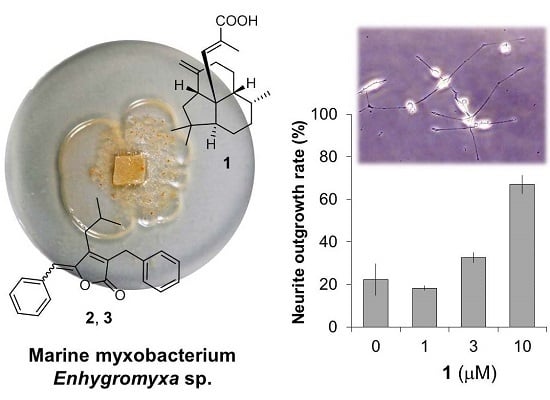
An Unusual Diterpene—Enhygromic Acid and Deoxyenhygrolides from a Marine Myxobacterium, Enhygromyxa sp.
Abstract
:1. Introduction
2. Results and Discussion
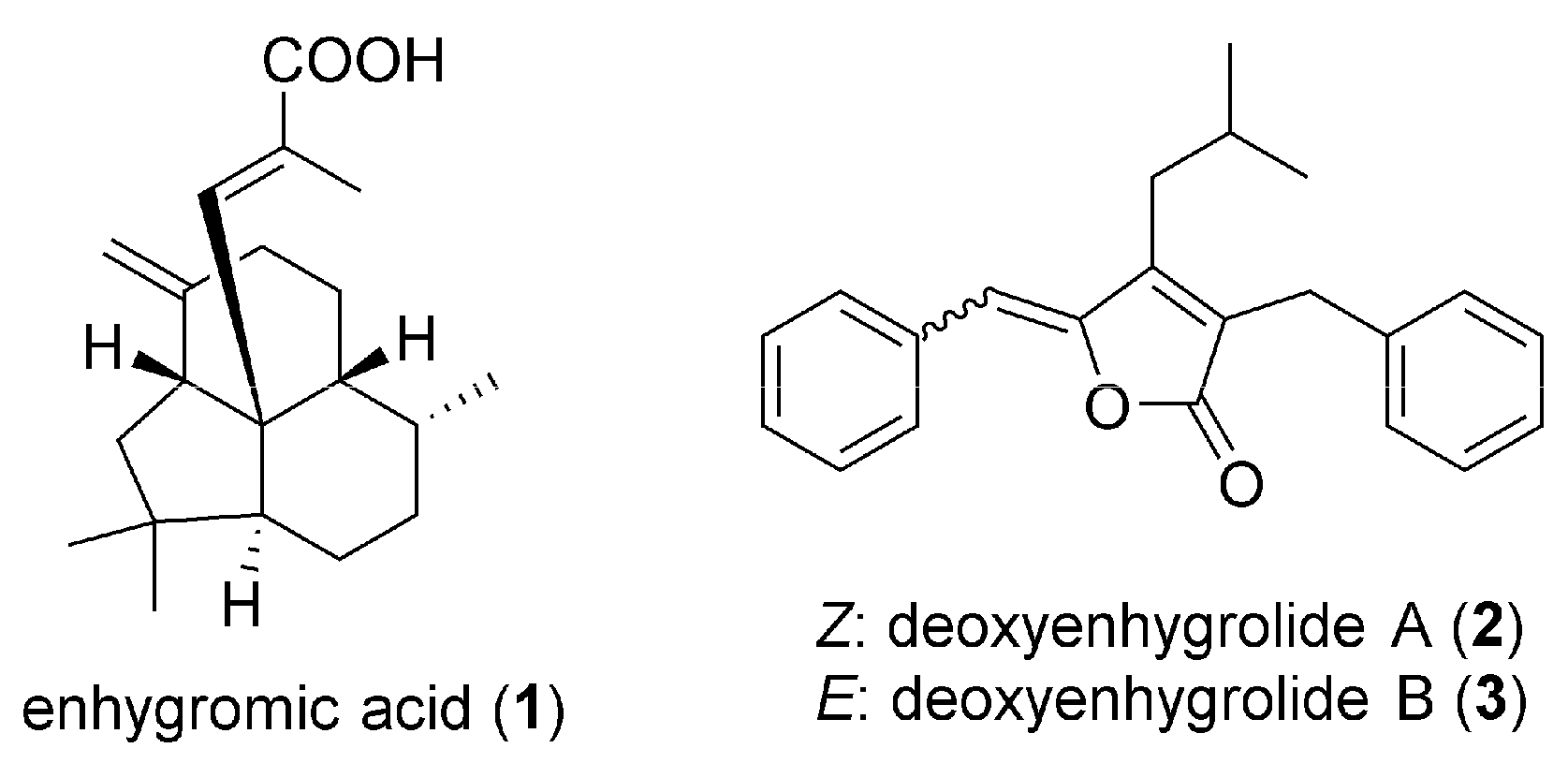
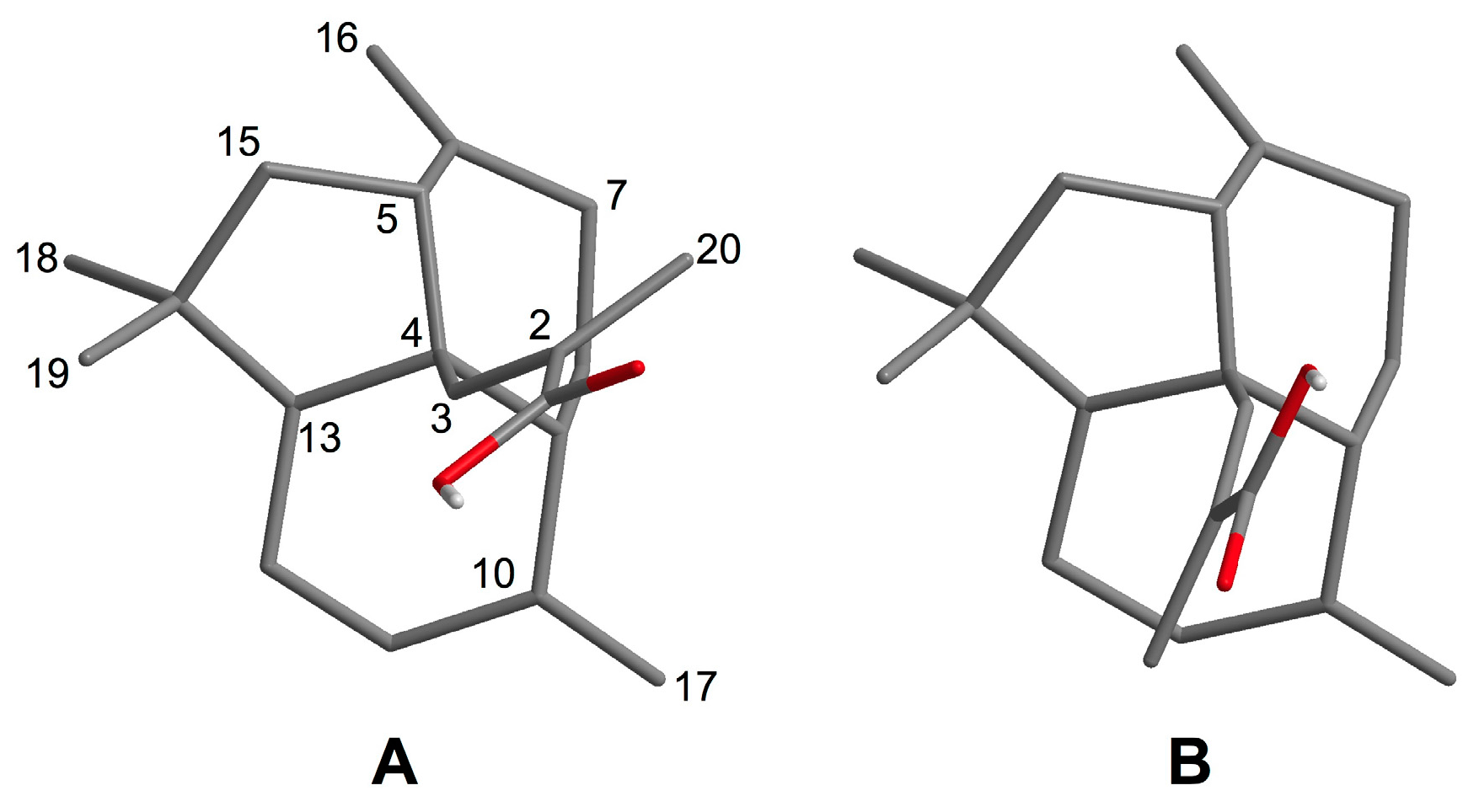
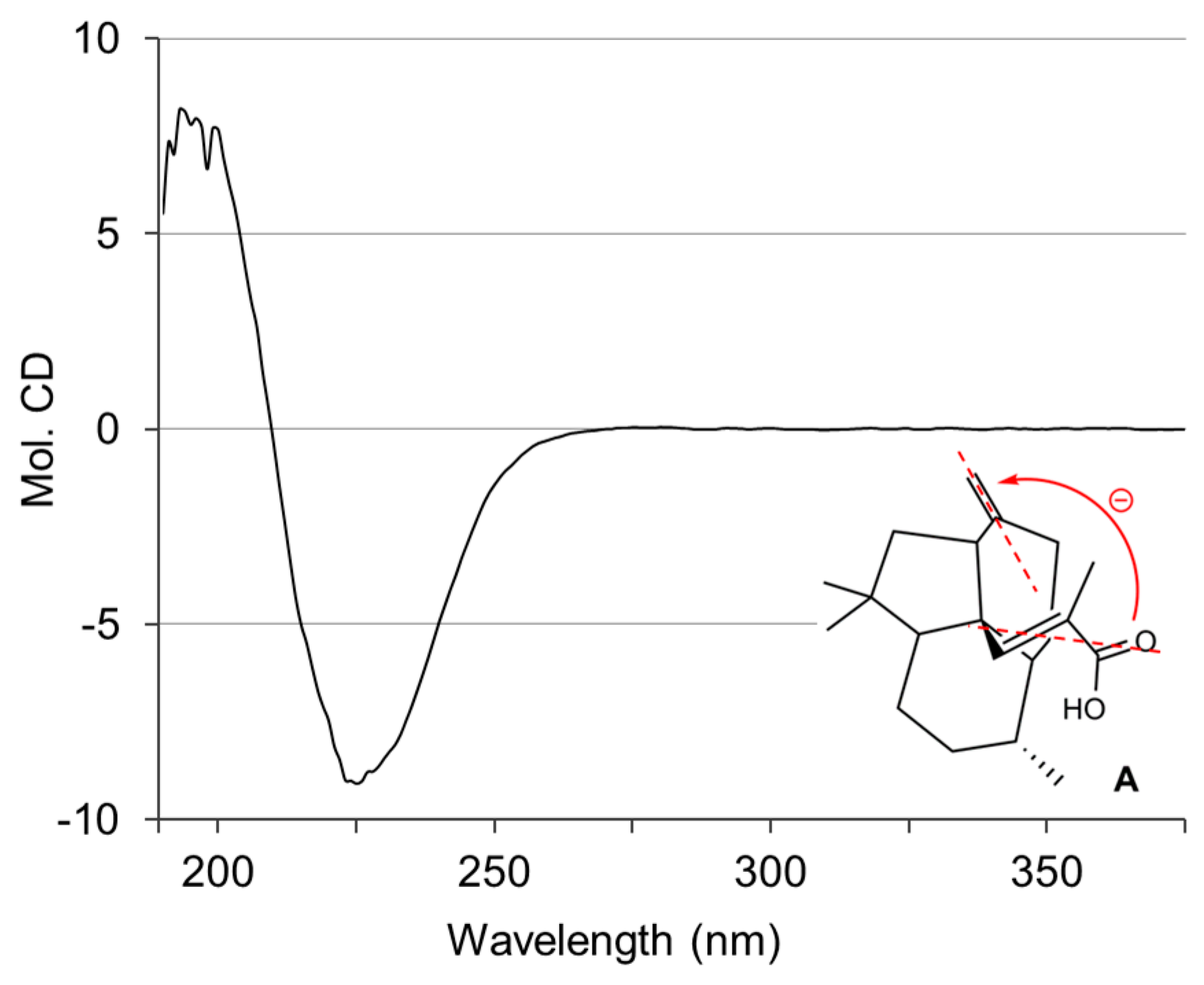
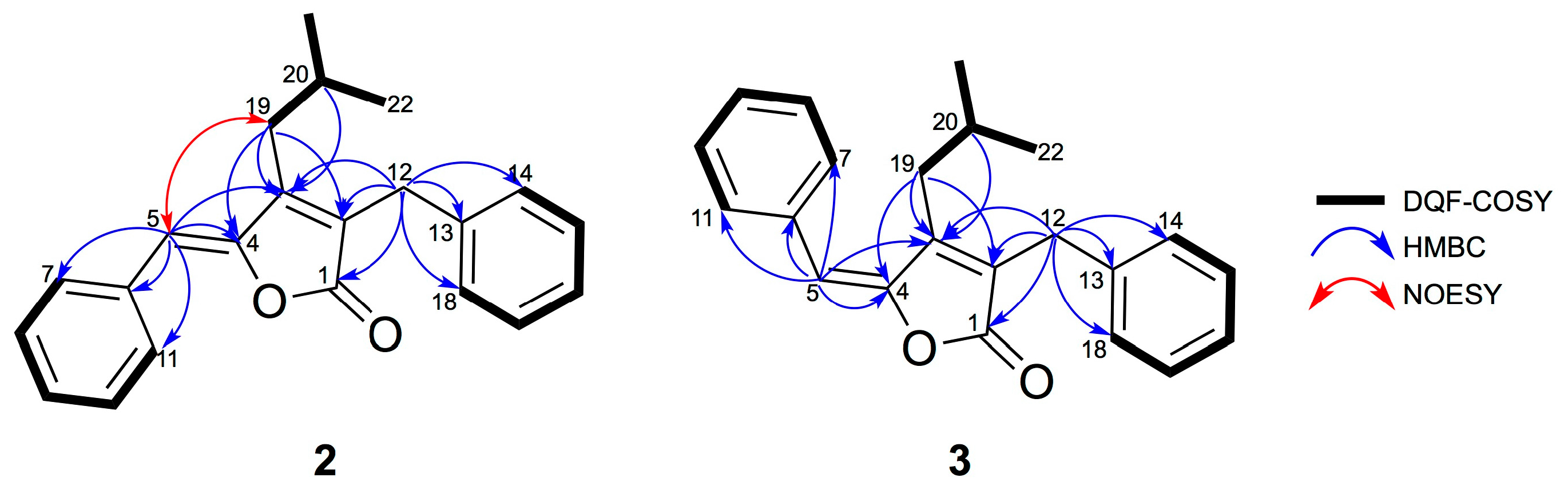
2.1. Isolation and Structural Elucidation of 1–3
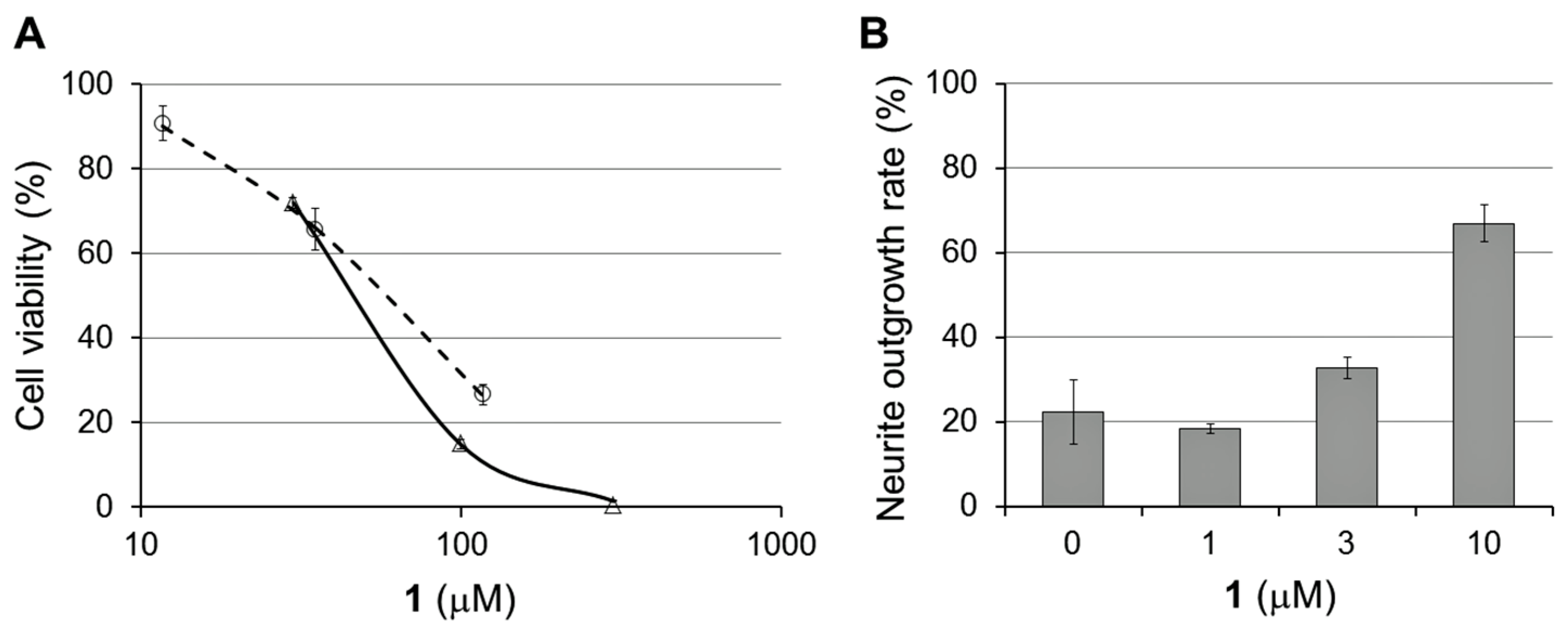
2.2. Bioactivities of 1–3
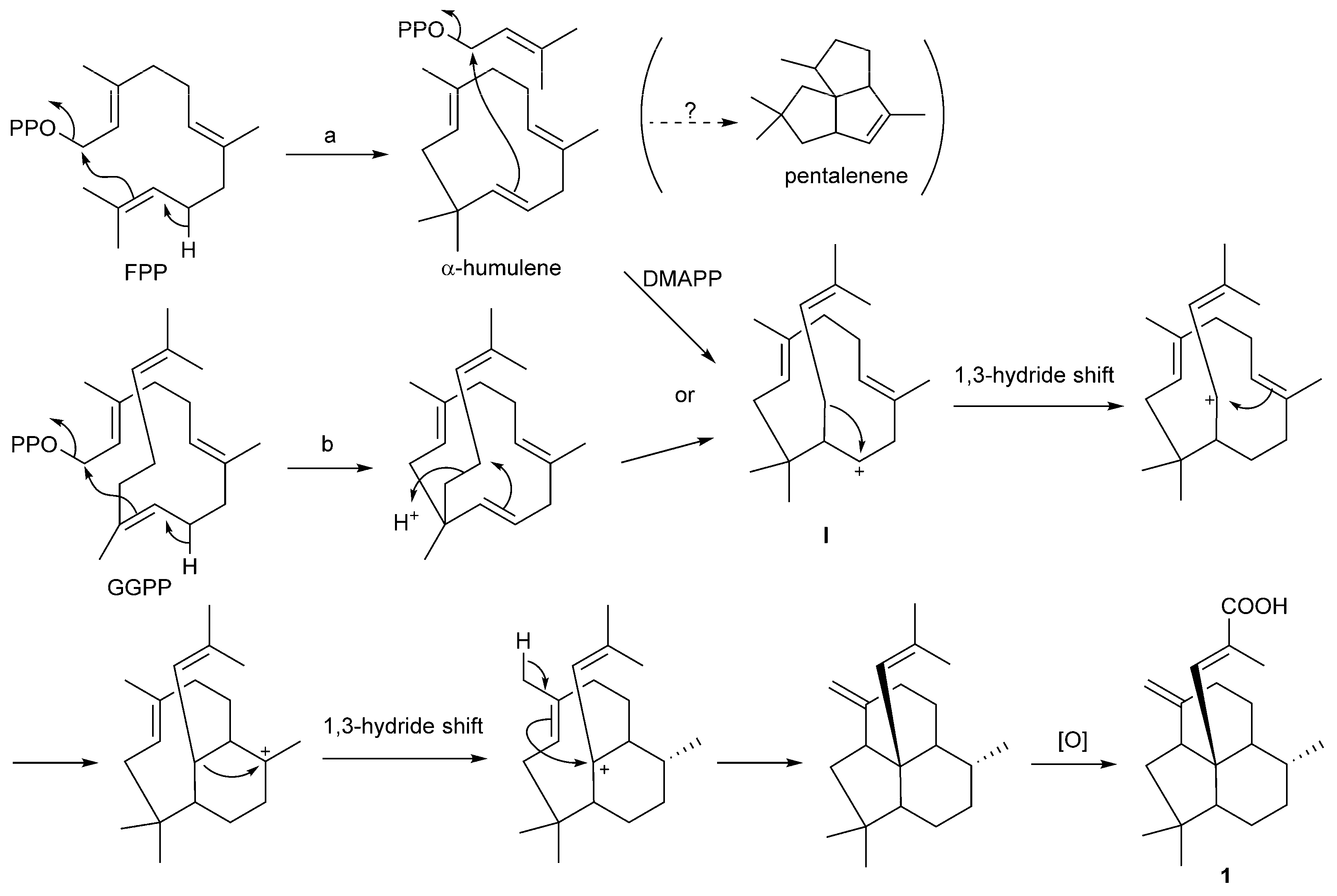
2.3. Putative Biosynthetic Mechanism for 1
3. Materials and Methods
3.1. General Procedures
3.2. Bacterial Strain
3.3. Isolation of 1–3
3.3.1. Enhygromic Acid (1)
3.3.2. Deoxyenhygrolide A (2)
3.3.3. Deoxyenhygrolide B (3)
3.4. Conformational Analysis
3.5. Cytotoxicity Test
3.6. NGF-Enhancing Activity for PC12 Cells
3.7. Anti-Phytophthora Assay
3.8. MIC Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reichenbach, H. The ecology of the myxobacteria. Environ. Microbiol. 1999, 1, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.C.; Müller, R. The biosynthetic potential of myxobacteria and their impact on drug discovery. Curr. Opin. Drug Discov. Dev. 2009, 12, 220–230. [Google Scholar]
- Weissman, K.J.; Müller, R. A brief tour of myxobacterial secondary metabolism. Bioorg. Med. Chem. 2009, 17, 2121–2136. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Céspedes, A.; Hufendiek, P.; Crüsemann, M.; Schäberle, T.F.; König, G.M. Marine-derived myxobacteria of the suborder Nannocystineae: An underexplored source of structurally intriguing and biologically active metabolites. Beilstein J. Org. Chem. 2016, 12, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Fudou, R.; Iizuka, T.; Nakajima, D.; Okazaki, K.; Shibata, D.; Ojika, M.; Harayama, S. PCR detection of type I polyketide synthase genes in myxobacteria. Appl. Environ. Microbiol. 2008, 74, 5571–5574. [Google Scholar] [CrossRef] [PubMed]
- Fudou, R.; Iizuka, T.; Yamanaka, S. Haliangicin, a novel antifungal metabolite produced by a marine myxobacterium 1. Fermentation and biological characteristics. J. Antibiot. 2001, 54, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Fudou, R.; Iizuka, T.; Sato, S.; Ando, T.; Shimba, N.; Yamanaka, S. Haliangicin, a novel antifungal metabolite produced by a marine myxobacterium 2. Isolation and structural elucidation. J. Antibiot. 2001, 54, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tomura, T.; Sato, J.; Iizuka, T.; Fudou, R.; Ojika, M. Isolation and biosynthetic analysis of haliamide, a new PKS-NRPS hybrid metabolite from the marine myxobacterium Haliangium ochraceum. Molecules 2016, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Fudou, R.; Jojima, Y.; Ogawa, S.; Yamanaka, S.; Inukai, Y.; Ojika, M. Miuraenamides A and B, novel antimicrobial cyclic depsipeptides from a new slightly halophilic myxobacterium: Taxonomy, production, and biological properties. J. Antibiot. 2006, 59, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ojika, M.; Inukai, Y.; Kito, Y.; Hirata, M.; Iizuka, T.; Fudou, R. Miuraenamides: Antimicrobial cyclic depsipeptides isolated from a rare and slightly halophilic myxobacterium. Chem. Asian J. 2008, 3, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Dreisigacker, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Wright, P.R.; Menche, D.; Schäberle, T.F.; König, G.M. Salimabromide: Unexpected chemistry from the obligate marine myxobacterium Enhygromyxa salina. Chem. Eur. J. 2013, 19, 9319–9324. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Schäberle, T.F.; König, G.M. Salimyxins and enhygrolides: Antibiotic, sponge-related metabolites from the obligate marine myxobacterium Enhygromyxa salina. ChemBioChem 2013, 14, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Schäberle, T.F.; Goralski, E.; Neu, E.; Erol, O.; Hölzl, G.; Dörmann, P.; Bierbaum, G.; König, G.M. Marine myxobacteria as a source of antibiotics–comparison of physiology, polyketide–type genes and antibiotic production of three new isolates of Enhygromyxa salina. Mar. Drugs 2010, 8, 2466–2479. [Google Scholar]
- Iizuka, T.; Jojima, Y.; Fudou, R.; Yamanaka, S. Isolation of myxobacteria from the marine environment. FEMS Microbiol. Lett. 1998, 169, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shimizu, Y.; Steiner, J.R.; Clardy, J. Nostoclide I and II, extracellular metabolites from a symbiotic cyanobacterium, Nostoc sp., from the lichen Peltigera canina. Tetrahedron Lett. 1993, 34, 761–764. [Google Scholar] [CrossRef]
- Mason, C.P.; Edwards, K.R.; Carlson, R.E.; Pignatello, J.; Gleason, F.K.; Wood, J.M. Isolation of chlorine-containing antibiotic from the freshwater cyanobacterium Scytonema hofmanni. Science 1982, 215, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Jojima, Y.; Hayakawa, A.; Fujii, T.; Yamanaka, S.; Fudou, R. Pseudenhygromyxa salsuginis gen. nov., sp. nov., a myxobacterium isolated from an estuarine marsh. Int. J. Syst. Exol. Microbiol. 2013, 63, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Furukawa, H.; Tomura, T.; Fudou, R.; Kaida, K.; Choi, B.K.; Imokawa, G.; Ojika, M. Bioactive maleic anhydrides and related diacids from the aquatic hyphomycete Tricladium castaneicola. J. Nat. Prod. 2015, 78, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Ojika, M.; Sakagami, Y. Linckosides A and B, two new neuritogenic steroid glycosides from the Okinawan starfish Linckia laevigata. Bioorg. Med. Chem. 2002, 10, 1961–1966. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard Ninth Edition, M07-A9; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard Second Edition, M27-A2; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Methods for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds; EUCAST, 2014. Available online: http://www.eucast.org/ (accessed on 6 April 2017).
- Da Silva Barros, M.E.; de Assis Santos, D.; Hamdan, J.S. Evaluation of susceptibility of Tricophyton mentagrophytes and Trichophyton rubrum clinical isolates to antifungal drugs using a modified CLSI microdilution method (M38-A). J. Med. Microbiol. 2007, 56, 514–518. [Google Scholar] [CrossRef] [PubMed]
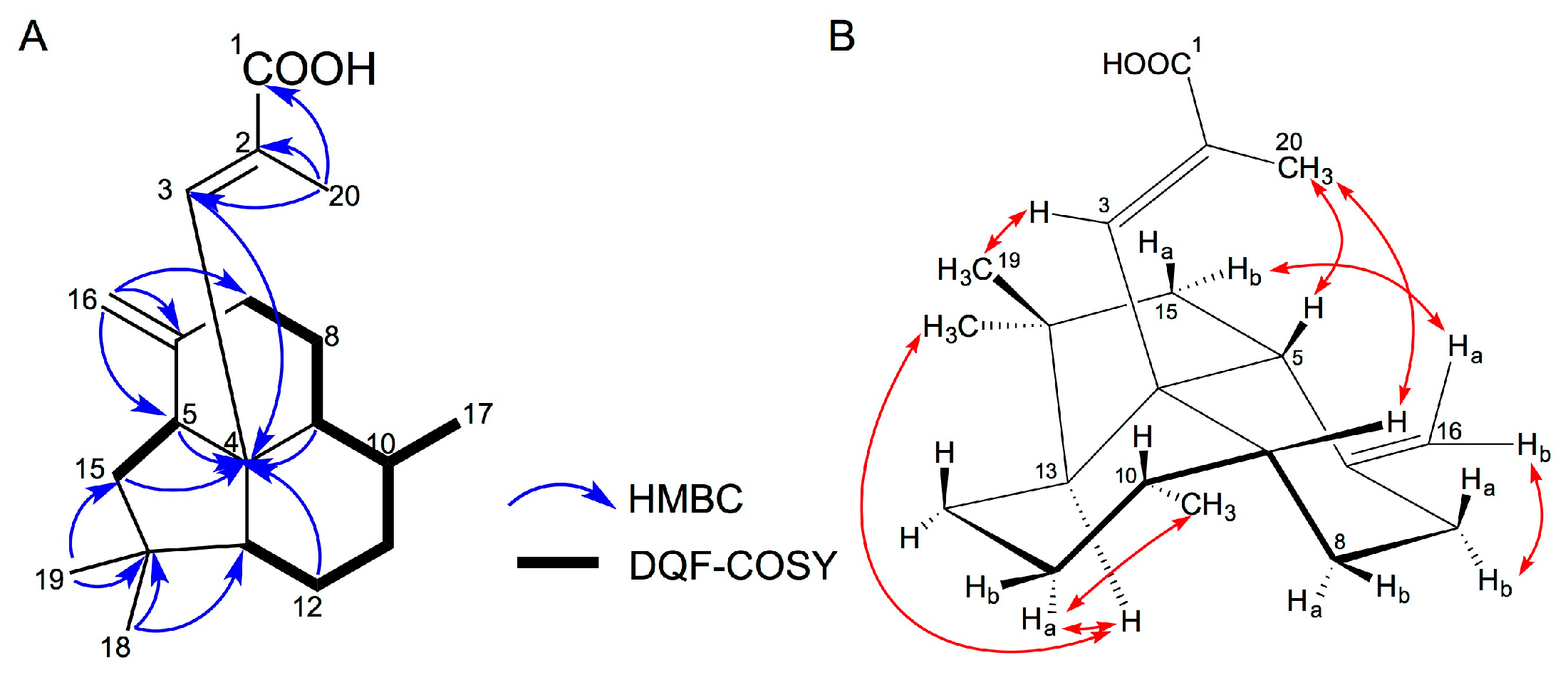
| Position | δC, Type | δH (J in Hz) | HMBC a |
|---|---|---|---|
| 1 | 170.2, C | ||
| 2 | 127.2 (br), C | ||
| 3 | 145.9 (br), CH | 7.27, s | 4 |
| 4 | 52.2, C | ||
| 5 | 46.7, CH | 2.81, brd (7.1) | 4 |
| 6 | 148.9, C | ||
| 7a | 35.3, CH2 | 2.08, m | |
| 7b | 2.39, dt (13.1, 3.0) | ||
| 8a | 21.7, CH2 | 1.36, m | |
| 8b | 1.55 m | ||
| 9 | 45.2, CH | 2.04, m | 4 |
| 10 | 31.7, CH | 1.55, m | |
| 11a | 29.4, CH2 | 1.09, m | |
| 11b | 1.42, m | ||
| 12 | 21.6, CH2 | 1.43, m; 1.45, m | 4 |
| 13 | 46.9, CH | 1.49, dd (12.7, 3.4) | |
| 14 | 35.6, C | ||
| 15a | 40.6, CH2 | 1.35, m | 4 |
| 15b | 1.92, m | ||
| 16a | 108.1, CH2 | 4.76, s | 5, 6, 7 |
| 16b | 4.85, d (1.5) | ||
| 17 | 19.6, CH3 | 0.76, d (6.9) | |
| 18 | 32.6, CH3 | 1.00, s | 13, 14 |
| 19 | 26.7, CH3 | 0.86, s | 14, 15 |
| 20 | 14.7, CH3 | 1.92, s | 1, 2, 3 |
| Deoxyenhygrolide A (2) | Deoxyenhygrolode B (3) | |||||
|---|---|---|---|---|---|---|
| Position | δC, Type | δH (J in Hz) | HMBC a | δC, Type | δH (J in Hz) | HMBC a |
| 1 | 169.8, C | 169.3, C | ||||
| 2 | 127.5, C | 132.5, C | ||||
| 3 | 152.0, C | 149.6, C | ||||
| 4 | 149.2, C | 150.2, C | ||||
| 5 | 109.0, CH | 5.70, s | 3, 4, 6, 7, 11 | 113.8, CH | 6.58, s | 3, 4, 6, 7, 11 |
| 6 | 133.8, C | 133.7, C | ||||
| 7, 11 | 130.7, CH | 7.74, d (7.5) | 129.5, CH | 6.82, m | ||
| 8, 10 | 129.0, CH | 7.12, t (7.5) | 128.3, CH | 6.96, m | ||
| 9 | 128.7, CH | 7.01, t (7.5) | 128.0, CH | 6.96, m | ||
| 12 | 30.1, CH2 | 3.51, s | 1, 2, 3, 13, 14, 18 | 30.2, CH2 | 3.56, s | 1, 2, 3, 13, 14, 18 |
| 13 | 138.4, C | 138.4, C | ||||
| 14, 18 | 128.9, CH | 7.18, d (7.5) | 128.9, CH | 7.25, d (7.5) | ||
| 15, 17 | 128.9, CH | 7.10, t (7.5) | 128.9, CH | 7.12, t (7.2) | ||
| 16 | 126.8, CH | 7.02, t (7.5) | 126.9, CH | 7.02, t (7.4) | ||
| 19 | 33.6, CH2 | 1.92, d (7.4) | 2, 3, 4 | 34.8, CH2 | 2.00, d (7.1) | 2, 3, 4 |
| 20 | 29.1, CH | 1.55, non (6.6) | 3 | 28.3, CH | 1.07, non (6.8) | 3 |
| 21, 22 | 22.6, CH3 | 0.62, d (6.6) | 22.0, CH3 | 0.33, d (6.6) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomura, T.; Nagashima, S.; Yamazaki, S.; Iizuka, T.; Fudou, R.; Ojika, M. An Unusual Diterpene—Enhygromic Acid and Deoxyenhygrolides from a Marine Myxobacterium, Enhygromyxa sp. Mar. Drugs 2017, 15, 109. https://doi.org/10.3390/md15040109
Tomura T, Nagashima S, Yamazaki S, Iizuka T, Fudou R, Ojika M. An Unusual Diterpene—Enhygromic Acid and Deoxyenhygrolides from a Marine Myxobacterium, Enhygromyxa sp. Marine Drugs. 2017; 15(4):109. https://doi.org/10.3390/md15040109
Chicago/Turabian StyleTomura, Tomohiko, Shiori Nagashima, Satoshi Yamazaki, Takashi Iizuka, Ryosuke Fudou, and Makoto Ojika. 2017. "An Unusual Diterpene—Enhygromic Acid and Deoxyenhygrolides from a Marine Myxobacterium, Enhygromyxa sp." Marine Drugs 15, no. 4: 109. https://doi.org/10.3390/md15040109