Terpenoids from Octocorals of the Genus Pachyclavularia
Abstract
:1. Introduction
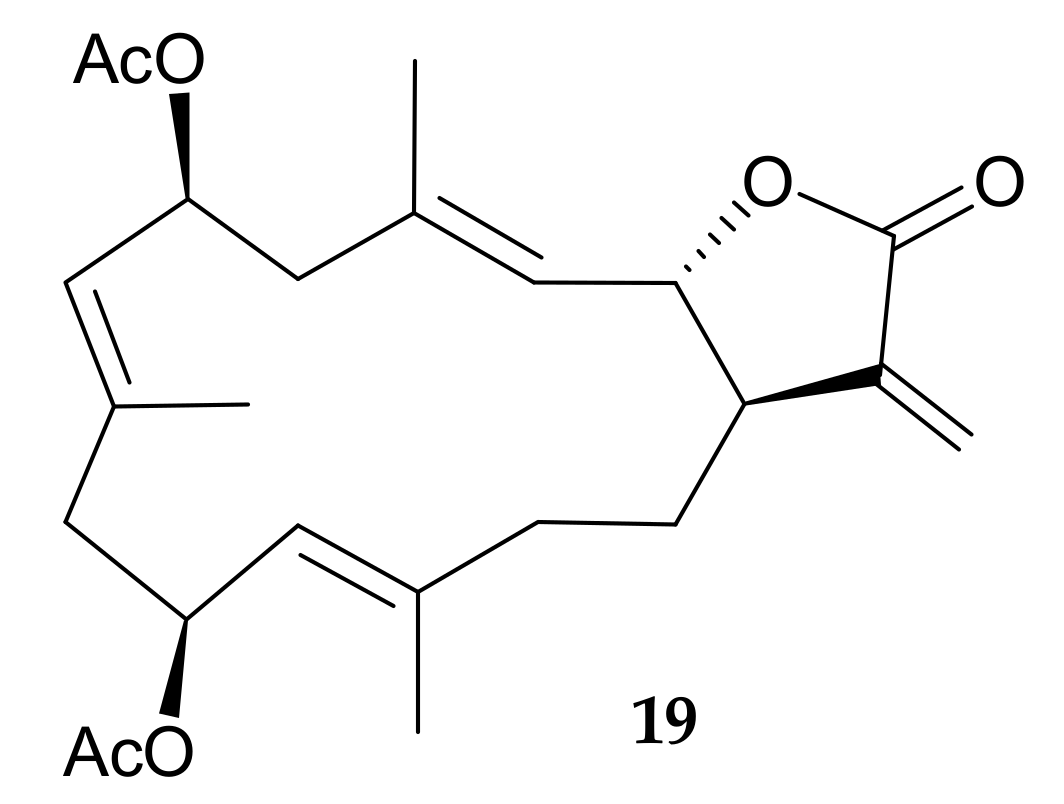
2. Cembranoids from Pachyclavularia violacea (Quoy and Gaimard, 1833) (=Clavularia violacea, Briareum violaceum)
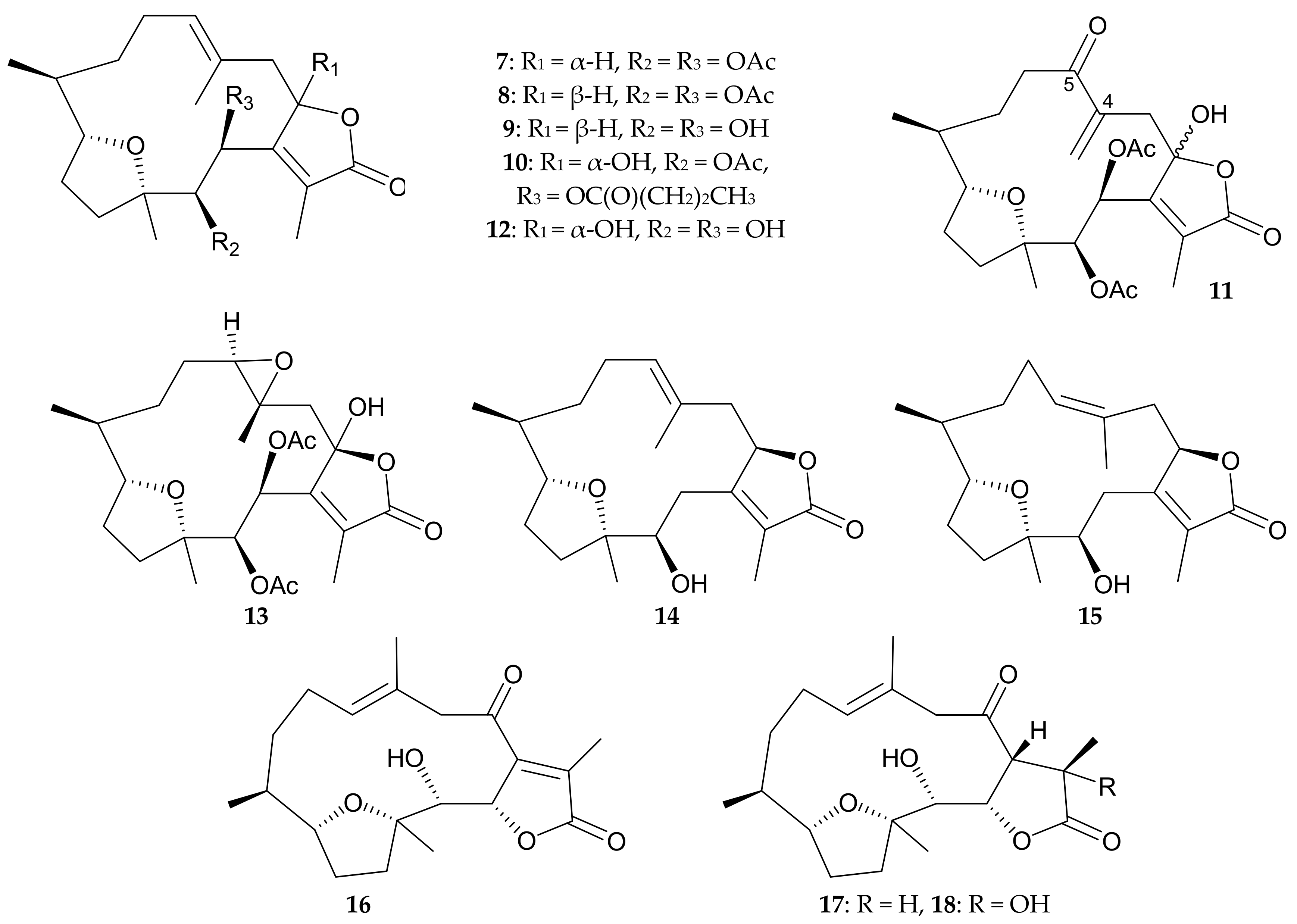
3. Briaranes from Pachyclavularia sp. and Pachyclavularia violacea
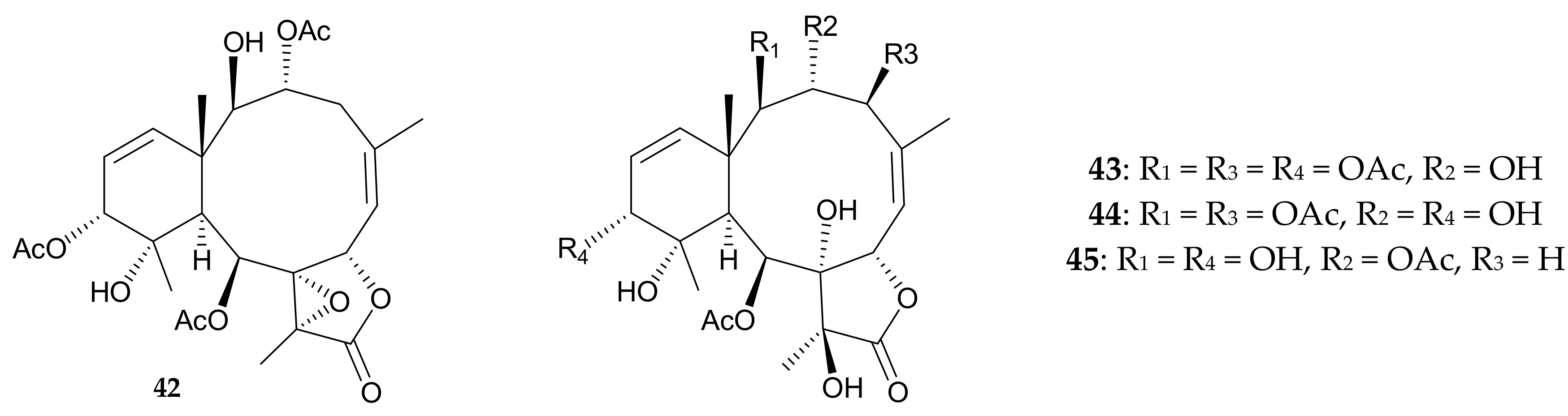
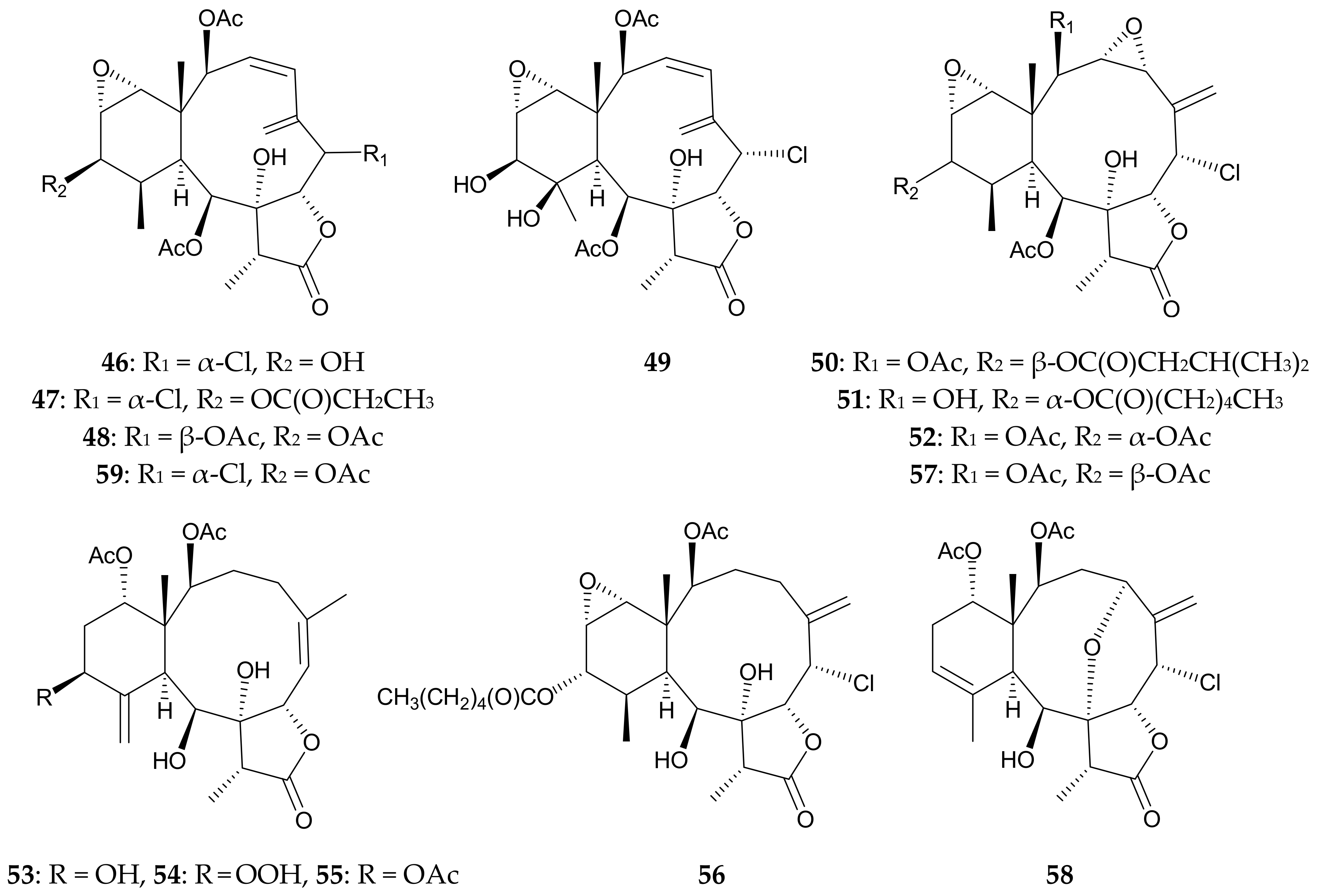
4. Briarellins from Pachyclavularia violacea
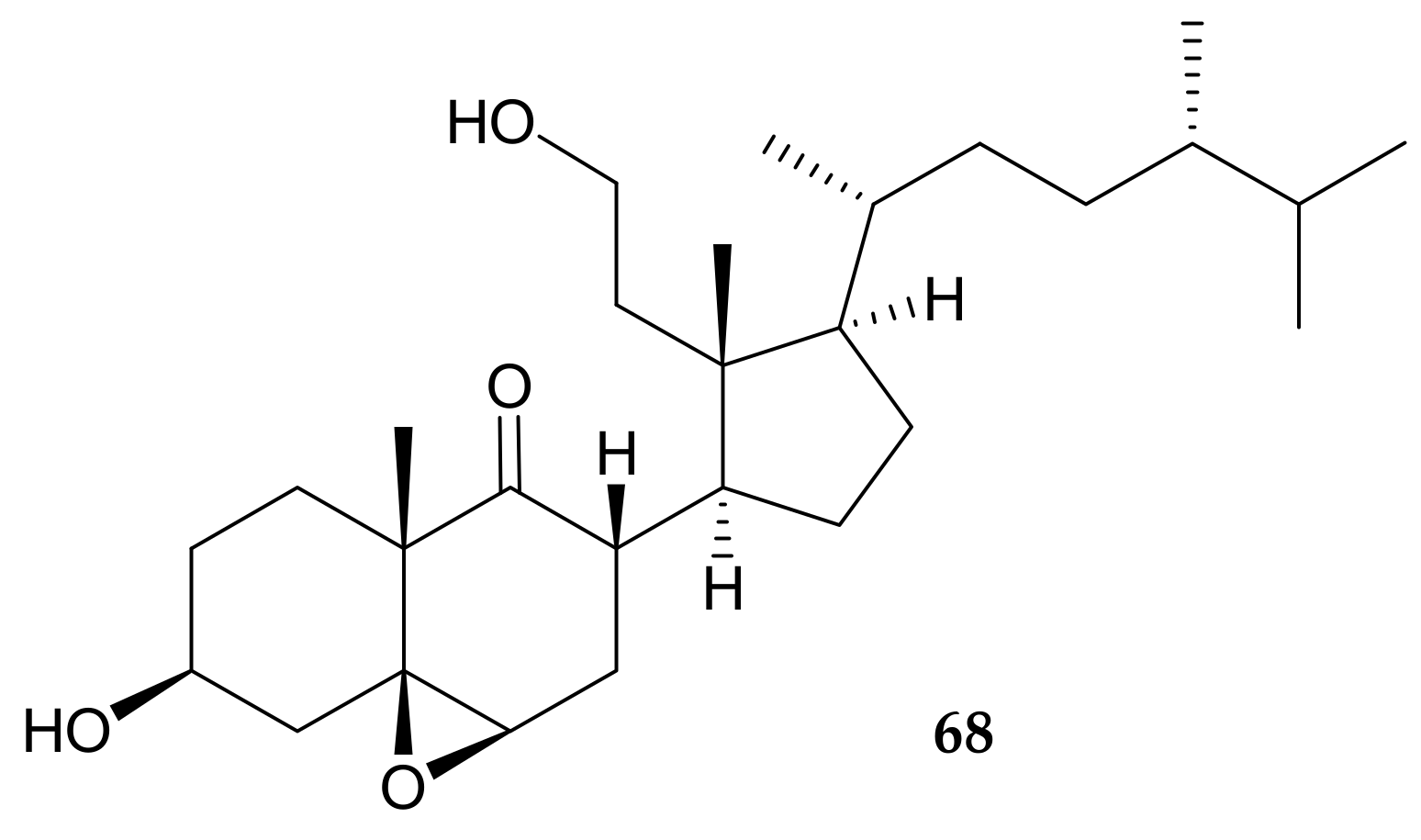
5. Secosterol from Pachyclavularia violacea
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. ZooKeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Raston, C.L.; Stokie, G.J.; White, A.H. Studies of Australian soft corals. XV. The structure of pachyclavulariadiol, a novel furano-diterpene from Pachyclavularia violacea. Aust. J. Chem. 1979, 32, 2265–2274. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, M.-C. The heterocyclic natural products of gorgonian corals of genus Briareum exclusive of briarane-type diterpenoids. Heterocycles 2002, 57, 1705–1715. [Google Scholar] [CrossRef]
- Inman, W.; Crews, P. The structure and conformational properties of a cembranolide diterpene from Clavularia violacea. J. Org. Chem. 1989, 54, 2526–2529. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Wang, G.-H.; Sung, P.-J.; Duh, C.-Y.; Chiang, M.Y. Pachyclavulariolides G–L and secopachyclavulariaenone A, seven novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron 2001, 57, 7639–7648. [Google Scholar] [CrossRef]
- Xu, L.; Patrick, B.O.; Roberge, M.; Allen, T.; van Ofwegen, L.; Andersen, R.J. New diterpenoids from the octocoral Pachyclavularia violacea collected in Papua New Guinea. Tetrahedron 2000, 56, 9031–9037. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Wang, G.-H.; Duh, C.-Y.; Soong, K. Pachyclavulariolides M–R, six novel diterpenoids from a Taiwanese soft coral Pachyclavularia violacea. J. Nat. Prod. 2003, 66, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.-Y.; El-Gamal, A.A.H.; Chu, C.-J.; Wang, S.-K.; Dai, C.-F. New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea. J. Nat. Prod. 2002, 65, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, I.-C.; Chiang, M.Y.-N.; Hwang, T.-L.; Kung, T.-H.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Briaviodiol A, a new cembranoid from a soft coral Briareum violacea. Chem. Pharm. Bull. 2010, 58, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Fukazawa, Y.; Bowden, B.F.; Coll, J.C. New diterpenes from an Australian Pachyclavularia species (Coelenterata, Anthozoa, Octocorallia). Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1989, 31, 548–553. [Google Scholar]
- Cardellina, J.H., II; James, T.R., Jr.; Chen, M.H.M.; Clardy, J. Structure of brianthein W, from the soft coral Briareum polyanthes. J. Org. Chem. 1984, 49, 3398–3399. [Google Scholar] [CrossRef]
- Pordesimo, E.O.; Schmitz, F.J.; Ciereszko, L.S.; Hossain, M.B.; van der Helm, D. New briarein diterpenes from the Caribbean gorgonians Erythropodium caribaeorum and Briareum sp. J. Org. Chem. 1991, 56, 2344–2357. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; König, G.M. Studies of Australian soft corals. XLVIII. New briaran diterpenoids from the gorgonian coral Junceella gemmacea. Aust. J. Chem. 1990, 43, 151–159. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Wratten, S.J.; Faulkner, D.J. Some diterpenes from the sea pen Stylatula sp. Tetrahedron 1979, 35, 1907–1912. [Google Scholar] [CrossRef]
- Iwasaki, J.; Ito, H.; Aoyagi, M.; Sato, Y.; Iguchi, K. Briarane-type diterpenoids from the Okinawan soft coral Pachyclavularia violacea. J. Nat. Prod. 2006, 69, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Iwasaki, J.; Sato, Y.; Aoyagi, M.; Iguchi, K.; Yamori, T. Marine diterpenoids with a briarane skeleton from the Okinawan soft coral Pachyclavularia violacea. Chem. Pharm. Bull. 2007, 55, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, J.; Ito, H.; Nakamura, M.; Iguchi, K. A synthetic study of briarane-type marine diterpenoid, pachyclavulide B. Tetrahedron Lett. 2006, 47, 1483–1486. [Google Scholar] [CrossRef]
- Ishiyama, H.; Okubo, T.; Yasuda, T.; Takahashi, Y.; Iguchi, K.; Kobayashi, J. Brianodins A–D, briarane-type diterpenoids from soft coral Pachyclavularia sp. J. Nat. Prod. 2008, 71, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Takayama, K.; Okamura, H.; Nakatani, M.; Doe, M.; Takemura, K.; Shiro, M. Cytotoxic briarane diterpenes from a gorgonacean Briareum sp. Heterocycles 1999, 51, 2619–2625. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hirose, T.; Takayama, K.; Okamura, H.; Nakatani, M.; Doe, M.; Takemura, K. Violides N–P, new briarane diterpenes from a gorgonacean Briareum sp. Heterocycles 2000, 53, 1789–1792. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M.Y.; Sheu, J.-H. Briaexcavatolides K–N, new briarane diterpenes from the gorgonian Briareum excavatum. J. Nat. Prod. 2001, 64, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Hu, W.-P.; Wu, S.-L.; Su, J.-H.; Fang, L.-S.; Wang, J.-J.; Sheu, J.-H. Briaexcavatolides X–Z, three new briarane-related derivatives from the gorgonian coral Briareum excavatum. Tetrahedron 2004, 60, 8975–8979. [Google Scholar] [CrossRef]
- Iwagawa, T.; Babazono, K.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Briarane diterpenes from a gorgonian Briareum sp. Heterocycles 2005, 65, 607–617. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, Y.-D.; Li, G.-Y.; Chiang, M.Y.; Lin, M.-R.; Huang, I.-C.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Excavatoids A–D, new polyoxygenated briaranes from the octocoral Briareum excavatum. Tetrahedron 2009, 65, 6918–6924. [Google Scholar] [CrossRef]
- Groweiss, A.; Look, S.A.; Fenical, W. Solenolides, new antiinflammatory and antiviral diterpenoids from a marine octocoral of the genus Solenopodium. J. Org. Chem. 1988, 53, 2401–2406. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Cheng, M.-C.; Liu, H.-Y.; Fang, L.-S.; Duh, C.-Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A–E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Liu, H.-Y.; Duh, C.-Y.; Chiang, M.Y. Briaexcavatolides A–J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Cheng, Y.-B.; Lin, Y.-S.; Kuo, Y.-H.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from Taiwanese soft coral Briareum violacea. Mar. Drugs 2014, 12, 4677–4692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-F.; Yamamura, S.; Terada, Y. Stereochemistry of the brianolide acetate (=solenolide D) by the molecular mechanics calculations. Tetrahedron Lett. 1992, 33, 101–104. [Google Scholar] [CrossRef]
- Wang, G.-H.; Sheu, J.-H.; Chiang, M.Y.; Lee, T.-J. Pachyclavulariaenones A–C, three novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron Lett. 2001, 42, 2333–2336. [Google Scholar] [CrossRef]
- Wang, G.-H.; Sheu, J.-H.; Duh, C.-Y.; Chiang, M.Y. Pachyclavulariaenones D–G, new diterpenoids from the soft coral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Anta, C.; González, N.; Rodríguez, J.; Jiménez, C. A new secosterol from the Indonesian octocoral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chang, P.-J.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-type diterpenoids-Part II. Heterocycles 2005, 65, 195–204. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R.; et al. Survey of briarane-type diterpenoids-Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-type diterpenoids-Part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C.; Su, J.-H.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; et al. Briarane diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Terpenoids from Octocorals of the Genus Pachyclavularia. Mar. Drugs 2017, 15, 382. https://doi.org/10.3390/md15120382
Chang Y-C, Sheu J-H, Wu Y-C, Sung P-J. Terpenoids from Octocorals of the Genus Pachyclavularia. Marine Drugs. 2017; 15(12):382. https://doi.org/10.3390/md15120382
Chicago/Turabian StyleChang, Yu-Chia, Jyh-Horng Sheu, Yang-Chang Wu, and Ping-Jyun Sung. 2017. "Terpenoids from Octocorals of the Genus Pachyclavularia" Marine Drugs 15, no. 12: 382. https://doi.org/10.3390/md15120382




