecular data from Porifera. Proc. R. Soc. Lond. B 1999, 266, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Wörheide, G.; Dohrmann, M.; Erpenbeck, D.; Larroux, C.; Maldonado, M.; Voigt, O.; Borchiellini, C.; Lavrov, D.V. Deep phylogeny and evolution of sponges (phylum Porifera). Adv. Mar. Biol. 2012, 61, 1–78. [Google Scholar] [PubMed]
- Schäcke, H.; Schröder, H.C.; Gamulin, V.; Rinkevich, B.; Müller, I.M.; Müller, W.E.G. Molecular cloning of a receptor tyrosine kinase from the marine sponge Geodia cydonium: A new member of the receptor tyrosine kinase class II family in invertebrates. Mol. Membr. Biol. 1994, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z.; Kruse, M.; Müller, I.; Müller, W.E.G. On the origin of adhesion receptors of metazoa: Cloning of the integrin α subunit cDNA from the sponge Geodia cydonium. Mol. Biol. Evol. 1997, 14, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Seack, J.; Pancer, Z.; Müller, I.M.; Müller, W.E.G. Molecular cloning and primary structure of a Rh (Rhesus) antigen-like protein from the marine sponge Geodia cydonium. Immunogenetics 1997, 46, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Blumbach, B.; Diehl-Seifert, B.; Seack, J.; Steffen, R.; Müller, I.M.; Müller, W.E.G. Cloning and expression of new receptors belonging to the immunoglobulin superfamily from the marine sponge Geodia cydonium. Immunogenetics 1999, 49, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G. Molecular phylogeny of Metazoa (animals): Monophyletic origin. Naturwissenschaften 1995, 82, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G. (Ed.) Molecular Evolution: Evidence for Monophyly of Metazoa. In Progress in Molecular and Subcellular Biology; Springer: Berlin, Germany, 1998; Volume 19. [Google Scholar]
- Müller, W.E.G. (Ed.) Molecular Evolution: Towards the Origin of Metazoa. In Progress in Molecular and Subcellular Biology; Springer: Berlin, Germany, 1998; Volume 21. [Google Scholar]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein alpha: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef] [PubMed]
- Krasko, A.; Gamulin, V.; Seack, J.; Steffen, R.; Schröder, H.C.; Müller, W.E.G. Cathepsin, a major protease of the marine sponge Geodia cydonium: Purification of the enzyme and molecular cloning of cDNA. Mol. Mar. Biol. Biotechnol. 1997, 6, 296–307. [Google Scholar] [PubMed]
- Krasko, A.; Lorenz, B.; Batel, R.; Schröder, H.C.; Müller, I.M.; Müller, W.E.G. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur. J. Biochem. 2000, 267, 4878–4887. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schloßmacher, U.; Schröder, H.C.; Müller, W.E.G. Biologically-induced transition of bio-silica sol to mesoscopic gelatinous flocs: A biomimetic approach to a controlled fabrication of bio-silica structures. Soft Matter 2013, 9, 654–664. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wang, X.H.; Manfrin, A.; Yu, S.H.; Grebenjuk, V.A.; Korzhev, M.; Wiens, M.; Schloßmacher, U.; Müller, W.E.G. Silicatein: Acquisition of structure-guiding and structure-forming properties during maturation from the pro-silicatein to the silicatein form. J. Biol. Chem. 2012, 287, 22196–22205. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Schlossmacher, U.; Neufurth, M.; Geurtsen, W.; Korzhev, M.; Wang, X.H. The enzyme carbonic anhydrase as an integral component of biogenic Ca-carbonate formation in sponge spicules. FEBS Open Bio 2013, 3, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schlossmacher, U.; Schröder, H.C.; Lieberwirth, I.; Glasser, G.; Korzhev, M.; Neufurth, M.; Wang, X.H. Enzyme-accelerated and structure-guided crystallization of Ca-carbonate: Role of the carbonic anhydrase in the homologous system. Acta Biomater. 2014, 10, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Nancollas, G.H. How to control the size and morphology of apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22369–22370. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E. Biominerals—Hierarchical nanocomposites: The example of bone. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Teng, P.N.; Jayaraman, T.; Onishi, S.; Li, J.; Bannon, L.; Huang, H.; Close, J.; Sfeir, C. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J. Biol. Chem. 2011, 286, 29462–29469. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Lundgren, T. From serum to the mineral phase. The role of the odontoblast in calcium transport and mineral formation. Int. J. Dev. Biol. 1995, 39, 213–222. [Google Scholar] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Neufurth, M.; Wang, S.; Link, T.; Al-Nawas, B.; Wang, X.H. A new printable and durable N,O-carboxymethyl chitosan-Ca2+-polyphosphate complex with morphogenetic activity. J. Mater. Chem. B 2015, 3, 1722–1730. [Google Scholar] [CrossRef]
- Mann, S.; Parker, S.B.; Ross, M.D.; Skarnulis, A.J.; Williams, R.J. The ultrastructure of the calcium carbonate balance organs of the inner ear: An ultra-high resolution electron microscopy study. Proc. R. Soc. Lond. B Biol. Sci. 1983, 218, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Pisam, M.; Jammet, C.; Laurent, D. First steps of otolith formation of the zebrafish: Role of glycogen? Cell Tissue Res. 2002, 310, 163–168. [Google Scholar] [PubMed]
- Murayama, E.; Takagi, Y.; Ohira, T.; Davis, J.G.; Greene, M.I.; Nagasawa, H. Fish otoliths contains a unique structural protein, otolin-1. Eur. J. Biochem. 2002, 269, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Deans, M.R.; Peterson, J.M.; Wong, G.W. Mammalian otolin: A multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein otoconin-90 and cerebellin-1. PLoS ONE 2010, 5, e12765. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.; Lins, U.; Farina, M.; Kachar, B.; Thalmann, R. Immunogold TEM of otoconin 90 and otolin—Relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo. Hear. Res. 2012, 292, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.D.; Biltz, R.M. Calcium carbonate in medullary bone. Calcif. Tissue Res. 1970, 6, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Azari, F.; Vali, H.; Guerquin-Kern, J.L.; Wu, T.D.; Croisy, A.; Sears, S.K.; Tabrizian, M.; McKee, M.D. Intracellular precipitation of hydroxyapatite mineral and implications for pathologic calcification. J. Struct. Biol. 2008, 162, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Kim, H.M.; Gerstenfeld, L.; Glimcher, M.J. Characterization of the apatite crystals of bone and their maturation in osteoblast cell culture: Comparison with native bone crystals. Connect. Tissue Res. 1996, 35, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of vertebrate skeletal mineralization by polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Betts, F.; Blumenthal, N.C. Properties of nucleating systems. Metab. Bone Dis. Relat. Res. 1978, 1, 179–183. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.S.; Zhou, P.P.; Cao, L.; Yu, L.J. Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase. Colloids Surf. B Biointerfaces 2013, 102, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Schlossmacher, U.; Grebenjuk, V.A.; Ushijima, H.; Wang, X.H. Induction of carbonic anhydrase in SaOS-2 cells, exposed to bicarbonate and consequences for calcium phosphate crystal formation. Biomaterials 2013, 34, 8671–8680. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Tolba, E.; Neufurth, M.; Diehl-Seifert, B.; Wang, X.H. Mineralization of bone-related SaOS-2 cells under physiological hypoxic conditions. FEBS J. 2016, 283, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Mahamid, J.; Politi, Y.; Ma, Y.; Addadi, L. Overview of the amorphous precursor phase strategy in biomineralization. Front. Mater. Sci. China 2009, 3, 104–108. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Neufurth, M.; Huang, J.; Wang, K.; Feng, Q.; Schröder, H.C.; Diehl-Seifert, B.; Muñoz-Espí, R.; Wang, X.H. Non-enzymatic transformation of amorphous CaCO3 into calcium phosphate mineral after exposure to sodium phosphate in vitro: Implications for in vivo hydroxyapatite bone formation. ChemBioChem 2015, 16, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Imsiecke, G.; Münkner, J.; Lorenz, B.; Bachinski, N.; Müller, W.E.G.; Schröder, H.C. Inorganic polyphosphates in the developing freshwater sponge Ephydatia muelleri: Effect of stress by polluted waters. Environ. Toxicol. Chem. 1996, 15, 1329–1334. [Google Scholar] [CrossRef]
- Lorenz, B.; Batel, R.; Bachinski, N.; Müller, W.E.G.; Schröder, H.C. Purification of two exopolyphosphatases from the marine sponge Tethya lyncurium. Biochim. Biophys. Acta 1995, 1245, 17–28. [Google Scholar] [CrossRef]
- Kaandorp, J.A.; Kübler, J.E. The Algorithmic Beauty of Seaweeds, Sponges and Corals; Springer: Berlin, Germany, 2001. [Google Scholar]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, B.; Schröder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Feng, Q.; Schröder, H.C.; Markl, J.S.; Kokkinopoulou, M.; Wang, X.H. Amorphous Ca2+ polyphosphate nanoparticles regulate the ATP level in bone-like SaOS-2 cells. J. Cell. Sci. 2015, 128, 2202–2207. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, X.H. Polyphosphate: A morphogenetically active implant material serving as metabolic fuel for bone regeneration. Macromol. Biosci. 2015, 15, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Polyphosphate as a metabolic fuel in Metazoa: A foundational breakthrough invention for biomedical applications. Biotechnol. J. 2016, 11, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Pallerla, S.R.; Knebel, S.; Polen, T.; Klauth, P.; Hollender, J.; Wendisch, V.F.; Schoberth, S.M. Formation of volutin granules in Corynebacterium glutamicum. FEMS Microbiol. Lett. 2005, 243, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; de Souza, W.; Miranda, K.; Rohloff, P.; Moreno, S.N. Acidocalcisomes—Conserved from bacteria to man. Nat. Rev. Microbiol. 2005, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X.H. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Wang, X.H.; Ackermann, M.; Wang, S.; Tolba, E.; Neufurth, M.; Feng, Q.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate/amorphous calcium carbonate implant material with enhanced bone healing efficacy in a critical-size-defect in rats. Biomed. Mater. 2016, 11, 035005. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Muñoz-Espí, R.; Diehl-Seifert, B.; Wang, X.H. Amorphous polyphosphate-hydroxyapatite: A morphogenetically active substrate for bone-related SaOS-2 cells in vitro. Acta Biomater. 2016, 31, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Tolba, E.; Müller, W.E.G.; El-Hady, B.M.A.; Neufurth, M.; Wurm, F.; Wang, S.; Schröder, H.C.; Wang, X.H. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J. Mater. Chem. B 2016, 4, 376–386. [Google Scholar] [CrossRef]
- Wang, X.H.; Schröder, H.C.; Wang, K.; Kaandorp, J.A.; Müller, W.E.G. Genetic, biological and structural hierarchies during sponge spicule formation: From soft sol-gels to solid 3D silica composite structures. Soft Matter 2012, 8, 9501–9518. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Burghard, Z.; Pisignano, D.; Wang, X.H. Silicateins—A novel paradigm in bioinorganic chemistry: Enzymatic synthesis of inorganic polymeric silica. Chem. Eur. J. 2013, 19, 5790–5804. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Macis, L.; Reitner, J.; Degnan, B.M.; Wörheide, G. Sponge paleogenomics reveals an ancient role for carbonic anhydrase in skeletogenesis. Science 2007, 316, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Enzymatically synthesized inorganic polymers as morphogenetically active bone scaffolds: Application in regenerative medicine. Int. Rev. Cell Mol. Biol. 2014, 313, 27–77. [Google Scholar] [PubMed]
- Wang, X.H.; Schröder, H.C.; Schlossmacher, U.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Müller, W.E.G. Modulation of the initial mineralization process of SaOS-2 cells by carbonic anhydrase activators and polyphosphate. Calcif. Tissue Int. 2014, 94, 495–509. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, W.E.G.; Schröder, H.C.; Wang, X. The Understanding of the Metazoan Skeletal System, Based on the Initial Discoveries with Siliceous and Calcareous Sponges. Mar. Drugs 2017, 15, 172. https://doi.org/10.3390/md15060172
Müller WEG, Schröder HC, Wang X. The Understanding of the Metazoan Skeletal System, Based on the Initial Discoveries with Siliceous and Calcareous Sponges. Marine Drugs. 2017; 15(6):172. https://doi.org/10.3390/md15060172
Chicago/Turabian StyleMüller, Werner E. G., Heinz C. Schröder, and Xiaohong Wang. 2017. "The Understanding of the Metazoan Skeletal System, Based on the Initial Discoveries with Siliceous and Calcareous Sponges" Marine Drugs 15, no. 6: 172. https://doi.org/10.3390/md15060172