New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade
Abstract
:1. Introduction
2. Linear Peptides
2.1. Linear Depsipeptides
2.2. Other Linear Peptides
3. Cyclic Peptides
3.1. Cyclic Depsipeptides
3.2. Other Cyclic Peptides
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silipo, A.; Molinaro, A.; Molteni, M.; Rossetti, C.; Parrilli, M.; Lanzetta, R. Full Structural Characterization of an Extracellular Polysaccharide Produced by the Freshwater Cyanobacterium Oscillatoria planktothrix FP1. Eur. J. Org. Chem. 2010, 29, 5594–5600. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Mahmood, N.A.; Hyde, E.G. Natural toxins from cyanobacteria (Blue-Green Algae). In Marine Toxins: Origin, Structure and Molecular Pharmacology, 1st ed.; Hall, S., Strichartz, G., Eds.; ACS: Washington, DC, USA, 1990; Volume 52, pp. 87–106. [Google Scholar]
- Martins, J.; Vasconcelos, V. Cyanobactins from cyanobacteria: Current genetic and chemical state of knowledge. Mar. Drugs 2015, 13, 6910–6946. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Watanabe, J.; Horiuchi, T.; Kobayashi, M. Combination effect of TZT-1027 (soblidotin) with other anticancer drugs. Anticancer Res. 2006, 26, 1145–1152. [Google Scholar] [PubMed]
- Deng, C.C.; Pan, B.Q.; O’Connor, O.A. Brentuximab Vedotin. Clin. Cancer Res. 2013, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Reyesac, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Rottacker, E.C.; Kaštovský, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus Okeania gen. nov. (Oscillatoriales, Cyanoprokaryota). J. Phycol. 2013, 49, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Tronholm, A.; Salvador-Reyes, L.A.; Luesch, H.; Paul, V.J. Caldora penicillata gen. nov., comb. nov. (cyanobacteria), a pantropical marine species with biomedical relevance. J. Phycol. 2015, 51, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rein, K.S. New Peptides Isolated from Lyngbya Species: A Review. Mar. Drugs 2010, 8, 1817–1837. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A–C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Zhang, W.; Ding, N.; Lo, J.; Liu, Y.X.; Clare-Salzler, M.J.; Luesch, H.; Li, Y.X. Total synthesis of grassystatin A, a probe for cathepsin E function. Bioorg. Med. Chem. 2012, 20, 4774–4780. [Google Scholar] [CrossRef] [PubMed]
- Mevers, E.; Liu, W.T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Suda, S.; Suenaga, K. Maedamide, a novel chymotrypsin inhibitor from a marine cyanobacterial assemblage of Lyngbya sp. Tetrahedron Lett. 2014, 55, 4126–4128. [Google Scholar] [CrossRef]
- Takayanag, A.; Iwasaki, A.; Suenaga, K. Total synthesis and stereochemical reassignment of maedamide. Tetrahedron Lett. 2015, 56, 4947–4949. [Google Scholar] [CrossRef]
- Simmons, T.L.; Engene, N.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, L.; Gerwick, W.H. Viridamides A and B, lipodepsipeptides with anti-protozoa activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008, 71, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Guo, J.T.; Linington, G.R.; Hunt, N.H.; Payne, R.J. Total synthesis, stereochemical assignment, and antimalarial activity of gallinamide A. Chem. Eur. J. 2011, 17, 13544–13552. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Togna, G.D.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A–C: Discovery and development of a new class of Leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Bayona, L.M.; Castellanos, L.; Puyana, M.; Camargo, P.; Aristizábal, F.; Edwards, C.; Tabudravu, J.N.; Jaspars, M.; Ramos, F.A. Almiramide D, cytotoxic peptide from the marine cyanobacterium Oscillatoria nigroviridis. Bioorg. Med. Chem. 2014, 22, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- McPhail, K.L.; Correa, J.; Linington, R.G.; González, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Ross, C.; Paul, V.J.; Matthew, S.; Luesch, H. Dragonamides C and D, linear lipopeptides from the marine cyanobacterium brown Lyngbya polychroa. J. Nat. Prod. 2008, 71, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Gunasekera, S.P.; Gerwick, W.H.; Paula, V.J. Phylogenetic inferences reveal a large extent of novel biodiversity in chemically rich tropical marine cyanobacteria. Appl. Environ. Microbiol. 2013, 79, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Salvador, L.A.; Schupp, P.J.; Paul, V.J.; Luesch, H. Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam. J. Nat. Prod. 2010, 73, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Ogawa, H.; Nguyen, K.A.; Suenaga, K. Jahanyne, an apoptosis-inducing lipopeptide from the marine cyanobacterium Lyngbya sp. Org. Lett. 2015, 17, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Teruya, T.; Fukazawa, H.; Suenaga, K. Revised structure and structureeactivity relationship of bisebromoamide and structure of norbisebromoamide from the marine cyanobacterium Lyngbya sp. Tetrahedron 2011, 67, 990–994. [Google Scholar] [CrossRef]
- Gao, X.G.; Liu, Y.Q.; Kwong, S.Q.; Xu, Z.S.; Ye, T. Total synthesis and stereochemical reassignment of bisebromoamide. Org. Lett. 2010, 12, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Yu, S.Y.; Jin, M.Z.; Xia, H.G.; Ma, D. Total synthesis and cytotoxicity of bisebromoamide and its analogues. Tetrahedron Lett. 2011, 52, 2124–2127. [Google Scholar] [CrossRef]
- Mevers, E.; Haeckl, J.; Boudreau, P.D.; Byrum, T.; Dorrestein, P.C.; Valeriote, F.A.; Gerwick, W.H. Lipopeptides from the tropical marine cyanobacterium Symploca sp. J. Nat. Prod. 2014, 77, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Pereiraa, A.R.; Kalea, A.J.; Fenleyb, A.T.; Byruma, T.; Debonsia, H.M.; Gilsonb, M.K.; Valerioted, F.A.; Moorea, B.S.; Gerwicka, W.H. The Carmaphycins, new proteasome inhibitors exhibiting an α,β-epoxyketone warhead from a marine cyanobacterium. Chembiochem 2012, 4, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Pereira, A.R.; Cao, Z.Y.; Shuman, C.F.; Engene, N.; Byrum, T.; Matainaho, T.; Murray, T.F.; Mangoni, A.; Gerwick, W.H. The hoiamides, structurally intriguing neurotoxic lipopeptides from Papua New Guinea marine cyanobacteria. J. Nat. Prod. 2010, 73, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.S.; Ye, T. Total synthesis of hoiamide C. Org. Lett. 2011, 13, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Malloy, K.L.; Choi, H.; Fiorilla, C.; Valeriote, F.A.; Matainahoe, T.; Gerwick, W.H. Hoiamide D, a marine cyanobacteria-derived inhibitor of p53/MDM2 interaction. Bioorg. Med. Chem. Lett. 2012, 22, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Mevers, E.; Byrumb, T.; Valerioted, F.A.; Gerwick, W.H. Lyngbyabellins K-N from two palmyra atoll collections of the marine cyanobacterium Moorea bouillonii. Eur. J. Org. Chem. 2012, 27, 5141–5150. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Suda, S.; Suenaga, K. Kurahyne, an acetylene-containing lipopeptide from a marine cyanobacterial assemblage of Lyngbya sp. RSC Adv. 2014, 4, 12840–12843. [Google Scholar] [CrossRef]
- Okamoto, S.; Iwasaki, A.; Ohno, O.; Suenaga, K. Isolation and structure of kurahyne B and total synthesis of the kurahynes. J. Nat. Prod. 2015, 78, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Imperial, L.; Garst, C.; Ratnayake, R.; Dang, L.H.; Paul, V.J.; Luesch, H. Caldoramide, a modified pentapeptide from the marine cyanobacterium Caldora penicillata. J. Nat. Prod. 2016, 7, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.A.; Biggs, J.S.; Paul, V.J.; Luesch, H. Veraguamides A-G, cyclic hexadepsipeptides from a dolastatin16-producing cyanobacterium Symploca cf. hydnoides from Guam. J. Nat. Prod. 2011, 74, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Jia, X.; Zhang, A. Total synthesis of the proposed structure of cyclic hexadepsipeptide veraguamide A. Org. Biomol. Chem. 2012, 10, 7027–7030. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Ross, C.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatin 4, a dolastatin 13 analog with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007, 70, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.M.; Chen, Q.Y.; Luesch, H. Total synthesis of the potent marine-derived elastase inhibitor lyngbyastatin 7 and in vitro biological evaluation in model systems for pulmonary diseases. J. Org. Chem. 2016, 2, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Taori, K.; Paul, V.J.; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Cyclic depsipeptides, grassypeptolides D and E and ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008, 71, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Rocca, J.R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Total Structure Determi nation of Grassypeptolide, a New Marine Cyanobacterial Cytotoxin. Org. Lett. 2008, 10, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Ratnayake, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Grassypeptolides A–C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides. J. Org. Chem. 2010, 75, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.Q.; Wang, Z.; Xing, X.Y.; Maguire, A.R.; Luesch, H.; Zhang, H.; Xu, Z.S.; Ye, T. Total synthesis and biological evaluation of grassypeptolide A. Chem. Eur. J. 2013, 19, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Popplewell, W.L.; Ratnayake, R.; Wilson, J.A.; Beutler, J.A.; Colburn, N.H.; Henrich, C.J.; McMahon, J.B.; McKee, T.C. Grassypeptolides F and G, cyanobacterial peptides from Lyngbya majuscula. J. Nat. Prod. 2011, 74, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C–F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptin A, a cytotoxic cyclic depsipeptide from a Singapore collection of Lyngbya majuscula. J. Nat. Prod. 2009, 72, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptins B and C, cytotoxic cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, B.; Lei, H.H.; Wang, Z.; Liu, Y.Q.; Xu, Z.S.; Ye, T. Total synthesis and stereochemical revision of lagunamide A. Chem. Commun. 2012, 48, 8697–8699. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Owle, C.S.; Montaser, R.; Luesch, H.; Paul, V.J. Malyngamide 3 and Cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from Cocos Lagoon, Guam. J. Nat. Prod. 2011, 74, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Bingnan, H.; Gross, H.; McPhail, K.L.; Goeger, D.; Maier, C.S.; Gerwick, W.H. Wewakamide A and guineamide G, cyclic depsipeptides from the marine cyanobacteria Lyngbya semiplena and Lyngbya majuscula. J. Microbiol. Biotechnol. 2011, 21, 930–936. [Google Scholar]
- Rubio, B.K.; Parrish, S.M.; Yoshida, W.; Schupp, P.J.; Schils, T.; Williams, P.G. Depsipeptides from a Guamanian marine cyanobacterium, Lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron 2010, 51, 6718–6721. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.Y.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Paul, V.J.; Luesch, H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry 2009, 70, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Ross, C.; Paul, V.J.; Luesch, H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Vansach, T.; Yoshida, W.Y.; Sakamoto, B.; Pörzgen, P.; Horgen, F.D. Halogenated fatty acid amides and cyclic depsipeptides from an eastern caribbean collection of the cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2009, 72, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Lv, C.S.; Liu, J.Y.; Tang, L.J.; Feng, J.M.; Tang, S.B.; Wang, Z.; Liu, Y.Q.; Meng, Y.; Ye, T.; et al. Total synthesis of the proposed structure for itralamide B. Synlett 2014, 25, 1014–1018. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.C.; Cowley, E.S.; Sikorska, J.; Shaala, L.A.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Apratoxin H and apratoxin A sulfoxide from the Red Sea cyanobacterium Moorea producens. J. Nat. Prod. 2013, 76, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Okino, T.; Gerwick, W.H. Bouillonamide: A mixed polyketide–peptide cytotoxin from the marine cyanobacterium Moorea bouillonii. Mar. Drugs 2013, 11, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Vining, O.B.; Medina, R.A.; Mitchell, E.A.; Videau, P.; Li, D.; Serrill, J.D.; Kelly, J.X.; Gerwick, W.H.; Proteau, P.J.; Ishmael, J.E.; et al. Depsipeptide companeramides from a Panamanian marine cyanobacterium associated with the coibamide producer. J. Nat. Prod. 2015, 3, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Okeania sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Kanamori, Y.; Iwasaki, A.; Sumimoto, S.; Suenaga, K. Urumamide, a novel chymotrypsin inhibitor with a b-amino acid from a marine cyanobacterium Okeania sp. Tetrahedron 2016, 57, 4213–4216. [Google Scholar] [CrossRef]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a Potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.Y.; Pan, Z.Y.; Wu, C.L.; Wang, W.; Fang, L.J.; Su, W. Efficient synthesis and stereochemical revision of coibamide A. J. Am. Chem. Soc. 2015, 42, 13488–13491. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.D.; Byrum, T.; Liu, W.; Dorrestein, P.C.; Gerwick, W.H. Viequeamide A, a cytotoxic member of the kulolide superfamily of cyclic depsipeptides from a marine button cyanobacterium. J. Nat. Prod. 2012, 75, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Song, S.S.; Tian, Y.; Xu, Y.J.; Miao, Z.H.; Zhang, A. Total synthesis of the marine cyclic depsipeptide viequeamide A. J. Nat. Prod. 2013, 76, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A.M.; Engene, N.; Spadafora, C.; Gerwick, W.H.; Balunas, M.J. Medusamide A, a Panamanian cyanobacterial depsipeptide with multiple β-Amino acids. Org. Lett. 2016, 3, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Miller, M.W.; Kwan, J.C.; Luesch, H.; Paul, V.J. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010, 73, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Pörzgen, P.; Pittman, E.; Yoshida, W.Y.; Westenburg, H.E.; Horgen, F.D. Isolation and structure determination of malevamide E, a dolastatin 14 analogue, from the marine cyanobacterium Symploca laete-Wiridis. J. Nat. Prod. 2008, 71, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Cao, Z.Y.; Murray, T.F.; Gerwick, W.H. Hoiamide A, a sodium channel activator of unusual architecture from a consortium of two Papua New Guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Błaszczyk, J.M.; Cegłowska, M.; Marzec, H.M. Structures and activity of new anabaenopeptins produced by Baltic Sea cyanobacteria. Mar. Drugs 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Maru, N.; Ohno, O.; Uemura, D. Lyngbyacyclamides A and B, novel cytotoxic peptides from marine cyanobacteria Lyngbya sp. Tetrahedron 2010, 51, 6384–6387. [Google Scholar] [CrossRef]
- Boyaud, F.; Mahiout, Z.; Lenoir, C.; Tang, S.; Wdzieczak-Bakala, J.; Witczak, A.; Bonnard, I.; Banaigs, B.; Ye, T. First total synthesis and stereochemical revision of laxaphycin B and Its Extension to lyngbyacyclamide A and Nicolas Inguimbert. Org. Lett. 2013, 15, 3898–3901. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; González, J.; Ureña, L.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.V.; Al-Lihaibi, S.S.; Alarif, W.M.; Abdel-Lateff, A.; Nogata, Y.; Washio, K.; Morikawa, M.; Okino, T. Wewakazole B, a Cytotoxic Cyanobactin from the Cyanobacterium Moorea producens Collected in the Red Sea. J. Nat. Prod. 2016, 79, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Long, B.H.; Zhang, J.Z.; Tang, X.D.; Wu, Z.Z. Total synthesis of wewakazole B. Org. Biomol. Chem. 2016, 14, 9712–9715. [Google Scholar] [CrossRef] [PubMed]
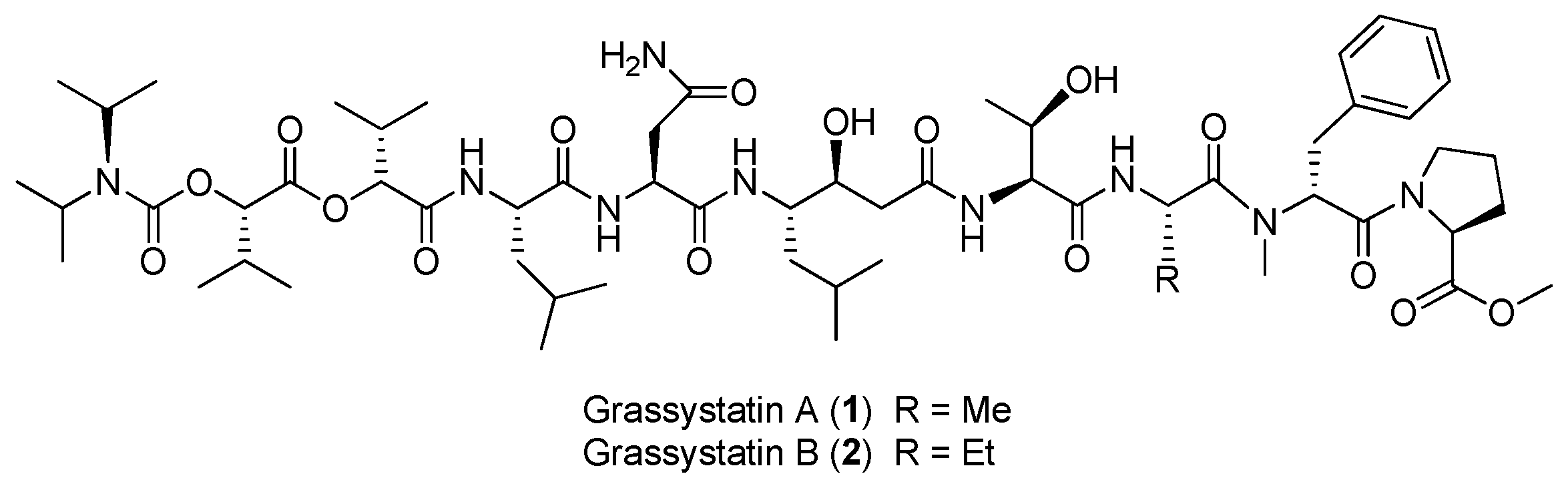
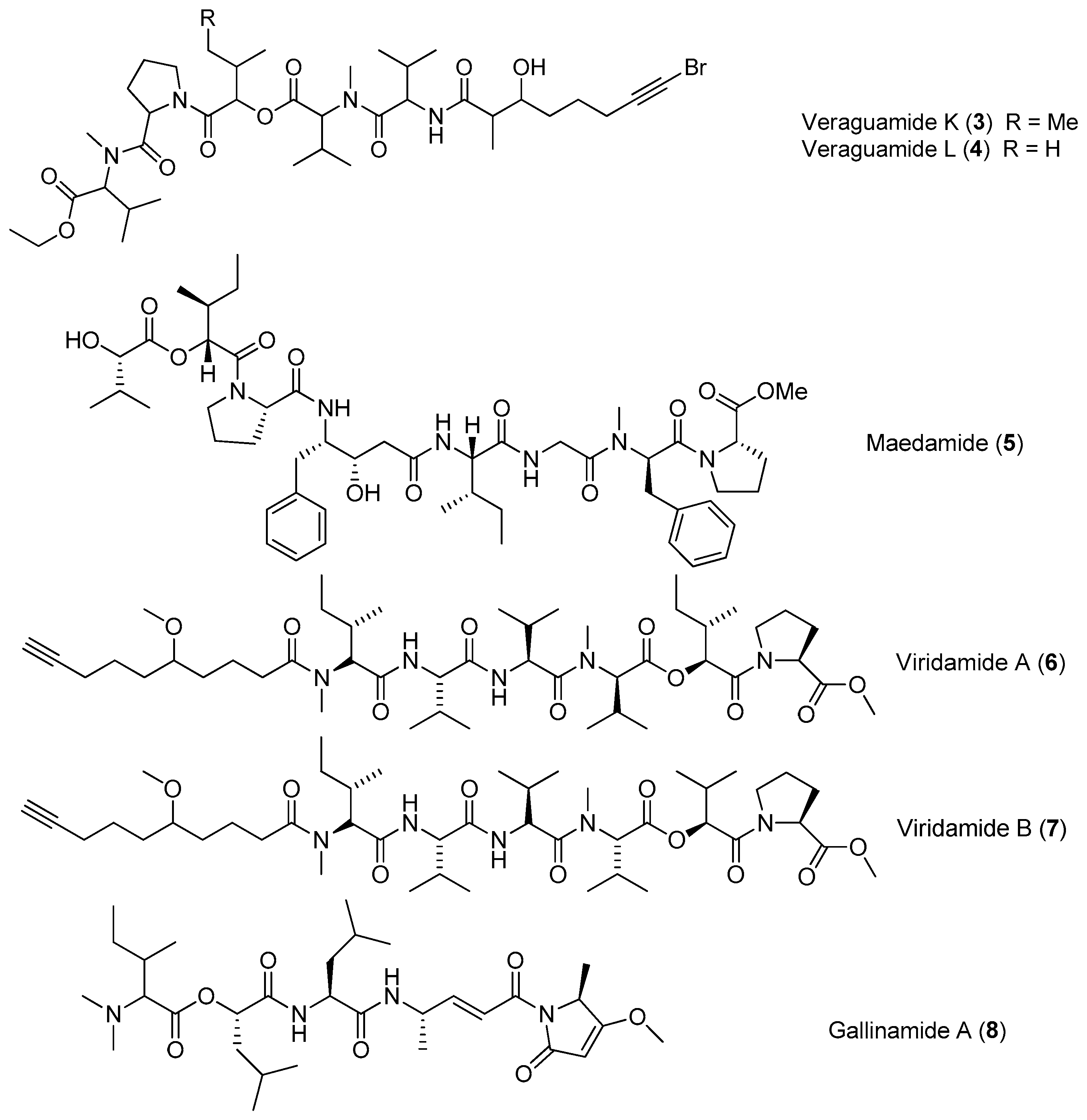

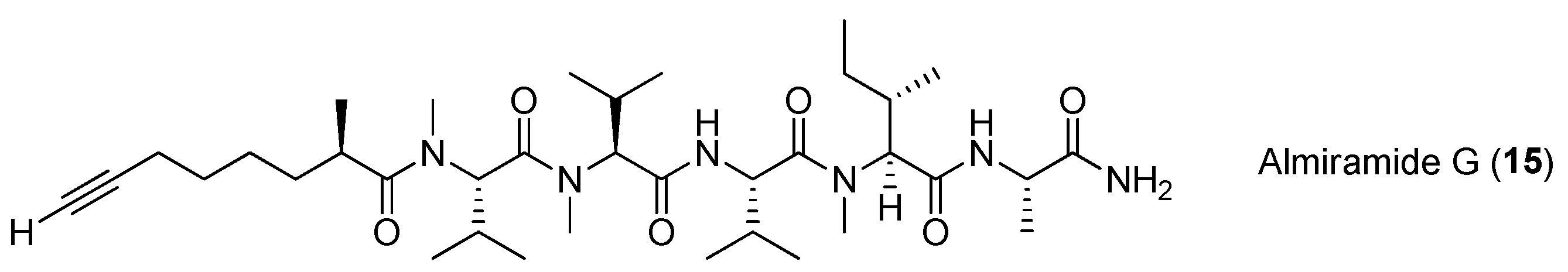
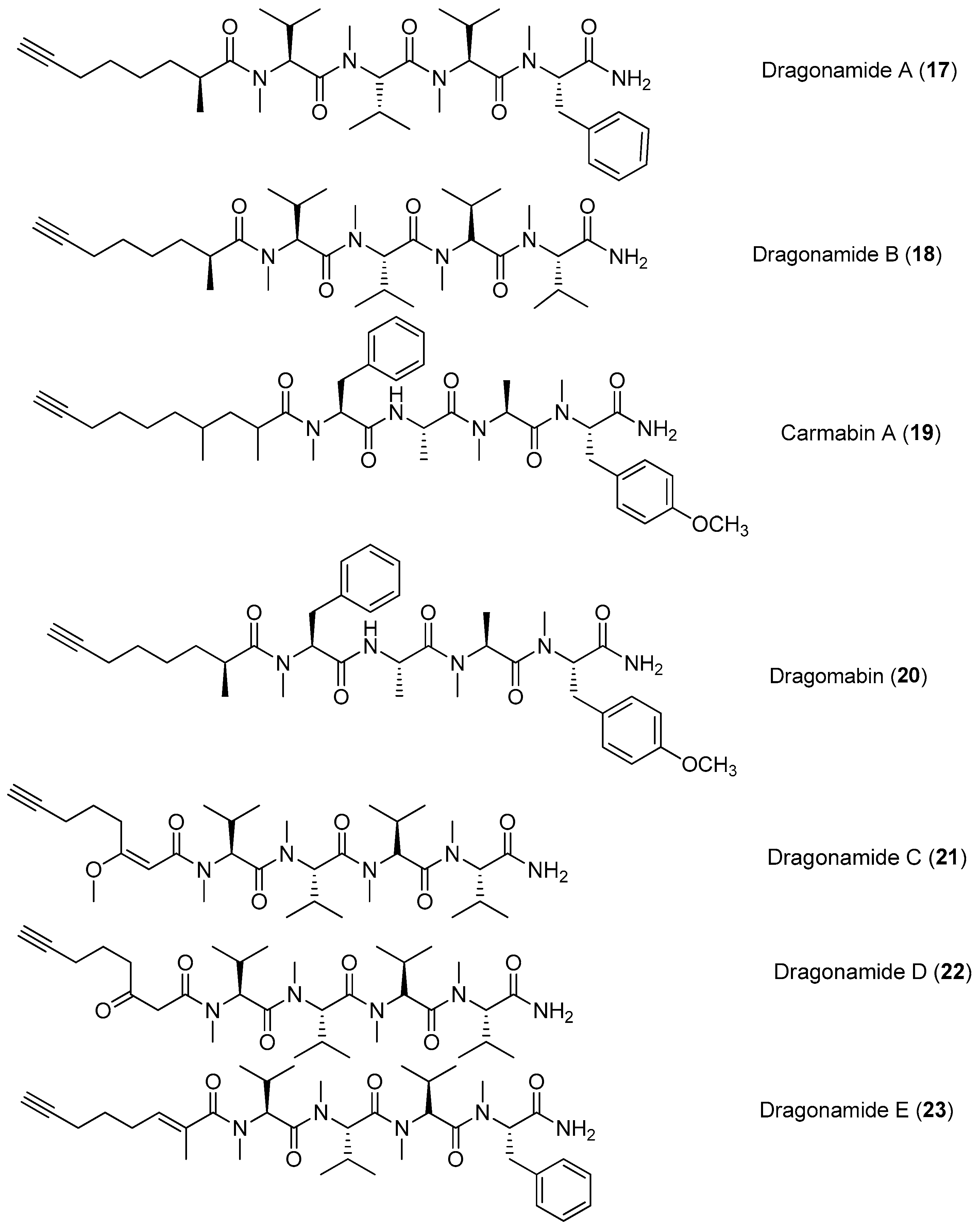






| Metabolites | Sources | Activities | References |
|---|---|---|---|
| Grassystatins A–B (1–2) | Okeania lorea (formerly Lyngbya cf. confervoides) | Cathepsin inhibition | [12,13] |
| Veraguamides K–L (3–4) | cf. Oscillatoria margaritifera Coiba, Panama | nd a | [14] |
| Maedamide (5) | Lyngbya sp. | Potent antitumor cytotoxicity Protease inhibition | [15,16] |
| Viridamides A–B (6–7) | Okeania comitata (formerly Oscillator nigroviridis) Panama | Antitrypanosomal activity Antileishmanial activity | [17] |
| Gallinamide A (8) | Schizothrix iedras Gallinas | Antimalarial activity | [18,19] |
| Metabolites | Sources | Bioactivities | References |
|---|---|---|---|
| Almiramides A–C (9–11) | Lyngbya majuscula Panama | General antileishmanial activity Antitumor cytotoxicity | [20,21] |
| Almiramide D (12) | Oscillatoria nigroviridis Island of Providence (Colombia, S.W. Caribbean Sea) | Antitumor cytotoxicity | [21] |
| Almiramide E–H (13–16) | Oscillatoria nigroviridis Island of Providence (Colombia, S.W. Caribbean Sea) | nd a | [21] |
| Dragonamides A–B (17–18) Carmabin A (19) Dragomabin (20) | Moorea producens (formerly Lyngbya polychroa) Panama | Antimalarial activity | [22] |
| Dragonamides C–D (21–22) | Moorea producens (formerly Lyngbya polychroa) Florida, Fort Lauderdale, Hollywood | Weak antitumor cytotoxicity | [23,24] |
| Dragonamide E (23) | Lyngbya majuscula | Antileishmanial activity | [25] |
| Lyngbyapeptin D (24) | Moorea bouillonii (formerly Lyngbya bouillonii) Apra Harbor, Guam | nd a | [26] |
| Jahanyne (25) | Lyngbya sp. | Potent antitumor cytotoxicity | [27] |
| Bisebromoamide (26) | Lyngbya sp. | Protein kinase inhibition | [28,29,30,31] |
| Norbisebromoamide (27) | Lyngbya sp. | nd a | [29] |
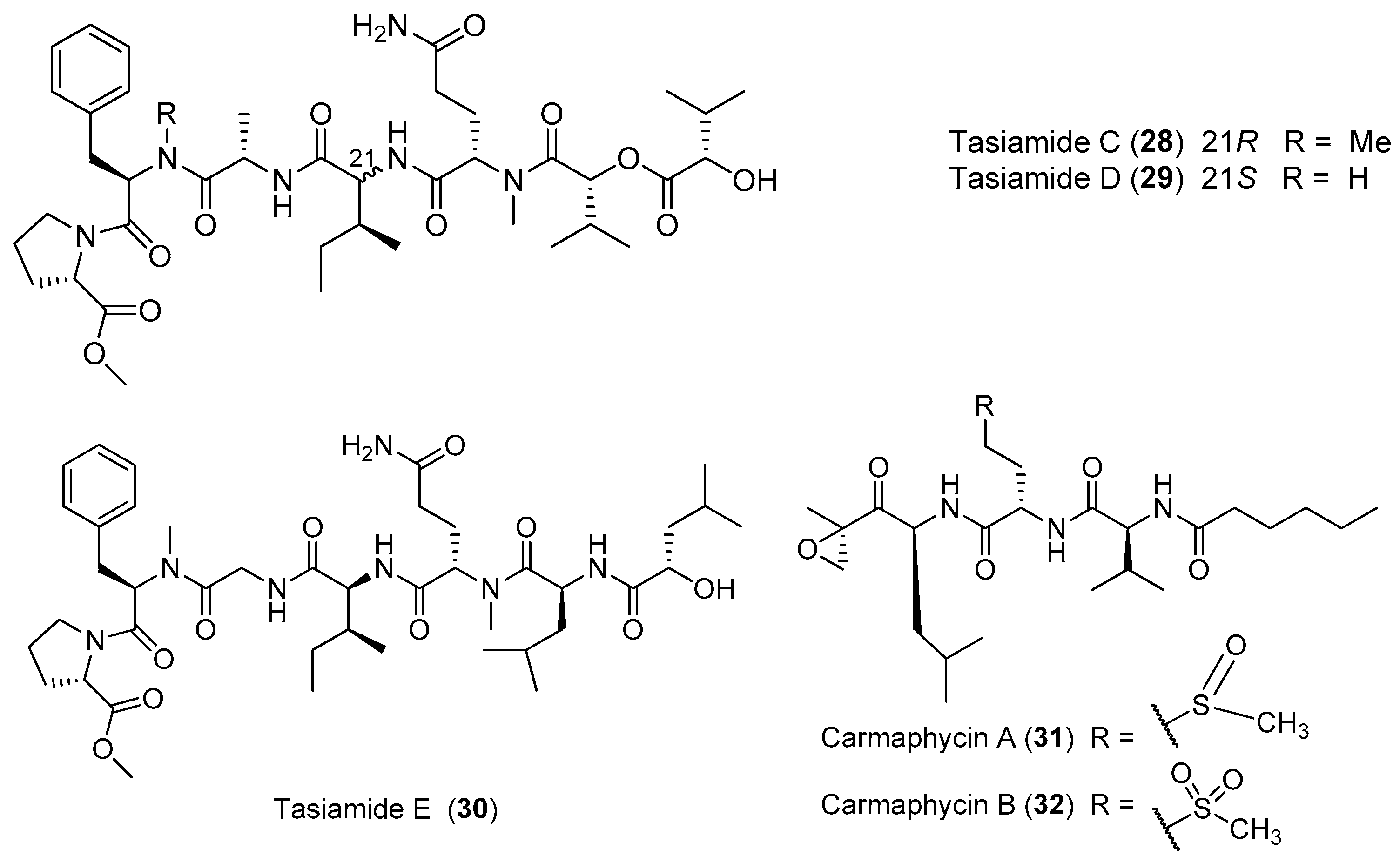
| Tasiamides C–D (28–29) | Symploca sp. Papua New Guinea | Weak antitumor cytotoxicity | [32] |
| Tasiamide E (30) | Symploca sp. Papua New Guinea | nd a | [32] |
| Carmaphycins A–B (31–32) | Symploca sp. Curacao | Protease inhibition Potent antitumor cytotoxicity | [33] |
| Hoiamides C–D (33–34) | Cyanobacterium Papua New Guinea | nd a | [34,35,36] |
| Lyngbyabellin M (35) | cyanobacterium from Palmyra Atoll Central Pacific Ocean | nd a | [37] |
| Kurahyne (36) | cyanobacterial mixture | Antitumor cytotoxicity | [38] |
| Kurahyne B (37) | Okeania sp. | Mild antitumor cytotoxicity | [39] |
| Caldoramide (38) | Caldora penicillata Florida | Antitumor cytotoxicity | [40] |
| Grassystatin C (39) | Okeania lorea (formerly Lyngbya cf. confervoides) | Cathepsin inhibition | [12] |
| Structure Class | Metabolites | Sources | Activities | References |
|---|---|---|---|---|
| Veraguamides | Veraguamides A–G (40–46) | cf. Oscillatoria margaritifera, Panama Symploca cf. hydnoides Cetti Bay, Guam | Weak antitumor cytotoxicity | [14,41,42] |
| Veraguamides H–J (47–49) | cf. Oscillatoria margaritifera Panama | nd a | [14] | |
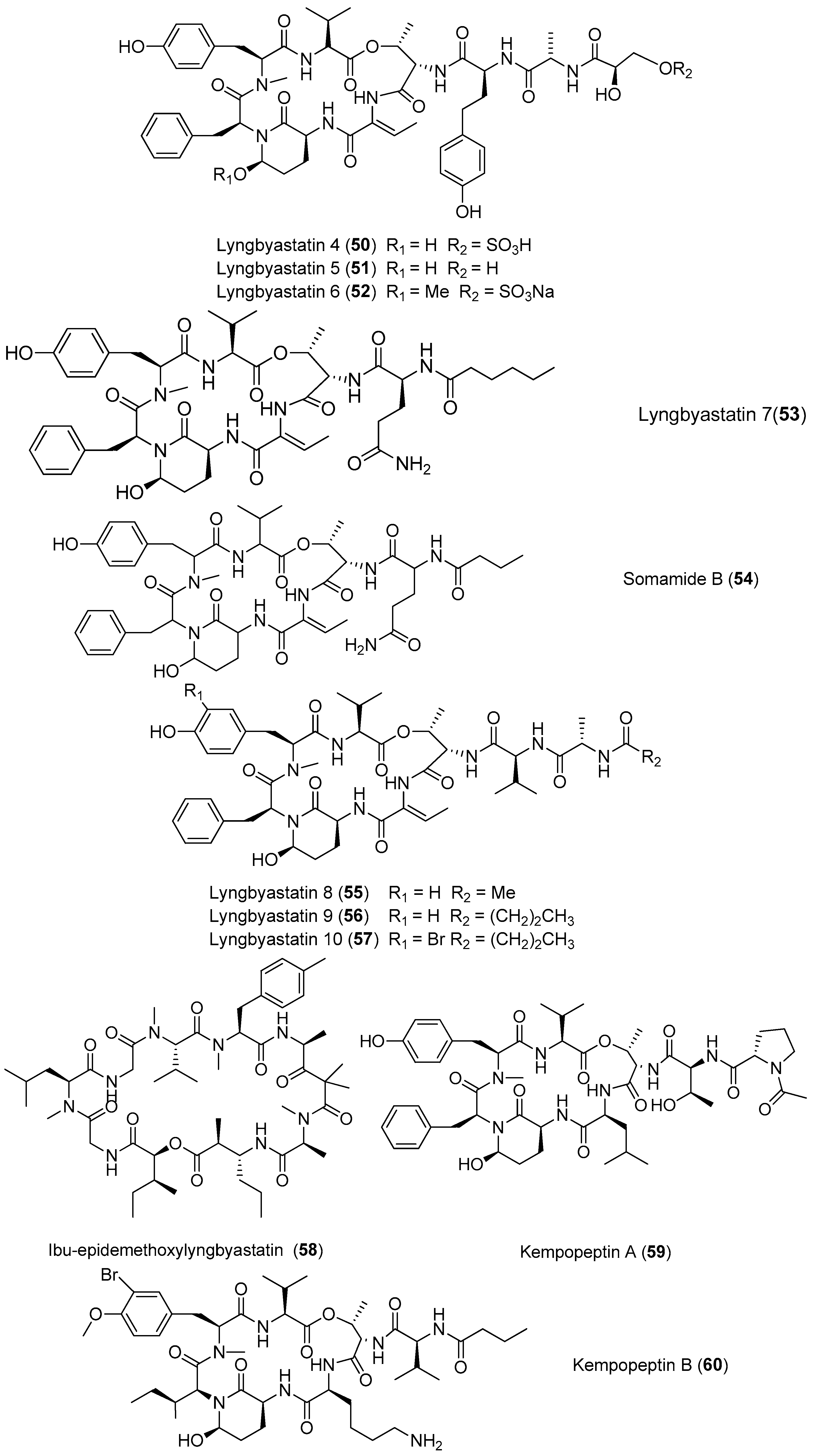
| Lyngbyastatins | Lyngbyastatins 4–6 (50–52) | Lyngbya confervoides off the coast of Florida | Potent protease inhibition | [43,44] |
| Lyngbyastatin 7 (53) Somamide B (54) | Lyngbya sp. from Florida | Potent protease inhibition | [44,45] | |
| Lyngbyastatins 8–10 (55–57) | Lyngbya semiplena Tumon Bay, Guam | Potent protease inhibition | [46] | |
| Ibu-epidemethoxylyngbyastatin 3 (58) | Leptolyngbya sp. SS Thistlegorm shipwreck, Red Sea | Weak cytotoxicity to neuro-2a cells | [47] | |
| Kempopeptins A and B (59, 60) | Lyngbya sp. Florida | Potent protease inhibition | [48] | |
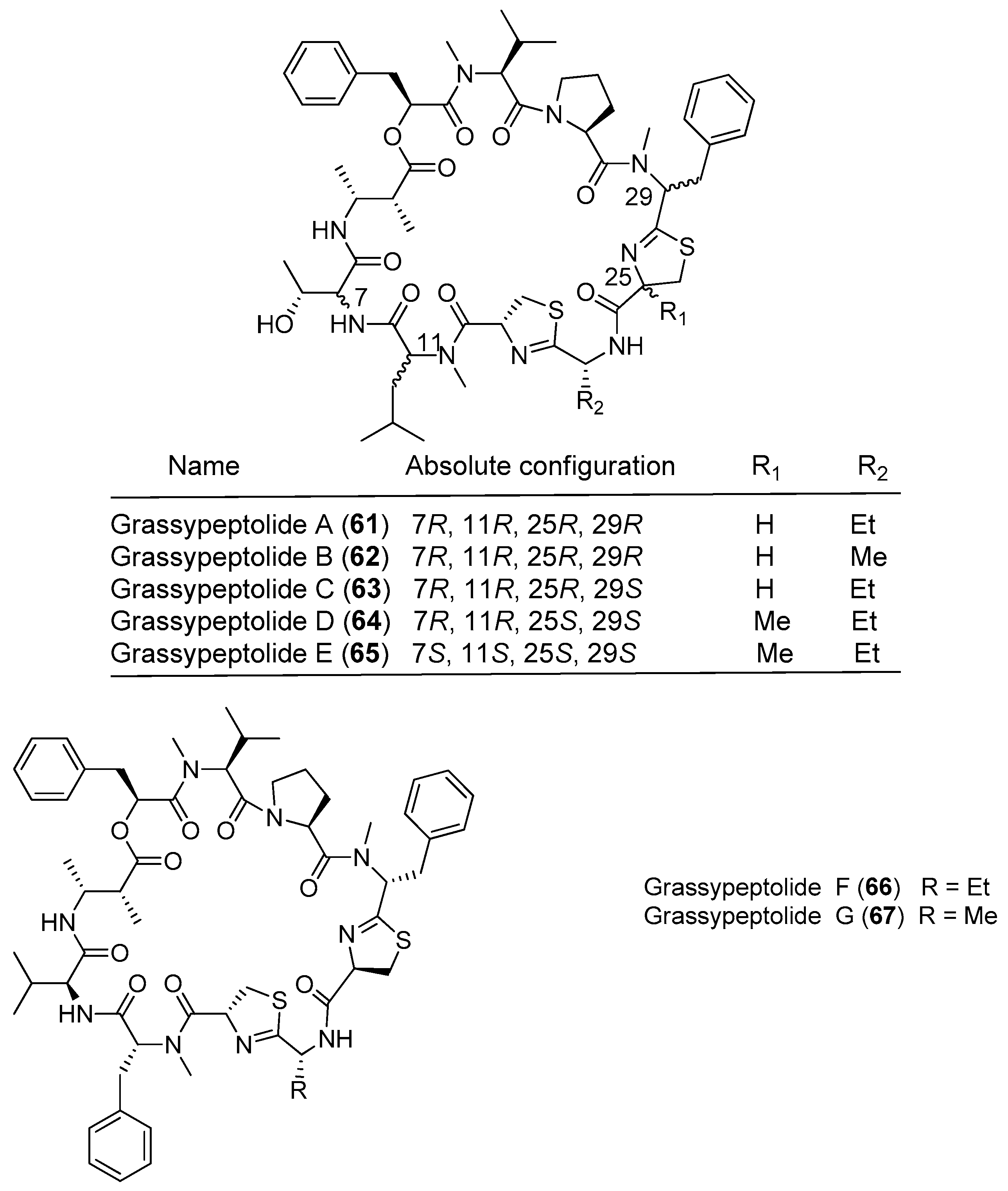
| Grassypeptolide A (61) | Okeania lorea (formerly Lyngbya confervoides) off Grassy Key in Florida | Antitumor cytotoxicity | [49] | |
| Grassypeptolides A–C (61–63) | Okeania lorea (formerly Lyngbya confervoides) | Cause G1 and G2/M phase cell cycle arrest | [50,51] | |
| Grassypeptolides | Grassypeptolides D and E (64, 65) | Leptolyngbya sp. SS Thistlegorm shipwreck, Red Sea | Potent antitumor cytotoxicity | [47] |
| Grassypeptolides F and G (66, 67) | Lyngbya majuscula Panama | Moderate inhibitory activity against the transcription factor AP-1 | [52] |
| Sources | Metabolites | Sources/Location | Activities | References |
|---|---|---|---|---|
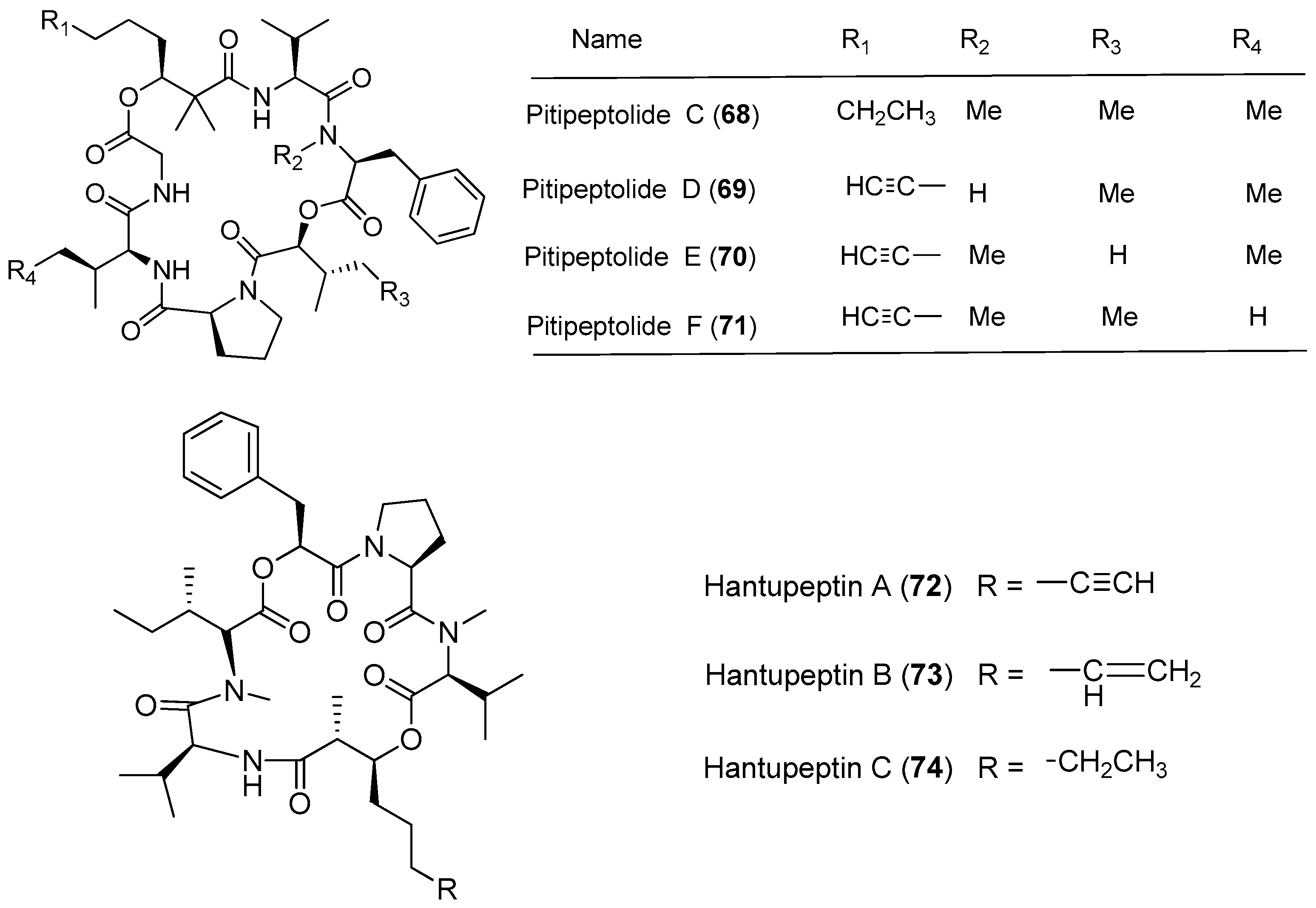
| Lyngbya majuscula | Pitipeptolides C–E (68–70) | Guam, Piti Bomb Holes | nd a | [53] |
| Pitipeptolide F (71) | Guam, Piti Bomb Holes | Antibacterial activity | [53] | |
| Hantupeptins A–C (72–74) | Pulau Hantu Besar Singapore | Moderate antitumor cytotoxicity | [54,55] | |
| Lagunamides A–C (75–77) | Pulau Hantu Besar Singapore | Antimalarial activity Potent antitumor cytotoxicity | [56,57,58] | |
| Cocosamides A and B (78, 79) | Cocos Lagoon, Guam | Slight antitumor cytotoxicity | [59] | |
| Desmethoxymajusculamide C (80) | Fijian | Potent antitumor cytotoxicity | [60] | |
| Pitiprolamide (81) | Piti Bomb Holes, Guam | Weak antitumor cytotoxicity Weak antibacterial activity | [61] | |
| Guineamide G (82) | Papua New Guinea | Brine shrimp toxicity Cytotoxicity against neuroblastoma cell | [62] | |
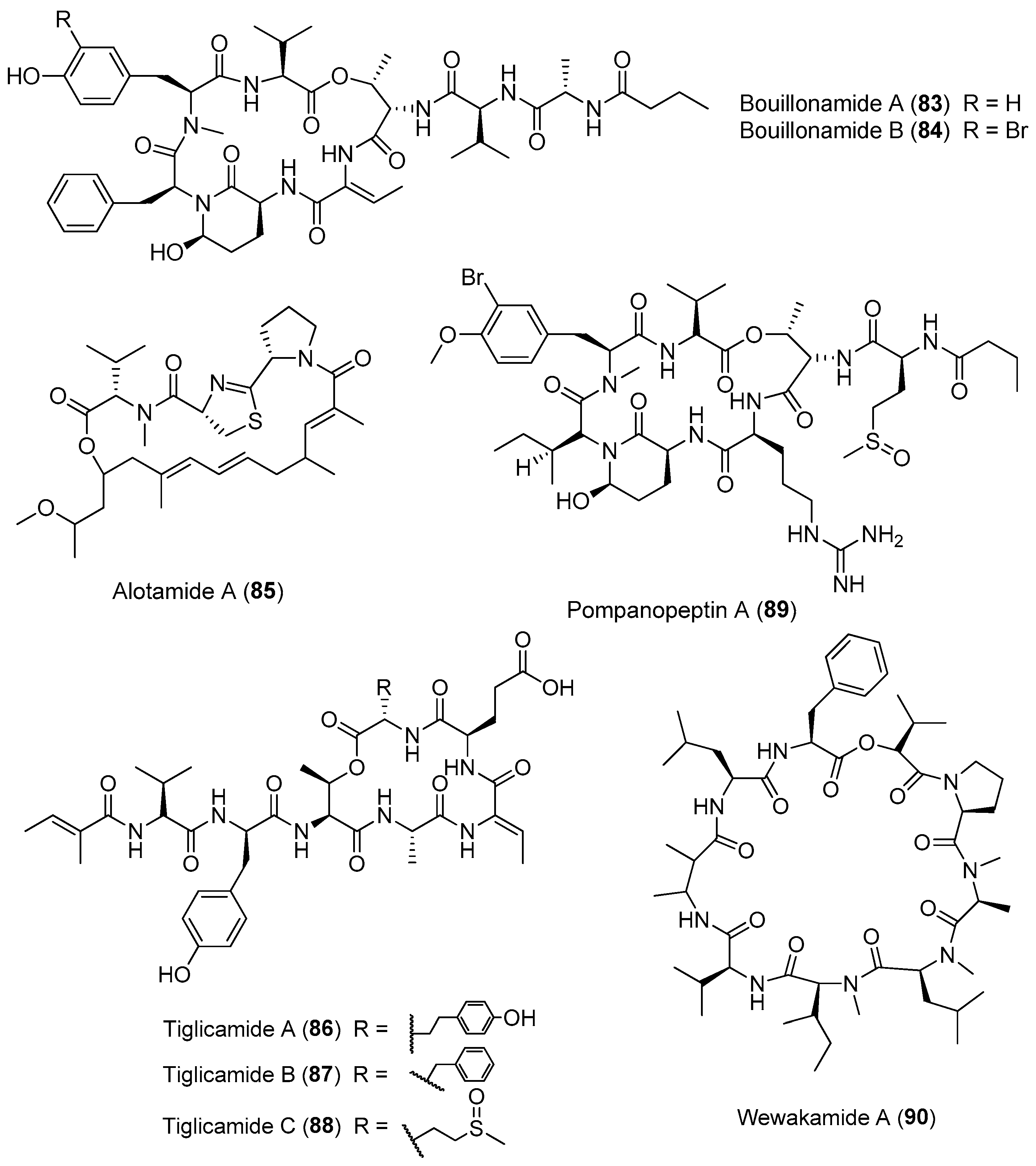
| Genus Lyngbya | Bouillomides A and B (83, 84) | Lyngbya bouillonii, Guam | Protease inhibition | [63] |
| Alotamide A (85) | Lyngbya bouillonii Papua New Guinea | Influx of Ca2+ in murine cerebrocortical neurons | [64] | |
| Tiglicamides A–C (86–88) | Lyngbya confervoides Florida | Protease inhibition | [65] | |
| Pompanopeptin A (89) | Lyngbya confervoides Florida | Protease inhibition | [66] | |
| wewakamide A (90) | Lyngbya semiplena Papua New Guinea | Potent brine shrimp toxicity | [62] | |
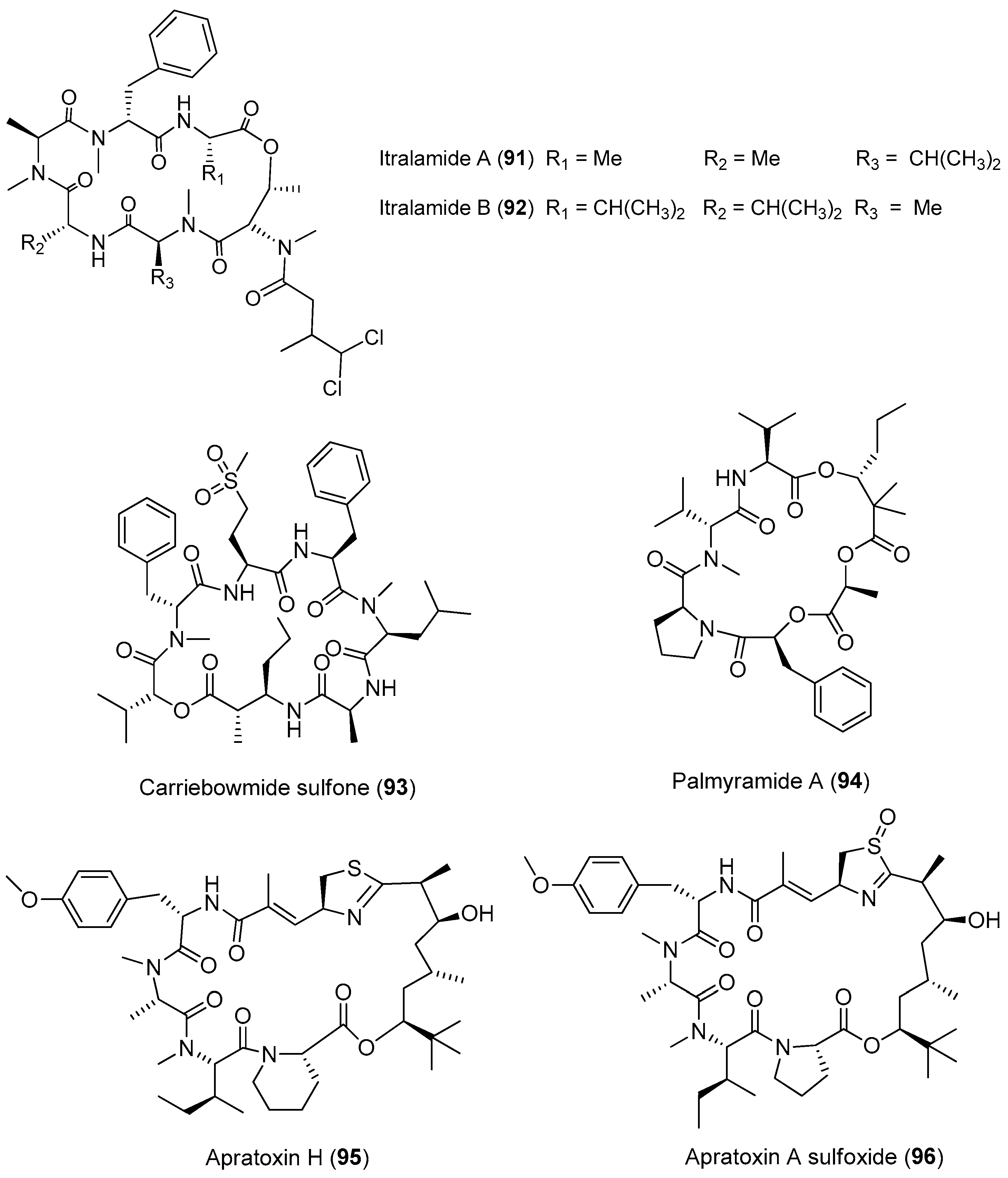
| Moorea producens | Itralamides A and B (91, 92) | eastern Caribbean | Antitumor cytotoxicity | [67,68] |
| Carriebowmide sulfone (93) | eastern Caribbean | nd a | [67] | |
| Palmyramide A (94) | Palmyra Atoll | Blocks sodium channel in neuro-2a cells Antitumor cytotoxicity | [69] | |
| Apratoxin H (95) Apratoxin A sulfoxide (96) | Gulf of Aqaba, Nabq Mangrovs | Potent antitumor cytotoxicity | [70] | |
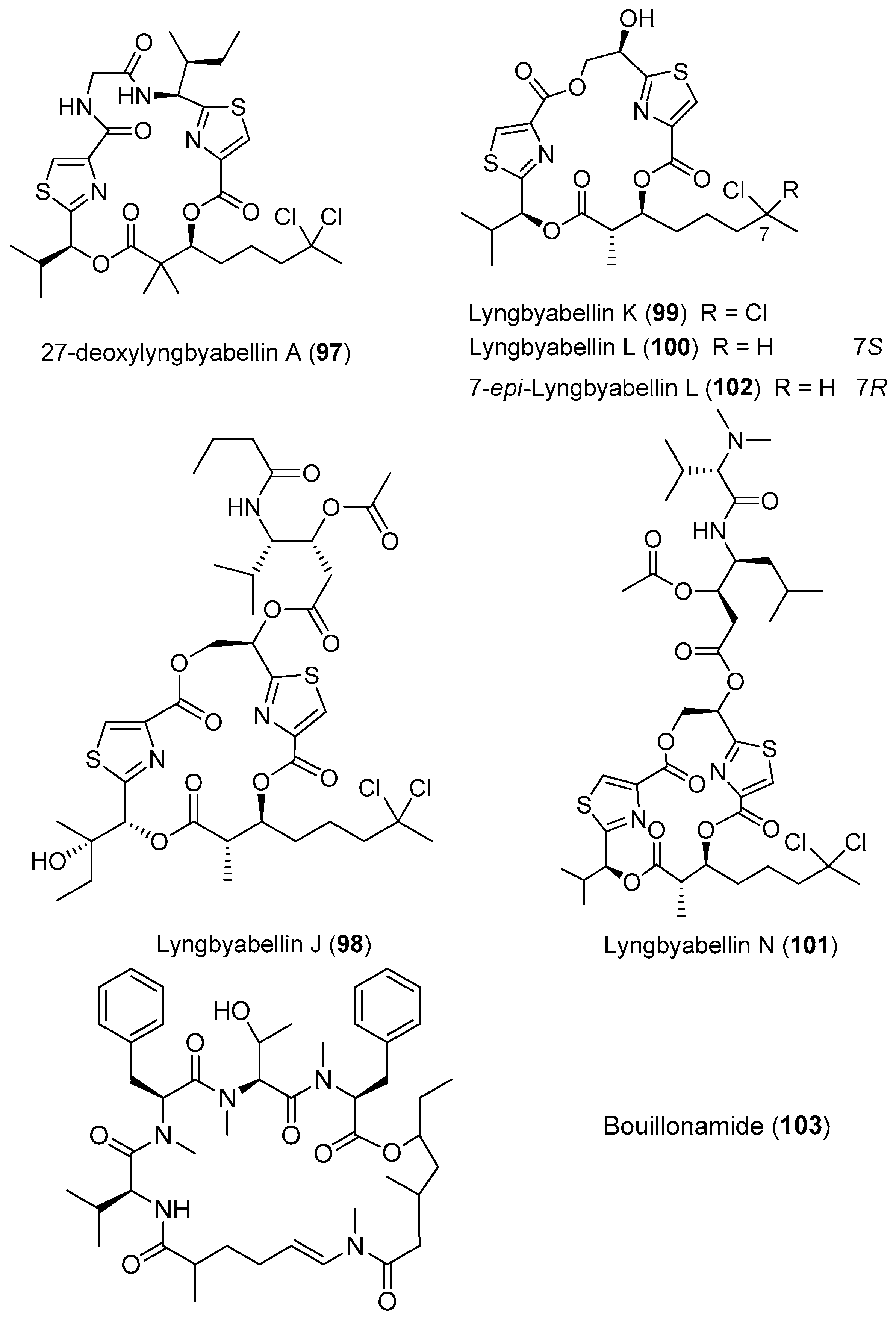
| Moorea bouillonii | 27-deoxylyngbyabellin A (97) Lyngbyabellin J (98) | Apra Bay, Guam | Moderate antitumor cytotoxicity | [26] |
| Lyngbyabellins K–L, N (99–101) 7-epi-Lyngbyabellin L (102) | Palmyra Atoll Central Pacific Ocean | Antitumor cytotoxicity | [37] | |
| Bouillonamide (103) | New Britain, Papua New Guinea | Mild toxicity to neuron 2a cells | [71] | |
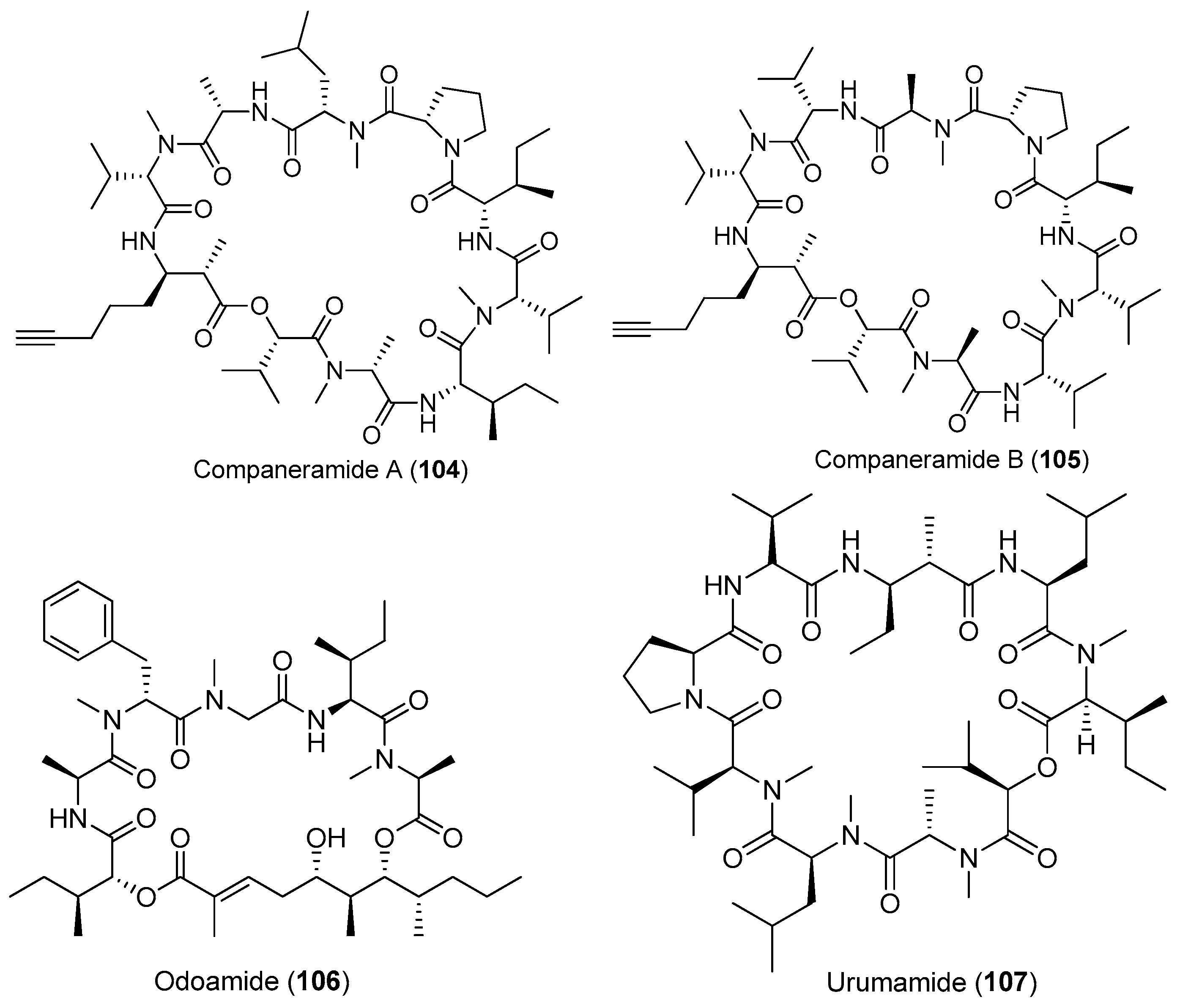
| Other marine cyanobacteria | Companeramides A andB (104, 105) | cyanobacterium from Panama | Moderate antimalaria parasites | [72] |
| Odoamide (106) | Okeania sp. | Potent antitumor cytotoxicity | [73] | |
| Urumamide (107) | Okeania sp. | Mild antitumor cytotoxicity | [74] | |
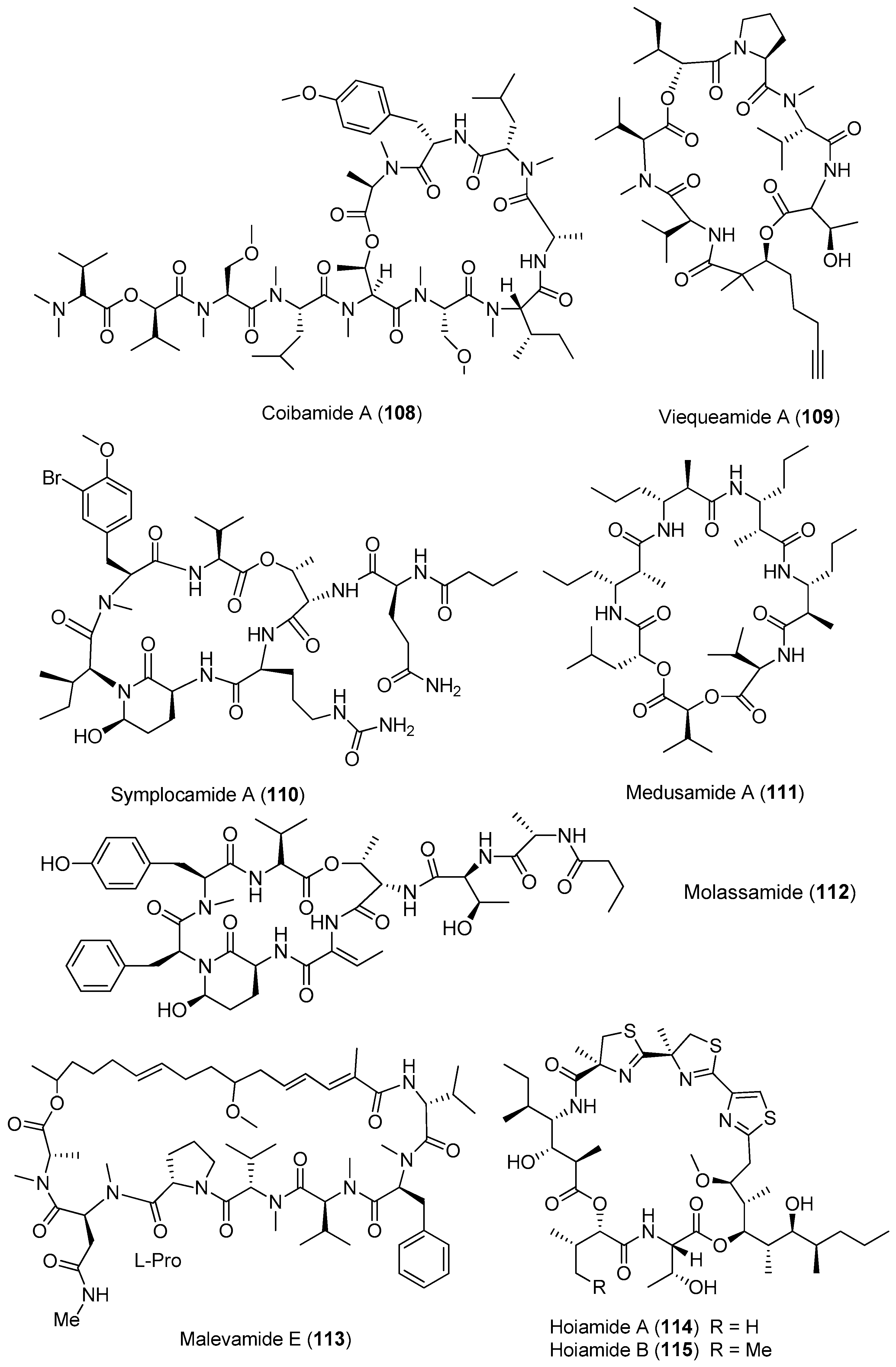
| Coibamide A (108) | Caldora penicillata (formerly Leptolyngbya sp.) Panama | Antitumor cytotoxicity | [75,76] | |
| Viequeamide A (109) | Rivularia sp. viequeamides uerto Rico, Vieque | Potent antitumor cytotoxicity | [77,78] | |
| Symplocamide A (110) | Symploca sp. Papua New Guinea | Potent antitumor cytotoxicity | [79] | |
| Medusamide A (111) | cyanobacterium from Panama | nd a | [80] | |
| Molassamide (112) | Dichothrix utahensis Molasses Reef, Key Largo, Florida | Protease inhibition | [81] | |
| Malevamide E (113) | Symploca laeteviridis | Inhibits Ca2+ release activated Ca2+ (CRAC) channels | [82] | |
| hoiamide A (114) | Lyngbya majuscula and Phormidium gracile Papua New Guinea | nd a | [83] | |
| Hoiamide B (115) | two different cyanobacterium from Papua New Guinea | Activates sodium chanal in mouse neocortical neurons Suppresses spontaneous Ca2+ oscillations in neocortical neurons | [34] |
| Metabolites | Sources | Activities | References |
|---|---|---|---|
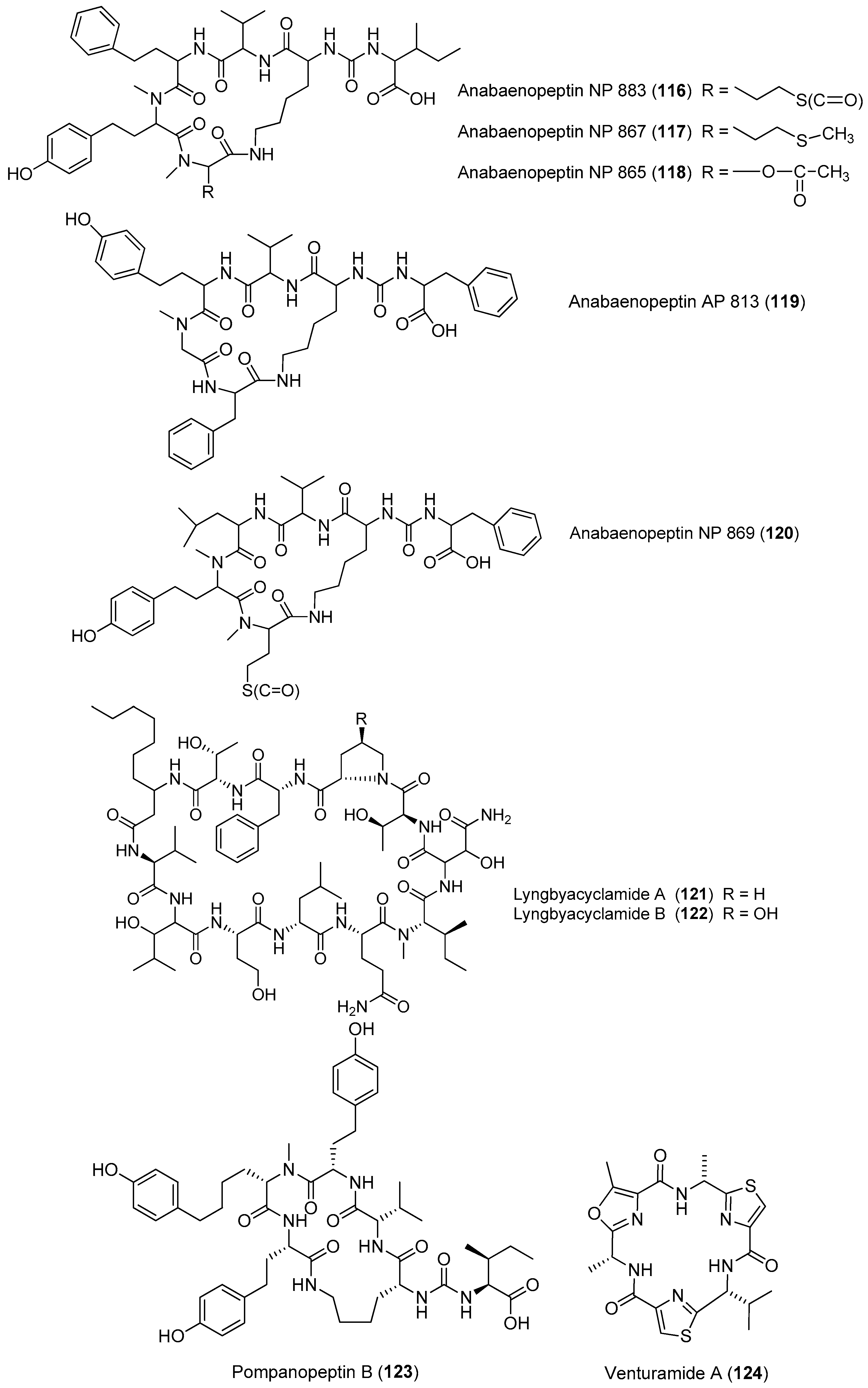
| Anabaenopeptins NP 883, NP 867, NP 865, AP813, NP 869 (116–120) | bloom sample of marine cyanobacteria Baltic Sea | nd a | [84] |
| Lyngbyacyclamides A–B (121–122) | Lyngbya sp. Okinawa, Japan | Moderate antitumor cytotoxicity | [85,86] |
| Pompanopeptin B (123) | Lyngbya confervoides Florida | Protease inhibition | [66] |
| Venturamides A and B (124, 125) | Oscillatoria sp. | Antimalaria parasites | [87] |
| Wewakazole B (126) | Moorea producens Red Sea | Moderate antitumor cytotoxicity | [88] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Zhang, J.; He, S.; Yan, X. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Mar. Drugs 2017, 15, 132. https://doi.org/10.3390/md15050132
Mi Y, Zhang J, He S, Yan X. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Marine Drugs. 2017; 15(5):132. https://doi.org/10.3390/md15050132
Chicago/Turabian StyleMi, Yue, Jinrong Zhang, Shan He, and Xiaojun Yan. 2017. "New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade" Marine Drugs 15, no. 5: 132. https://doi.org/10.3390/md15050132






