An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders
Abstract
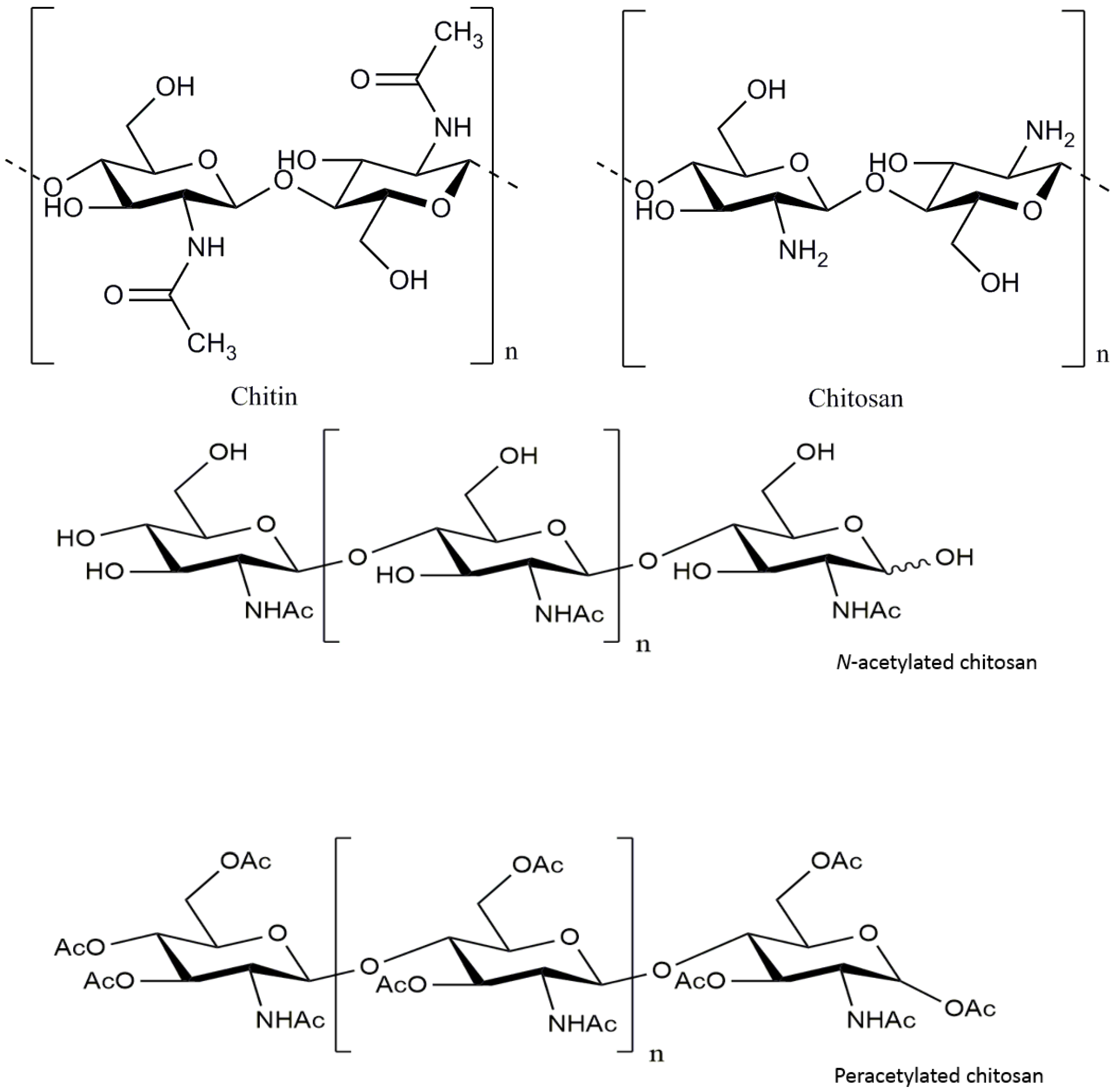
:1. Introduction
2. Update on Pathogenesis and Therapy for Neuronal Disorders
2.1. The Pathogenesis of Neuronal Disorders
2.2. Current Treatments and Therapies for Neuronal Disorders
3. The Potential Protective Effects of Chitosan and Its Derivatives against Neuronal Disorders
3.1. Potential Applications of Chitosan and Its Derivatives in Alzheimer’s Disease Therapy
3.2. The Inhibitory Effects of Chitosan and Its Derivatives against Parkinson’s Disease
3.3. The Inhibition Effects of Chitosan and Its Derivatives against Huntington’s Disease
3.4. The Inhibitory Effects of Chitosan and Its Derivatives against Other Neuronal Disorders
4. The Mechanisms of Neuroprotective Effects of Chitosan and Its Derivatives
4.1. Anti-Oxidative Stress Action
4.2. Suppressing Effect on Abeta Aggregation
4.3. Anti-Neuroinflammatory
4.4. Anti-Apoptosis Action
4.5. Anti-Excitotoxic Action
4.6. Other Mechanisms
5. Progress of the Clinical Studies on Chitosan and Its Derivatives
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-Amyloid peptide |
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| APPs | amyloid precursor proteins |
| BACE-1 | β-amyloid cleavage enzyme |
| CNS | central nervous system |
| COS | chitooligosaccharide |
| CS | chitosan |
| DA | dopaminergic |
| DBT | Dibutyltin |
| DCFH | 2′,7′-dichlorofluorescin |
| DEAE | diethylaminoethyl |
| DMAE | dimethyl aminoethyl |
| HD | Huntington’s disease |
| IL-1 | interleukin-1 |
| iNOS | inducible nitric oxide synthase |
| LDH | lactate dehydrogenase |
| MMP | mitochondrial membrane potential |
| PACOs | peracetylated chitosan oligosaccharides |
| PD | Parkinson’s disease |
| ROS | reactive oxygen species |
References
- Amor, S.; Peferoen, L.A.; Vogel, D.Y.; Breur, M.; van der Valk, P.; Baker, D.; van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.; Romer, K.; Wiltfang, J.; Kornhuber, J. Glutamate and the glutamate receptor system: A target for drug action. Int. J. Geriatr. Psychiatry 2003, 18 (Suppl. 1), S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Zablocka, A.; Janusz, M. The two faces of reactive oxygen species. Postep. Hig. Med. Doswiadczalnej 2008, 62, 118–124. [Google Scholar] [PubMed]
- Monaghan, D.T.; Bridges, R.J.; Cotman, C.W. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 365–402. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Gao, L.; Zhang, Y.; Wang, W.; Yu, G.; Guan, H.; Zhang, L.; Li, C. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway. Mar. Drugs 2015, 13, 1267–1289. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pee, K.H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. Engl. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Bavestrello, G.; Kurek, D.; Paasch, S.; Brunner, E.; Born, R.; Galli, R.; Stelling, A.L.; Sivkov, V.N.; Petrova, O.V.; et al. Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 2012, 51, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan Chitin as a Versatile Template for Extreme Biomimetics. Polymers 2015, 7, 235–365. [Google Scholar] [CrossRef]
- Hong, K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Sun, A. Application of Chitosan and Oligochitosan in the Field of Cosmetics. Chem. Ind. Eng. Prog. 2003, 32, 3026–3031. [Google Scholar]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective properties of chitosan and its derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by Abeta25–35 in PC12 cells. Bioorg. Med. Chem. Lett. 2009, 19, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, T.; Salim, C.; Rajini, P.S.; Suresh, P.V. Antioxidant and neuroprotective potential of chitooligomers in Caenorhabditis elegans exposed to Monocrotophos. Carbohydr. Polym. 2016, 135, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, Y.; Gu, X.; Ding, F. Chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. Neurosci. Lett. 2008, 444, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Ali, A.; Asif, M.; Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur. J. Med. Chem. 2016, 124, 1121–1141. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid beta-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Chami, L.; Checler, F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and beta-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, G. Mitochondrial dysfunction in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sinha, M.; Chanda, D.; Roy, T.; Banerjee, K.; Munshi, S.; Patro, B.S.; Chakrabarti, S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 2011, 1812, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Coppede, F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. 2009, 667, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Recchia, A.; Debetto, P.; Negro, A.; Guidolin, D.; Skaper, S.D.; Giusti, P. Alpha-synuclein and Parkinson’s disease. FASEB J. 2004, 18, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.L. Ubiquitin-proteasome system dysfunction in Parkinson’s disease: Current evidence and controversies. Expert Rev. Proteom. 2007, 4, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Singh, S.; Kumar, V.; Kumar, D.; Agarwal, S.; Rana, M.K. Huntington’s disease: An update of therapeutic strategies. Gene 2015, 556, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Spokes, E.G. Neurochemical alterations in Huntington’s chorea: A study of post-mortem brain tissue. Brain 1980, 103, 179–210. [Google Scholar] [CrossRef] [PubMed]
- Mallucci, G.; Collinge, J. Update on Creutzfeldt-Jakob disease. Curr. Opin. Neurol. 2004, 17, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, R.M.; Segura-Aguilar, J. Novel mechanisms and approaches in the study of neurodegeneration and neuroprotection. A review. Neurotox. Res. 2003, 5, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Bagetta, G. How to study neuroprotection? Cell. Death Differ. 2008, 15, 1084–1085. [Google Scholar] [CrossRef]
- Pellicciari, R.; Costantino, G.; Marinozzi, M.; Natalini, B. Modulation of glutamate receptor pathways in the search for new neuroprotective agents. Farmaco 1998, 53, 255–261. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002, 33, 182–191. [Google Scholar] [CrossRef]
- Agnello, D.; Bigini, P.; Villa, P.; Mennini, T.; Cerami, A.; Brines, M.L.; Ghezzi, P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002, 952, 128–134. [Google Scholar] [CrossRef]
- Gao, H.M.; Liu, B.; Zhang, W.; Hong, J.S. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol. Sci. 2003, 24, 395–401. [Google Scholar] [CrossRef]
- Volbracht, C.; van Beek, J.; Zhu, C.; Blomgren, K.; Leist, M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur. J. Neurosci. 2006, 23, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; An, L.; Wang, Y.; Zhao, H.; Gao, C. Neuroprotective effect of Alpinia oxyphylla Miq. fruits against glutamate-induced apoptosis in cortical neurons. Toxicol. Lett. 2003, 144, 205–212. [Google Scholar] [CrossRef]
- Kietzmann, T.; Knabe, W.; Schmidt-Kastner, R. Hypoxia and hypoxia-inducible factor modulated gene expression in brain: Involvement in neuroprotection and cell death. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [PubMed]
- Schwartz, G.; Fehlings, M.G. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J. Neurosurg. 2001, 94 (Suppl. 2), 245–256. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Fridkin, M.; Zheng, H. Novel bifunctional drugs targeting monoamine oxidase inhibition and iron chelation as an approach to neuroprotection in Parkinson’s disease and other neurodegenerative diseases. J. Neural Transm. 2004, 111, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, A.; Hider, R.C. The crucial role of metal ions in neurodegeneration: The basis for a promising therapeutic strategy. Br. J. Pharmacol. 2005, 146, 1041–1059. [Google Scholar] [PubMed]
- Tremblay, R.; Hewitt, K.; Lesiuk, H.; Mealing, G.; Morley, P.; Durkin, J.P. Evidence that brain-derived neurotrophic factor neuroprotection is linked to its ability to reverse the NMDA-induced inactivation of protein kinase C in cortical neurons. J. Neurochem. 1999, 72, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Gdalyahu, A.; Shani, Y.; Otten, U.; Lazarovici, P.; Cohen, I.R.; Schwartz, M. Production of neurotrophins by activated T cells: Implications for neuroprotective autoimmunity. J. Autoimmun. 2000, 15, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Akerud, P.; Canals, J.M.; Snyder, E.Y.; Arenas, E. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J. Neurosci. 2001, 21, 8108–8118. [Google Scholar] [PubMed]
- Woo, M.S.; Park, J.S.; Choi, I.Y.; Kim, W.K.; Kim, H.S. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J. Neurochem. 2008, 106, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, K.; Mehrabian, Z.; Spinnewyn, B.; Chinopoulos, C.; Drieu, K.; Fiskum, G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry 2003, 36 (Suppl. 1), S89–S94. [Google Scholar] [PubMed]
- Handley, O.J.; Naji, J.J.; Dunnett, S.B.; Rosser, A.E. Pharmaceutical, cellular and genetic therapies for Huntington’s disease. Clin. Sci. (Lond.) 2006, 110, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zeng, Y.; Lu, H.; Zhang, L. Determination of the degree of acetylation and the distribution of acetyl groups in chitosan by HPLC analysis of nitrous acid degraded and PMP labeled products. Carbohydr. Res. 2015, 413, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chang, P.; Zhu, Q.; Liu, W.; Sun, Y.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides protect rat primary hippocampal neurons from oligomeric beta-amyloid 1–42-induced neurotoxicity. Neurosci. Lett. 2013, 554, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-beta1–42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Miao, J.; Yan, C.; Ge, R.; Liang, T.; Liu, E.; Li, Q. Chitosan attenuates dibutyltin-induced apoptosis in PC12 cells through inhibition of the mitochondria-dependent pathway. Carbohydr. Polym. 2016, 151, 996–1005. [Google Scholar] [PubMed]
- Xu, W.; Huang, H.C.; Lin, C.J.; Jiang, Z.F. Chitooligosaccharides protect rat cortical neurons against copper induced damage by attenuating intracellular level of reactive oxygen species. Bioorg. Med. Chem. Lett. 2010, 20, 3084–308. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010, 213 Pt 9, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gong, L.; Gu, X.; Ding, F. Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peroneal nerve crush injury model. Microsurgery 2009, 29, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhuge, X.; Yang, Y.; Gu, X.; Ding, F. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neurosci. Lett. 2009, 454, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Khodagholi, F.; Eftekharzadeh, B.; Maghsoudi, N.; Rezaei, P.F. Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: Involvement of transcription factors Nrf2 and NF-kappaB. Mol. Cell Biochem. 2010, 337, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Evin, G. Future Therapeutics in Alzheimer’s Disease: Development Status of BACE Inhibitors. BioDrugs 2016, 30, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J. Emerging amyloid beta (Ab) peptide modulators for the treatment of Alzheimer’s disease (AD). Expert Opin. Emerg. Drugs 2008, 13, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Suemoto, T.; Shiomitsu, T.; Suzuki, M.; Shimadzu, H.; Akatsu, H.; Yamamoto, T.; Arai, H.; Sasaki, H.; Yanai, K.; et al. A novel imaging probe for in vivo detection of neuritic and diffuse amyloid plaques in the brain. J. Mol. Neurosci. 2004, 24, 247–255. [Google Scholar] [CrossRef]
- Hampel, H.; Shen, Y. Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) as a biological candidate marker of Alzheimer’s disease. Scand. J. Clin. Lab. Investig. 2009, 69, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hou, W.; Sun, Y.; Gao, Z.; Zhu, S.; Jiang, Z. Chitosan Oligosaccharides Inhibit/Disaggregate Fibrils and Attenuate Amyloid beta-Mediated Neurotoxicity. Int. J. Mol. Sci. 2015, 16, 10526–10536. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Kim, S.K. Water-soluble chitosan derivatives as a BACE1 inhibitor. Bioorg. Med. Chem. 2005, 13, 6551–6555. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.-G.; Kim, Y.-T.; Park, P.-J.; Lin, X.; Kim, S.-K. Chitooligosaccharides as a novel β-secretase inhibitor. Carbohydr. Polym. 2005, 61, 198–202. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Sung, M.J.; Seo, S.B.; Yoo, S.J.; Lim, W.K.; Kim, H.M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 2002, 321, 105–109. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.M.; Yang, C.H. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Twomey, C.; McCarthy, J.V. Pathways of apoptosis and importance in development. J. Cell. Mol. Med. 2005, 9, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Intern. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.N.; Jeong, H.J.; Hong, S.H.; Choi, J.H.; An, N.H.; Kim, H.M. High molecular weight water-soluble chitosan protects against apoptosis induced by serum starvation in human astrocytes. J. Nutr. Biochem. 2002, 13, 245–249. [Google Scholar] [CrossRef]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing! Age Ageing 2006, 35, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Andre, C.; Thomassin, M.; Guillaume, Y.C. Association mechanism of four acetylcholinesterase inhibitors (AChEIs) with human serum albumin: A biochromatographic approach. J. Pharm. Biomed. Anal. 2008, 48, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Castro, A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2006, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Ngo, D.-N.; Kim, S.-K. Acetylcholinesterase inhibitory activity of novel chitooligosaccharide derivatives. Carbohydr. Polym. 2009, 78, 869–872. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov (accessed on 8 November 2016).
- Stern, R. Go Fly a Chitin: The Mystery of Chitin and Chitinases in Vertebrate Tissues. Front. Biosci. (Landmark Ed.) 2017, 22, 580–595. [Google Scholar] [CrossRef] [PubMed]


| Specific Polysaccharides | Anti-Neuronal Disorder Effects | Mechanisms | References |
|---|---|---|---|
| Chitosans (CSs) | Anti-Parkinson’s disease; Anti-spinal cord injury | Anti-apoptosis action; Anti-oxidative stress | [63,65,68] |
| Chitooligosaccharides (COSs) | Anti-Alzheimer’s disease | β-amyloid inhibitory activities; Anti-neuroinflammation; Anti-apoptosis action | [23,24,25,26,61,62,73,74,75] |
| Anti-Parkinson’s disease | Anti-exitotoxic action; Anti-apoptosis action | [26] | |
| Anti-Huntington’s disease | Anti-exitotoxic action; Anti-oxidative stress | [64] | |
| Anti- nerve crush injury | Promoting nerve regeneration; Anti-neuroinflammation | [66,67,78] | |
| Peracetylated chitosan oligosaccharides | Anti-Alzheimer’s disease; Anti-Parkinson’s disease | Anti-oxidative stress; Anti-apoptosis action | [8] |
| Water-soluble chitosans | Anti-Alzheimer’s disease | β-amyloid inhibitory activities; Anti-apoptosis | [77,82] |
| COS derivatives | Anti-Alzheimer’s disease | Anti-AChE and BACE-1 enzyme activities | [74,75,87] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Mar. Drugs 2017, 15, 89. https://doi.org/10.3390/md15040089
Hao C, Wang W, Wang S, Zhang L, Guo Y. An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders. Marine Drugs. 2017; 15(4):89. https://doi.org/10.3390/md15040089
Chicago/Turabian StyleHao, Cui, Wei Wang, Shuyao Wang, Lijuan Zhang, and Yunliang Guo. 2017. "An Overview of the Protective Effects of Chitosan and Acetylated Chitosan Oligosaccharides against Neuronal Disorders" Marine Drugs 15, no. 4: 89. https://doi.org/10.3390/md15040089




