Enhancement of Biomass and Lipid Productivities of Water Surface-Floating Microalgae by Chemical Mutagenesis
Abstract
:1. Introduction
2. Results
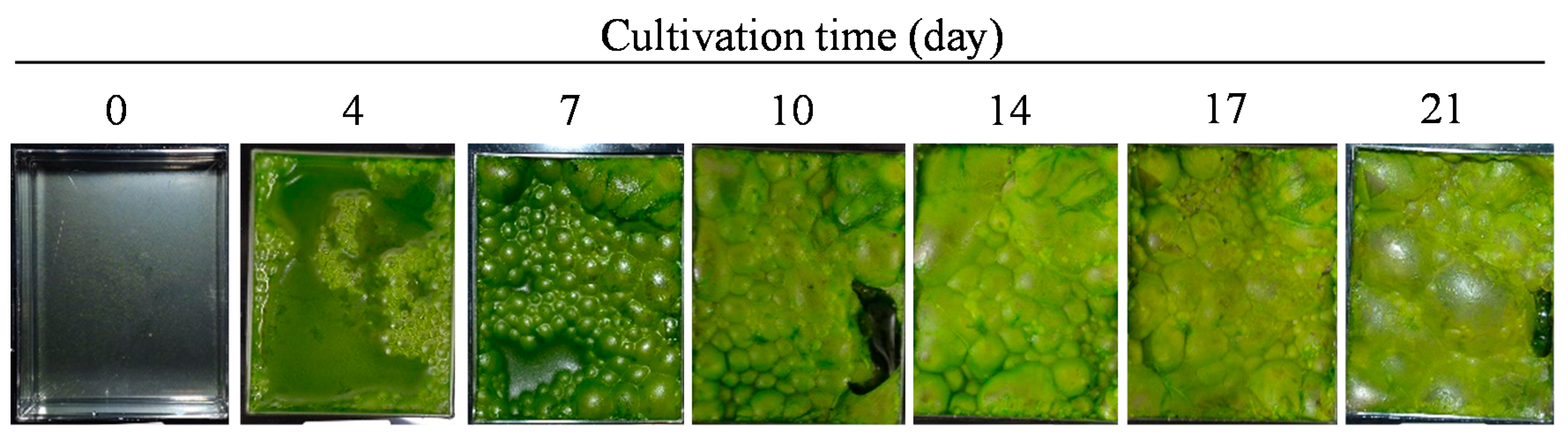
2.1. Characterization of Strain FFG039
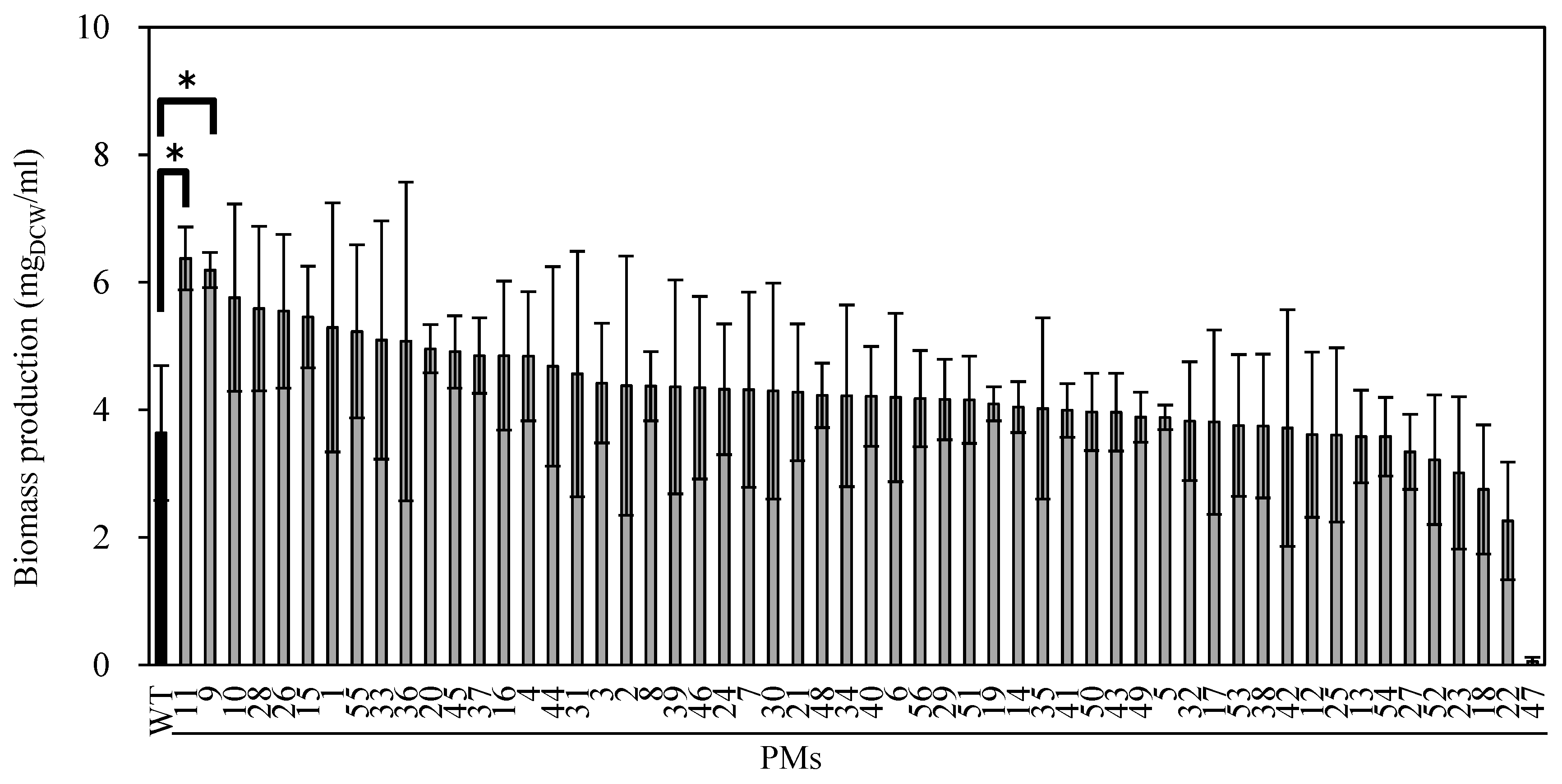
2.2. Mutagenesis of Water Surface-Floating Microalgae
2.3. Characterization of FFG039 PMs
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. Chemical Mutagenesis and Screening
4.3. Quantitative Analysis of Chlorophyll, Biomass, and Lipids
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.; Dastidar, M. Production and characterization of biodiesel from algae. Fuel Process. Technol. 2014, 120, 79–88. [Google Scholar] [CrossRef]
- Gnansounou, E.; Raman, J.K. Life cycle assessment of algae biodiesel and its co-products. Appl. Energy 2016, 161, 300–308. [Google Scholar] [CrossRef]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- De Baerdemaeker, T.; Lemmens, B.; Dotremont, C.; Fret, J.; Roef, L.; Goiris, K.; Diels, L. Benchmark study on algae harvesting with backwashable submerged flat panel membranes. Bioresour. Technol. 2013, 129, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Liu, T. Harvesting of microalgae Scenedesmus sp. using polyvinylidene fluoride microfiltration membrane. Desalination Water Treat. 2012, 45, 177–181. [Google Scholar] [CrossRef]
- Rios, S.D.; Salvado, J.; Farriol, X.; Torras, C. Antifouling microfiltration strategies to harvest microalgae for biofuel. Bioresour. Technol. 2012, 119, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hou, J.; Bowden, D.; Belovich, J.M. Evaluation of an inclined gravity settler for microalgae harvesting. J. Chem. Technol. Biotechnol. 2014, 89, 714–720. [Google Scholar] [CrossRef]
- Che, R.; Huang, L.; Yu, X. Enhanced biomass production, lipid yield and sedimentation efficiency by iron ion. Bioresour. Technol. 2015, 192, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Tateishi, T.; Niwa, Y.; Muto, M.; Yoshino, T.; Kisailus, D.; Tanaka, T. Peptide-mediated microalgae harvesting method for efficient biofuel production. Biotechnol. Biofuels 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zenouzi, A.; Ghobadian, B.; Hejazi, M.; Rahnemoon, P. Harvesting of microalgae Dunaliella salina using electroflocculation. J. Agric. Sci. Technol. 2013, 15, 879–887. [Google Scholar]
- Coward, T.; Lee, J.G.; Caldwell, G.S. Harvesting microalgae by ctab-aided foam flotation increases lipid recovery and improves fatty acid methyl ester characteristics. Biomass Bioenergy 2014, 67, 354–362. [Google Scholar] [CrossRef]
- Muto, M.; Nojima, D.; Yue, L.; Kanehara, H.; Naruse, H.; Ujiro, A.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Potential of water surface-floating microalgae for biodiesel production: Floating-biomass and lipid productivities. J. Biosci. Bioeng. 2016, 123, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.Y.; Obbard, J.P. Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Algal Res. 2012, 1, 17–21. [Google Scholar] [CrossRef]
- Shin, W.-S.; Lee, B.; Jeong, B.-R.; Chang, Y.K.; Kwon, J.-H. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J. Appl. Phycol. 2016, 28, 3193–3202. [Google Scholar] [CrossRef]
- Zhang, Y.; He, M.; Zou, S.; Fei, C.; Yan, Y.; Zheng, S.; Rajper, A.A.; Wang, C. Breeding of high biomass and lipid producing Desmodesmus sp. by ethylmethane sulfonate-induced mutation. Bioresour. Technol. 2016, 207, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Tsuzuki, M.; Ueda, R. Improved productivity by reduction of the content of light-harvesting pigment in Chlamydomonas perigranulata. J. Appl. Phycol. 2001, 13, 95–101. [Google Scholar] [CrossRef]
- Polle, J.E.; Kanakagiri, S.; Jin, E.; Masuda, T.; Melis, A. Truncated chlorophyll antenna size of the photosystems—A practical method to improve microalgal productivity and hydrogen production in mass culture. Int. J. Hydrog. Energy 2002, 27, 1257–1264. [Google Scholar] [CrossRef]
- Manandhar-Shrestha, K.; Hildebrand, M. Development of flow cytometric procedures for the efficient isolation of improved lipid accumulation mutants in a Chlorella sp. Microalga. J. Appl. Phycol. 2013, 25, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Power, M.E. Benthic turfs vs floating mats of algae in river food webs. Oikos 1990, 58, 67–79. [Google Scholar] [CrossRef]
- Menéndez, M.; Martínez, M.; Comín, F.A. A comparative study of the effect of pH and inorganic carbon resources on the photosynthesis of three floating macroalgae species of a mediterranean coastal lagoon. J. Exp. Mar. Biol. Ecol. 2001, 256, 123–136. [Google Scholar] [CrossRef]
- Patidar, S.K.; Mishra, S.K.; Bhattacharya, S.; Ghosh, T.; Paliwal, C.; Goel, S.; Mishra, S. Naturally floating microalgal mat for in situ bioremediation and potential for biofuel production. Algal Res. 2015, 9, 275–282. [Google Scholar] [CrossRef]
- Hillebrand, H. Development and dynamics of floating clusters of filamentous algae. In Periphyton of Freshwater Ecosystems; Springer: Dordrecht, The Netherlands, 1983; pp. 31–39. [Google Scholar]
- Kawasaki, Y.; Nakada, T.; Tomita, M. Taxonomic revision of oil-producing green algae, Chlorococcum oleofaciens (Volvocales, Chlorophyceae), and its relatives. J. Phycol. 2015, 51, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.; van der Zwart, M.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Sorting cells of the microalga Chlorococcum littorale with increased triacylglycerol productivity. Biotechnol. Biofuels 2016, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Schnackenberg, J.; Ikemoto, H.; Miyachi, S. Photosynthesis and hydrogen evolution under stress conditions in a CO2-tolerant marine green alga, Chlorococcum littorale. J. Photochem. Photobiol. B 1996, 34, 59–62. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yang, G.; Han, J.; Thomsen, L.; Pan, K. Breeding 3 elite strains of Nannochloropsis oceanica by nitrosoguanidine mutagenesis and robust screening. Algal Res. 2016, 19, 104–108. [Google Scholar] [CrossRef]
- Vigeolas, H.; Duby, F.; Kaymak, E.; Niessen, G.; Motte, P.; Franck, F.; Remacle, C. Isolation and partial characterization of mutants with elevated lipid content in Chlorella sorokiniana and Scenedesmus obliquus. J. Biotechnol. 2012, 162, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kawaroe, M.; Sudrajat, A.O.; Hwangbo, J.; Augustine, D. Chemical mutagenesis of microalgae Nannochloropsis sp. using ems (ethyl methanesulfonate). Br. J. Appl. Sci. Technol. 2015, 8, 494–505. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Yang, L.; Na, H.; Zhang, P.; Zhang, H.; Wang, Y.; Chen, Y.; Yu, J.; Huo, C.; et al. Isolating lipid droplets from multiple species. Nat. Protoc. 2013, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Beacham, T.A.; Macia, V.M.; Rooks, P.; White, D.A.; Ali, S.T. Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following ems and uv induced mutagenesis. Biotechnol. Rep. 2015, 7, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]

| Strain | Biomass Productivity gDCW/(m2 day) | Lipid Content (%) | Lipid Productivity gDCW/(m2 day) |
|---|---|---|---|
| Botryosphaerella sp. AVFF007 | 5.2 ± 0.6 | 24.0 ± 1.1 | 1.3 ± 0.1 |
| Chlorococcum sp. FFG039 | 3.2 ± 1.0 | 31.1 ± 2.8 | 1.0 ± 0.3 |
| Chlorococcum sp. FFG039-PM9 | 5.2 ± 0.4 | 34.5 ± 2.1 | 1.8 ± 0.1 |
| Chlorococcum sp. FFG039-PM11 | 5.4 ± 0.2 | 34.7 ± 0.4 | 1.9 ± 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojima, D.; Ishizuka, Y.; Muto, M.; Ujiro, A.; Kodama, F.; Yoshino, T.; Maeda, Y.; Matsunaga, T.; Tanaka, T. Enhancement of Biomass and Lipid Productivities of Water Surface-Floating Microalgae by Chemical Mutagenesis. Mar. Drugs 2017, 15, 151. https://doi.org/10.3390/md15060151
Nojima D, Ishizuka Y, Muto M, Ujiro A, Kodama F, Yoshino T, Maeda Y, Matsunaga T, Tanaka T. Enhancement of Biomass and Lipid Productivities of Water Surface-Floating Microalgae by Chemical Mutagenesis. Marine Drugs. 2017; 15(6):151. https://doi.org/10.3390/md15060151
Chicago/Turabian StyleNojima, Daisuke, Yuki Ishizuka, Masaki Muto, Asuka Ujiro, Fumito Kodama, Tomoko Yoshino, Yoshiaki Maeda, Tadashi Matsunaga, and Tsuyoshi Tanaka. 2017. "Enhancement of Biomass and Lipid Productivities of Water Surface-Floating Microalgae by Chemical Mutagenesis" Marine Drugs 15, no. 6: 151. https://doi.org/10.3390/md15060151






