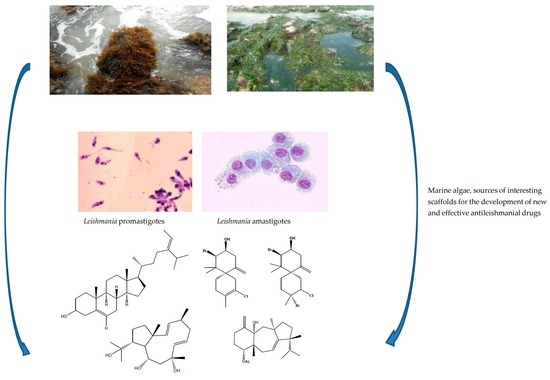
Marine Algae as Source of Novel Antileishmanial Drugs: A Review
Abstract
:1. Introduction
2. Marine Algae
3. Current Status of Antileishmanial Drug Discovery from Marine Macroalgae
3.1. Macroalgae with Antileishmanial Properties
3.2. Isolated Compounds from Marine Macroalgae Screened for Antileishmanial Properties
4. Approaches Used for Assessment of Antileishmanial Activity by the Authors
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steverdind, D. The history of Leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, P.D.; Alonso, A.; Larraga, V. Genome-wide gene expression profile induced by exposure to cadmium acetate in Leishmania infantum promastigotes. Int. Microbiol. 2011, 14, 1–11. [Google Scholar] [PubMed]
- Khraiwesh, M.; Leed, S.; Roncal, N.; Johnson, J.; Sciotti, R.; Smith, P.; Rrad, L.; Paris, R.; Hudson, T.; Hickman, M.; et al. Antileishmanial activity of compounds derived from the Medicines for Malaria Venture Open Access Box against intracellular Leishmania major amastigotes. Am. J. Trop. Med. Hyg. 2016, 94, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Armeli Minicante, S.; Michelet, S.; Bruno, F.; Castelli, G.; Vitale, F.; Sfriso, A.; Morabito, M.; Genovese, G. Bioactivity of phycocolloids against the mediterranean protozoan Leishmania infantum: An inceptive study. Sustainability 2016, 8, 1131. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 5, e35671. [Google Scholar] [CrossRef] [PubMed]
- Savoia, D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries 2015, 6, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.K.; Sen, P.; Roy, S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol. Biol. Int. 2011. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Control of the Leishmaniasis: Report of the Meeting of the WHO Expert Committee on the Control of Leishmaniases; WHO Technical Report Series; WHO: Geneva, Switzerland, 2010; pp. 22–26. [Google Scholar]
- Singh, O.P.; Singh, B.; Chakravarty, J.; Sundar, S. Current challenges in treatment options for visceral leishmaniasis in India: A public health perspective. Infect. Dis. Poverty 2016, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Lockwood, D.N. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151–158. [Google Scholar] [PubMed]
- Mishra, J.; Dey, A.; Singh, N.; Somvanshi, R.; Singh, S. Evaluation of toxicity and therapeutic efficscy of a new liposomal formulation of amphotericin B in a mouse model. Indian J. Med. Sci. 2013, 137, 767–776. [Google Scholar]
- Lakshmi, V.; Khare, P.; Misra, P.; Dube, A. Antileishmanial potential of a marine sponge Haliclona oculata against experimental visceral Leishmaniasis. J. Coast. Life Med. 2015, 3, 187–192. [Google Scholar]
- Meena, A.K.; Kandale, A.; Rao, M.M.; Panda, P.; Kaur, K.P. A Review on Leishmaniasis and treatment with natural drugs. Available online: https://www.researchgate.net/publication/234002706 (accessed on 20 October 2017).
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; de Palmer Paixão, I.C.N.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl. Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef] [PubMed]
- Dalimi, A.; Delavari, M.; Ghaffarifar, F.; Sadraei, J. In vitro and in vivo antileishmanial effects of aloe-emodin on Leishmania major. J. Tradit. Complement. Med. 2015, 5, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.A.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities: Affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Exp. Mar. Biol. Ecol. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Aenderson, H.J.; Hansen, E.; Albrigtsen, M.; Escarlera, L.; Esposito, F.; Helland, K.; Hansen, Ø.K.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-Diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Bajpai, V.K. Antimicrobial bioactive compounds from marine algae: A mini review. Indian J. Geomar. Sci. 2016, 45, 1076–1085. [Google Scholar]
- Singh, R.D.; Mody, S.K.; Patel, H.B.; Devi, S.; Sarvaiya, V.N.; Patel, H.A.; Patel, B.R. Antimicrobial drug discovery: Evident shifting from terrestrial to marine micro-organisms. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2322–2327. [Google Scholar] [CrossRef]
- Rovirosa, J.; Sepulveda, M.; Quezada, E.; San-Martin, A. Isoepitaondiol, a diterpenoid of Stypopodium flabelliforme and the insecticidal activity of stypotriol Epitaondiol and derivatives. Phytochemistry 1992, 31, 2679–2681. [Google Scholar] [CrossRef]
- Sabina, H.; Tasneem, S.; Kausar, Y.; Choudhary, M.I.; Aliya, R. Antileishmanial activity in the crude extract of various seaweed from the coast of Karachi, Pakistan. Pakistan J. Bot. 2005, 37, 163–168. [Google Scholar]
- dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Filho, B.P.D.; Sudatti, B.D.; Bianco, M.É.; Pereira, C.R.; Nakamura, V.C. Effect of Elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.A.E.; Passalacqua, T.G.; Velásquez, A.M.A.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A.S. New drugs with antiprotozoal activity from marine algae: A review. Rev. Bras. Farmacogn. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- Gallé, J.B.; Attioua, B.; Kaiser, M.; Rusig, A.M.; Lobstein, A.; Vonthron-Sénécheau, C. Eleganolone, a diterpene from the french marine alga Bifurcaria bifurcata inhibits growth of the human pathogens Trypanosoma brucei and Plasmodium falciparum. Mar. Drugs 2013, 11, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bianco, E.M.; de Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Guimaraes, T.R.; Bittencourt, F.; Gouvea, L.P.; Aresi, C.; de Almeida, R.M.T.; Moritz, G.M.I.; et al. Anti-infective potential of marine invertebrates and seaweeds from the brazilian coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Custódio, L.; Rodrigues, M.J.; de Sousa, C.B.; Oliveira, M.; Barreira, L.; Neng, D.N.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological activities and chemical composition of methanolic extracts of selected autochthonous microalgae strains from the red sea. Mar. Drugs 2015, 13, 3531–3549. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-Derived Biofuel: A Review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef] [Green Version]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Haimeur, A.; Guéno, F.; Meskini, N.; Tremblin, G. Marine microalgae used as food supplements and their implication in preventing cardiovascular diseases. OCL 2012, 22, 4. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kyoung, H.K.; Seo, H.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed]
- Noel, V.T.; Se-Kwon, K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar]
- Anand, N.; Rachel, D.; Thangaraju, N.; Anantharaman, P. Potential of marine algae (seaweeds) as source of medicinally important compounds. Plant Genet. Resour. 2016, 14, 303–313. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Sahayaraj, K. Biological values and conservation of marine algae: An overview. In Proceedings of the Conservation and Sustainable Utilization of Marine Resources, National Conference on Conservation and Sustainable Utilization of Marine Resources, Tamil Nadu, India, 22–23 January 2015; pp. 21–24. [Google Scholar]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Gerald, B.; Tasdemir, D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from british and irish waters. Phytother. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Vonthron-Sénécheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.M. Antiprotozoal activities of organic extracts from French marine seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Ainane, T.; Abourriche, A.; Bennamara, A.; Charrouf, M.; Lemrani, M. Anti-leishmanial activity of extracts from a brown seaweed Bifurcaria bifurcata the Atlantic coast of Morocco. Phytothérapie 2015, 1–6. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.D.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane diterpene from the Brazilian brown alga Canistrocarpus cervicornis as antileishmanial agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.B.; Katkam, G.N.; Morais, T.R.; Conserva, G.A.A.; Vizetto-Duarte, C.; Pereira, H.; Laurenti, M.D.; Campino, L.; Levy, D.; Uemi, M.; et al. Antileishmanial activity of meroditerpenoids from the macroalgae Cystoseira baccata. Exp. Parasitol. 2017, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Süzgeç-Selçuk, S.; Mericli, A.H.; Guven, K.C.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Tasdemir, D. Evaluation of Turkish seaweeds for antiprotozoal, antimycobacterial and cytotoxic activities. Phytother. Res. 2011, 25, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Freile-Pelegrin, Y.; Robledo, D.; Chan-Bacab, M.J.; Ortega-Morales, B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia 2008, 79, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Lira, M.L.F.; Lopes, R.; Gomes, A.P.; Barcellos, G.; Verícimo, M.; Osako, K.; Ortiz-Ramirez, F.A.; Ramos, C.J.B.; Cavalcanti, D.N.; Teixeira, V.L.; et al. Anti-leishmanial activity of Brazilian green, brown, and red algae. J. Appl. Phycol. 2015, 28, 591–598. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B.; Atici, T.; Brun, R.; Perozzo, R.; Tasdemir, D. Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine 2006, 13, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.A.S.; dos Anjos, K.F.; de Vasconcelos, R.T.N.; Higino, T.M.; Brelaz-de-Castro, M.C.; Bianco, E.M.; de Figueiredo, R.C. The in vitro biological activity of the Brazilian brown seaweed Dictyota mertensii against Leishmania amazonensis. Molecules 2014, 19, 14052–14065. [Google Scholar]
- Fouladvand, M.; Barazesh, A.; Farokhzad, F.; Malekizadeh, H.; Sartavi, K. Evaluation of in vitro anti-leishmanial activity of some brown, green and red algae from the Persian Gulf. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 597–600. [Google Scholar] [PubMed]
- Soares, D.C.; Szlachta, M.M.; Teixeira, V.L.; Soares, A.R.; Saraiva, E.M. The brown alga Stypopodium zonale (Dictyotaceae): A potential source of Anti-Leishmania drugs. Mar. Drugs 2016, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Tedone, L.; Hamann, M.; Morabito, M. The Mediterranean Red Alga Asparagopsis: A Source of Compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Genovese, G.; Bruno, F.; Castelli, G.; Piazza, M.; Migliazzo, A.; Morabito, M. Effectiveness of the red alga Asparagopsis taxiformis extracts against Leishmania infantum. Open Life Sci. 2015, 10, 490–496. [Google Scholar] [CrossRef]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three british and irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Sabina, H.; Aliya, R. Bioactive assessment of selected marine red algae against Leishmania major and chemical constituents of Osmundea pinnatifida. Pak. J. Bot. 2011, 43, 3053–3056. [Google Scholar]
- De Felício, R.; de Albuquerque, S.; Young, M.C.M.; Yokoya, N.S.; Debonsia, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.G.; Fidalgo, L.M.; Pasarón, O.C.; Delgado, N.G.; Hernández, A.P. Antileishmanial activity of six extracts from marine organisms. Rev. Cuba. Med. Trop. 2012, 64, 61–64. [Google Scholar]
- Lakshmi, V.; Khare, P.; Misra, P.; Srivastava, M.N.; Dube, A. Antileishmanial potential of Chondrococcus hornemanni against experimental visceral leishmaniasis. J. Mar. Biol. Oceanogr. 2014, 3, 4. [Google Scholar]
- Da Silva Machado, F.L.; Pacienza-Lima, W.; Duarte, H.M.; Rossi-Bergmann, B.; Gestinari, L.M.; Fujii, M.T.; Kaiser, C.R.; Soares, A.R. Chemical diversity and antileishmanial activity of crude extracts of Laurencia complex (Ceramiales, Rhodophyta) from Brazil. Rev. Bras. Farmacogn. 2014, 24, 635–643. [Google Scholar] [CrossRef]
- Da Silva Machado, F.L.; Pacienza-Limac, W.; Rossi-Bergmann, B.; de Souza Gestinari, L.M.; Fujii, M.T.; de Paula, J.C.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea. Planta Med. 2011, 77, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of some british green algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Roesener, H.U.; Koch, M.L. Antibiotics from algae. Part 16. Polyhydroxyoligophenyls and phenyl ether from Bifurcaria bifurcata. Phytochemistry 1976, 15, 1279–1281. [Google Scholar] [CrossRef]
- Valls, R.; Piovetti, L.; Deffo, P. Analysis of sterols and diterpenoids of brown algae (Cystoseiraceae). Oceanis 1991, 17, 305–307. [Google Scholar]
- Culioli, G.; Ortalo-Magne, A.; Daoudi, M.; Thomas-Guyon, H.; Valls, R.; Piovetti, L. Trihydroxylated linear diterpenes from the brown alga Bifurcaria bifurcata. Phytochemistry 2004, 65, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Ortalo-Magne, A.; Culioli, G.; Valls, R.; Pucci, B.; Piovetti, L. Polar acyclic diterpenoids from Bifurcaria bifurcata (Fucales, Phaeophyta). Phytochemistry 2005, 66, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Göthel, Q.; Lichte, E.; Köck, M. Further eleganolone-derived diterpenes from the brown alga Bifurcaria bifurcata. Tetrahedron Lett. 2012, 53, 1873–1877. [Google Scholar] [CrossRef]
- Meena, A.K.; Kandale, A.; Nigam, S.; Panda, P.; Singh, B.; Rao, M. Review on Marine organisms with antileishmanial activity. J. Pharm. Res. 2010, 3, 818–821. [Google Scholar]
- Suzuki, M.; Vairappan, C.S. Halogenated secondary metabolites from Japanese species of the red algal genus Laurencia (Rhodomelaceae, Ceramiales). Curr. Top. Phytochem. 2005, 7, 1–34. [Google Scholar]
- Alarif, W.M.; Al-Lihaibi, S.S.; Abdel-Lateff, A.; Ayyad, S.E. New antifungal cholestane and aldehyde derivatives from the red alga Laurencia papillosa. Nat. Prod. Commun. 2011, 6, 1821–1824. [Google Scholar] [PubMed]
- Al-Massarani, S.M. Phytochemical and biological properties of sesquiterpene constituents from the marine red seaweed Laurencia: A review. Nat. Prod. Chem. Res. 2014, 2, 147. [Google Scholar]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological activities of two macroalgae from Adriatic coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Yung-Qing, T.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva lactuca and its polysaccharides: Food and biomedical aspects. J. Biol. Agric. Healthc. 2016, 6, 2224–3208. [Google Scholar]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a new antiprotozoal acyclic diterpene from the brown alga Bifurcaria bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.; Boutefnouchet, S.; Córdoba, O.; Vitorino, G.P.; Brehu, L.; Lamour, I.; Laimay, F.; Efstathiou, A.; Smirlis, D.; Michel, S.; et al. Antileishmanial activity of fucosterol recovered from Lessonia vadosa Searles (Lessoniaceae) by SFE, PSE and CPC. Phytochem. Lett. 2015, 11, 418–423. [Google Scholar] [CrossRef]
- Kar, S.; Sharma, G.; Das, P.K. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J. Antimicrob. Chemother. 2011, 66, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt Pires, C.; Rodrigues, S.D.; Bristot, D.; Gaeta, H.H.; Toyama, D.O.; Farias, W.R.L.; Toyama, M.H. Evaluation of macroalgae sulfated polysaccharides on the Leishmania (L.) amazonensis Promastigote. Mar. Drugs 2013, 11, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Vallim, M.A.; Paula, J.C.D.; Pereira, R.C.; Teixeira, V.L. The diterpenes from Dictyotacean marine brown algae in the Tropical Atlantic American region. Biochem. Syst. Ecol. 2015, 33, 1–16. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.; Moo-Puc, R.; Freile-Pelegrín, Y.; Robledo, D. Cytotoxic and antiproliferative constituents from Dictyota ciliolata, Padina sanctae-crucis and Turbinaria tricostata. Pharm. Biol. 2014, 52, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Moura, L.; Almeida, A.C.M.; Domingos, T.F.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Teixeira, V.L.; Fuly, A.L. Antiplatelet and anticoagulant effects of diterpenes isolated from the marine alga, Dictyota menstrualis. Mar. Drugs 2014, 12, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Duarte, H.M.; Tinnoco, L.W.; Pereira, R.C.; Teixeira, V.L. Intraspecific variation of meroditerpenoids in the brown alga Stypopodium zonale guiding the isolation of new compounds. Rev. Bras. Farmacogn. 2015, 25, 627–633. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Jing, H.J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, A.F.; Samimi, H.; Jamshidi, H.R.; Dehghani, A.; Zarezadeh, R. Antileishmanial activity of Yazd Spirogyra spp extracts against Leishmania (L.) major [MRHO/IR/75/ER] promastigotes: An in vitro study. J. Chem. Pharm. Res. 2017, 9, 112–117. [Google Scholar]
- Bodley, A.L.; McGarry, M.W.; Shapiro, T.A. Drug cytotoxicity assay for African Trypanosomes and Leishmania Species. J. Infect. Dis. 1995, 172, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- De Muylder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, C.J.; Mckerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparaison to a promastigote screen and indentification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef] [PubMed]
- De Rycker, M.; Hallyburton, I.; Thomas, J.; Campbell, L.; Wyllie, S.; Joshi, D.; Cameron, S.; Gilbert, H.I.; Watt, G.P.; Frearson, A.J.; et al. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrob. Agents Chemother. 2013, 57, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- França, P.H.B.; da Silva-Júnior, E.F.; Santos, B.V.O.; Alexandre-Moreira, M.S.; Quintans-Júnior, L.J.; de Aquino, T.M.; de Araújo-Júnior, J.X. Antileishmanial marine compounds: A review. Rec. Nat. Prod. 2017, 11, 92–113. [Google Scholar]
- Van den Bogaart, E.; Schoone, G.J.; England, P.; Faber, D.; Orrling, K.M.; Dujardin, J.C.; Sundar, S.; Schallig, H.D.; Adams, E.R. Simple colorimetric trypanothione reductase-based assay for high-throughput screening of drugs against Leishmania intracellular amastigotes. Antimicrob. Agents Chemother. 2014, 58, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Peniche, A.G.; Osorio, Y.; Renslo, A.R.; Frantz, D.E.; Melby, P.C.; Travi, B.L. Development of an ex vivo lymph node explant model for identification of novel molecules active against Leishmania major. Antimicrob. Agents Chemother. 2014, 58, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peniche, A.G.; Renslo, A.R.; Melby, P.C.; Travi, B.L. Antileishmanial activity of disulfiram and thiuram disulfide analogs in an ex vivo model system is selectively enhanced by the addition of divalent metal ions. Antimicrob. Agents Chemother. 2015, 59, 6463–6470. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, M.B.; Viera, S.; Ibáñez, J.; Mulet, T.; Magán-Marchal, N.; Garuti, H.; Gómez, V.; Cortés-Gil, L.; Martínez, A.; Ferrer, S.; et al. A new in vivo screening paradigm to accelerate antimalarial drug discovery. PLoS ONE 2013, 8, e66967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef]
| Species of Marine Algae | Extraction Solvent | Leishmania Species | Parasites Stage | Method of Activity Evaluation | Activity (IC50 or % of Inhibition); Cytotoxicity (CC50) | References |
|---|---|---|---|---|---|---|
| Phaeophyceae | ||||||
| Ascophyllum nodosum (Linnaeus) Le Jolis | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 66.3 μg/mL(>90.0 μg/mL) | [40] |
| Bifurcaria bifurcata R. Ross | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 6.4 μg/mL (32.7 μg/mL) | [40] |
| Ethyl acetate extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 3.8 μg/mL (6.0 μg/mL) | [41] | |
| Hexane extract | L. infantum | Promastigote | In vitro: MTT assay | 46.83 μg/mL (21.8 μg/mL) | [42] | |
| Ether extract | L. infantum | Promastigote | In vitro: MTT assay | 51.64 μg/mL (40.46 μg/mL) | [42] | |
| Chloroform extract | L. infantum | Promastigote | In vitro: MTT assay | 63.83 μg/mL (37.0 μg/mL) | [42] | |
| Canistrocarpus cervicornis (Kützing) De Paula and De Clerck | Acetate extract | L. amazonensis | Promastigote | In vitro: Microscopic counting | 50.0 μg/mL (50.0 μg/mL) | [43] |
| Methanol extract | L. amazonensis | Promastigote | In vitro: Microscopic counting | 100.0 μg/mL (51.0 μg/mL) | [43] | |
| Dichloromethane extract | L. amazonensis | Promastigote | In vitro: Microscopic counting | 20.0 μg/mL (46.0 μg/mL) | [43] | |
| Ethyl acetate fraction | L. amazonensis | Promastigote | In vitro: Microscopic counting | 8.0 μg/mL (47.0 μg/mL) | [43] | |
| Acetone extract | L. braziliensis | Promastigote | In vitro: Microscopic counting | 85.8% inhibition at 50.0 μg/mL (>50.0 μg/mL) | [29] | |
| Chorda filum (Linnaeus) Stackhouse | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 21.1 μg/mL (>90.0 μg/mL) | [40] |
| Colpomenia peregrina Sauvageau | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 29.1 μg/mL (>90.0 μg/mL) | [40] |
| Cystoseira baccata (S.G. Gmelin) P.C. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 15.7 μg/mL (>90.0 μg/mL) | [40] |
| Hexane extract | L. infantum | Promastigote | In vitro: MTT assay | 74.0% inhibition at 250.0 μg/mL (ND) | [44] | |
| Cystoseira barbata (Stackhouse) C. Agardh | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 23.46–69.98 μg/mL (>90.0 μg/mL) | [45] |
| Cystoseira crinata (Desf.) Duby | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 28.21 μg/mL (>90.0 μg/mL) | [45] |
| Cystoseira tamariscifolia (Hudson) Papenf. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 19.6 μg/mL (62.5 μg/mL) | [40] |
| Dictyopteris polypodioides (A.P. de Candolle) J.V. Lamouroux | Ethyl acetate extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 10.8 μg/mL (87.0 μg/mL) | [41] |
| Dictyota caribaea Hörnig and Schnetter | Organic extract | L. mexicana | Promastigote | In vitro: Microscopic counting | 24.4 μg/mL (≥1000.0 μg/mL) | [46] |
| Dictyota ciliolata Sonder ex Kützing | Hexane extract | L. amazonensis | Promastigote | In vitro: MTT assay | 1.15 μg/mL (23.5 μg/mL) | [47] |
| Ethyl acetate extract | L. amazonensis | Promastigote | In vitro: MTT assay | 4.22 μg/mL (19.0 μg/mL) | [47] | |
| Dictyota dichotoma (Hudson) J.V. Lamouroux | Ethanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 52.0 μg/mL (>90.0 μg/mL) | [48] |
| Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 42.4 μg/mL (>90.0 μg/mL) | [40] | |
| Ethyl acetate extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 8.8 μg/mL (27.0 μg/mL) | [41] | |
| Dictyota menstrualis (Hoyt) Schnetter, Hörning and Weber-Peuker | Ethyl acetate extract | L. amazonensis | Promastigote | In vitro: MTT assay | 0.7–0.75 μg/mL (18.3–24.7 μg/mL) | [47] |
| Hexane extract | L. amazonensis | Promastigote | In vitro: MTT assay | 0.61 μg/mL (18.2 μg/mL) | [47] | |
| Dictyota mertensii (Martius) Kützing | Dichloromethane/methanol extract | L. amazonensis | Promastigote | In vitro: Microscopic counting | 71.60 μg/mL (233.10 μg/mL) | [49] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 81.4 μg/mL (233.10 μg/mL) | [49] | ||
| Dictyota sp. | Dichloromethane/methanol extract | L. braziliensis | Promastigote | In vitro: MTT assay | 93.3% inhibition at 50.0 μg/mL (>50.0 μg/mL) | [29] |
| Fucus ceranoides (Linnaeus) | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 25.3 μg/mL (>90.0 μg/mL) | [40] |
| Fucus serratus (Linnaeus) | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 34.1 μg/mL (>90.0 μg/mL) | [40] |
| Fucus spiralis (Linnaeus) | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 34.3 μg/mL (>90.0 μg/mL) | [40] |
| Fucus vesiculosus (Linnaeus) | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 33.0 μg/mL (>90.0 μg/mL) | [40] |
| Halidrys siliquosa (Linnaeus) Lyngb. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 8.6 μg/mL (45.0 μg/mL) | [40] |
| Himanthalia elongata (Linnaeus) S.F. Gray | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 64.7 μg/mL (>90.0 μg/mL) | [40] |
| Laminaria digitata (Hudson) J.V. Lamouroux | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 34.5 μg/mL (>90.0 μg/mL) | [40] |
| Leathesia difformis (Linnaeus) Aresch. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 77.4 μg/mL (>90.0 μg/mL) | [40] |
| Lobophora variegata (J.V. Lamouroux) Womersley ex E.C. Oliveira | Organic extracts | L. mexicana | Promastigote | In vitro: Microscopic counting | 49.9 μg/mL (≥1000.0 μg/mL) | [46] |
| Padina sp. | Acetone extract | L. braziliensis | Promastigote | In vitro: MTT assay | 80.9% inhibition at 50.0 μg/mL (300.4 μg/mL) | [29] |
| L. braziliensis | Intracellular amastigote | In vitro: Microscopic counting | 40.2 μg/mL (300.4 μg/mL) | [29] | ||
| Pelvetia canaliculata (Linnaeus) Decaisne and Thuret | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 35.7 μg/mL (>90.0 μg/mL) | [40] |
| Pylaiella littoralis (Linnaeus) Kjellm. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 47.1 μg/mL (>90.0 μg/mL) | [40] |
| Saccorhiza polyschides (Lightf.) Batt. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 31.8 μg/mL (>90.0 μg/mL) | [40] |
| Sargassum muticum (Yendo) Fensholt | Polysaccharide extracts | L. infantum | Promastigote | In vitro: MTT assay | ~98.5% inhibition at 160.0 μg/mL (ND) | [4] |
| Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 34.7 μg/mL (>90.0 μg/mL) | [40] | |
| Sargassum natans (Linnaeus) Gaill. | Ethanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 90.9 μg/mL (>90.0 μg/mL) | [48] |
| Sargassum oligocystum Montagne | Hot water extract | L. major | Promastigote | In vitro: MTT assay | 78.0 μg/mL (ND) | [50] |
| Scinaia furcellata (Turn.) J. Agardh | Ethanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 64.4 μg/mL (>90.0 μg/mL) | [48] |
| Scytosiphon lomentaria (Lyngb.) Link | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 34.3 μg/mL (>90.0 μg/mL) | [40] |
| Stypocaulon scoparium (L.) Kützing | Isopropyl alcohol- Trichloromethane/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 30.4 μg/mL (>90.0 μg/mL) | [40] |
| Stypopodium zonale (J.V. Lamouroux) Papenfuss | Dichloromethane extract | L. amazonensis | Promastigote | In vitro: Microscopic counting | 100.0% inhibition at 10.0 μg/mL (>50.0 μg/mL) | [51] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 0.27 μg/mL (>50.0 μg/mL) | [51] | ||
| Ethyl acetate-hexane extract | L. amazonensis | Promastigote | In vitro: MTT assay | 0.75 μg/mL (29.5 μg/mL) | [47] | |
| Turbinaria turbinata (Linnaeus) Kuntze | Organic extracts | L. mexicana | Promastigote | In vitro: Microscopic counting | 10.9 μg/mL (≥1000.0 μg/mL) | [46] |
| Undaria pinnatifida (Harvey) Suringar | Polysaccharide extracts | L. infantum | Promastigote | In vitro: MTT assay | ~97.5% inhibition at 160.0 μg/mL (ND) | [4] |
| Rhodophyta | ||||||
| Asparagopsis armata Harvey | Ethanol-hexane:ethyl acetate fraction | L. donovani | Promastigote | In vitro: Resazurin assay | 10.0 μg/mL (ND) | [52] |
| Ethanol-ethyl acetate fraction | L. donovani | Promastigote | In vitro: Resazurin assay | 19.0 μg/mL (ND) | [52] | |
| Asparagopsis taxiformis (Delile) Trevisan | Hexane extract | L. donovani | Promastigote | In vitro: Resazurin assay | 17.0 μg/mL (ND) | [52] |
| Dichloromethane extract | L. donovani | Promastigote | In vitro: Resazurin assay | 16.0 μg/mL (ND) | [52] | |
| Ethanol-hexane:ethyl acetate fraction | L. donovani | Promastigote | In vitro: Resazurin assay | 14.0 μg/mL (ND) | [52] | |
| Ethanol-ethyl acetate fraction | L. donovani | Promastigote | In vitro: Resazurin assay | 20.0 μg/mL (ND) | [52] | |
| Ethanol extract | L. infantum | Promastigotes | In vitro: Microscopic counting | 25.0 μg/mL (>90.0 μg/mL) | [53] | |
| L. infantum | Axenic amastigotes | In vitro: Microscopic counting | 9.0 μg/mL (>90.0 μg/mL) | [53] | ||
| Boergeseniella fruticulosa (Wulfen) Kylin | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 26.6 μg/mL (>90.0 μg/mL) | [54] |
| Botryocladia leptopoda (J.Agardh) Kylin | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 60.81 μg/mL (ND) | [25,55] |
| Bostrychia tenella (J.V. Lamouroux) J. Agardh | Hexane fractions | L. amazonensis | Promastigote | In vitro: MTT assay | 17.4 μg/mL (ND) | [56] |
| Hexane subfractions (HO1; HO2; HO3; HO5; HO6) | L. amazonensis | Promastigote | In vitro: MTT assay | 22.2; 1.5; 2.7; 31.7; 66.2 μg/mL (ND) | [56] | |
| Dichloromethane subfractions (DO1-DO2) | L. amazonensis | Promastigote | In vitro: MTT assay | 4.4–4.3 μg/mL (ND) | [56] | |
| Bryothamnion triquetrum (S.G. Gmelin) M. Howe | Aqueous extract | L. amazonensis | Promastigote | In vitro: Phosphatase assay | 78.6 μg/mL (258.1 μg/mL) | [57] |
| Calliblepharis jubata (Goodenough and woodward) Kützing | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 49.8 μg/mL (>90.0 μg/mL) | [54] |
| Centroceras clavulatum (C. Agardh) Montagne | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 57.89–57.85 μg/mL (ND) | [25,55] |
| Ceramium rubrum (Hudson) C. Agardh | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 16.76 μg/mL (>90.0 μg/mL) | [46] |
| Ceramium virgatum Roth | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 25.6 μg/mL (>90.0 μg/mL) | [54] |
| Chylocladia verticillata (Lightf.) Bliding | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 47.3 μg/mL (>90.0 μg/mL) | [54] |
| Chondrococcus hornemanni (Mert.) Schmitz | Ethanol extract | L. donovani | Promastigote | In vitro: Green fluorescent protein assay | 29.5 μg/mL (>200.0 μg/mL) | [58] |
| L. donovani | Intracellular amastigote | In vitro: Microscopic counting | 40.6 μg/mL (>200.0 μg/mL) | [58] | ||
| L. donovani | In vivo | 75.38% suppression at 250.0 μg/mL | [58] | |||
| n-butanol soluble fraction of ethanol extract | L. donovani | Promastigote | In vitro: Green fluorescent protein assay | 54.2 μg/mL (>200.0 μg/mL) | [58] | |
| L. donovani | Intracellular amastigote | In vitro: Microscopic counting | 61.0 μg/mL (>200.0 μg/mL) | [58] | ||
| Chromatographic fraction of n-butanol soluble fraction (F011) | L. donovani | Promastigote | In vitro: Green fluorescent protein assay | 53.7 μg/mL (>200.0 μg/mL) | [58] | |
| L. donovani | Intracellular amastigote | In vitro: Microscopic counting | 57.4 μg/mL (>200.0 μg/mL) | [58] | ||
| L. donovani | In vivo | 76.2% suppression at 100.0 μg/mL | [58] | |||
| Chromatographic fraction of n-butanol soluble fraction (F012) | L. donovani | Promastigote | In vitro: Green fluorescent protein assay | 54.5 μg/mL (>200.0 μg/mL) | [58] | |
| L. donovani | Intracellular amastigote | In vitro: Microscopic counting | 51.7 μg/mL (>200.0 μg/mL) | [58] | ||
| L. donovani | In vivo | 56.7% suppression at 100.0 μg/mL | [58] | |||
| Chondrus crispus Stackhouse | Ethanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 95.0% inhibition at 9.7 μg/mL (84.0 μg/mL) | [41] |
| Claviclonium ovatum (J.V. Lamouroux) Kraft and Min-Thein | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 61.2 μg/mL (>90.0 μg/mL) | [54] |
| Corallina officinalis Linnaeus | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 22.7 μg/mL (88.6 μg/mL) | [54] |
| Corallina granifera Ell. et Sol. | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 35.02 μg/mL (>90.0 μg/mL) | [45] |
| Cryptopleura ramosa (Hudson) L. Newton | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 85.6 μg/mL (>90.0 μg/mL) | [54] |
| Cystoclonium purpureum (Hudson) Batt. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 67.3 μg/mL (>90.0 μg/mL) | [54] |
| Dasya pedicellata (C. Agardh) C. Agardh | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 23.04 μg/mL (14.7 μg/mL) | [45] |
| Dilsea carnosa (Schmidel) Kuntze | Ethyl acetate extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 9.5 μg/mL (74.0 μg/mL) | [41] |
| Dumontia incrassata (O.F. Müll.) J.V. Lamouroux | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 68.6 μg/mL (>90.0 μg/mL) | [54] |
| Furcellaria lumbricalis (Hudson) J.V. Lamouroux | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 43.3 μg/mL (>90.0 μg/mL) | [54] |
| Gelidium crinale (Hare ex Turner) | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 19.95 μg/mL (>90.0 μg/mL) | [45] |
| Gelidium pulchellum (Turner) Kützing | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 32.5 μg/mL (>90.0 μg/mL) | [54] |
| Gracilaria bursa-pastoris (S.G. Gmelin) P.C. Silva | Polysaccharide extracts | L. infantum | Promastigote | In vitro: MTT assay | ~87.5.0% inhibition at 160.0 μg/mL (ND) | [4] |
| Gracilaria corticata (J. Agardh) J. Agardh | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 37.5 μg/mL (ND) | [25,55] |
| Cold water extract | L. major | Promastigote | In vitro: MTT assay | 65.0 μg/mL (ND) | [50] | |
| Hot water extract | L. major | Promastigote | In vitro: MTT assay | 38.0 μg/mL (ND) | [50] | |
| Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine and Farnham | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 53.3 μg/mL (>90.0 μg/mL) | [54] |
| Gracilaria salicornia (C. Agardh) E.Y. Dawson | Cold water extract | L. major | Promastigote | In vitro: MTT assay | 74.0 μg/mL (ND) | [50] |
| Hot water extract | L. major | Promastigote | In vitro: MTT assay | 46.0 μg/mL (ND) | [50] | |
| Gracilaria verrucosa (Hudson) Papenfuss | Methanol extract | L. donovani | Axenic amastigotes | In vitro: Resazurin assay | 36.02 μg/mL (>90.0 μg/mL) | [45] |
| Gracilaria viridis Sfriso, Wolf, Sciuto, Morabito, Andreoli and Moro | Polysaccharide extracts | L. infantum | Promastigote | In vitro: MTT assay | ~82.8% inhibition at 160.0 μg/mL (ND) | [4] |
| Halopitys incurvus (Hudson) Batt. | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 16.5 μg/mL (>90.0 μg/mL) | [54] |
| Halurus equisetifolius (Lightf.) Kützing | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 69.2 μg/mL (>90.0 μg/mL) | [54] |
| Jania rubens (Linnaeus) J.V. Lamouroux | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 60.7 μg/mL (>90.0 μg/mL) | [54] |
| Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 28.0 μg/mL (>90.0 μg/mL) | [45] | |
| Laurencia aldingensis Saito and Womersley | Dichloromethane/methanol extract | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 24.5 μg/mL (259.8 μg/mL) | [59] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 12.5 μg/mL (259.8 μg/mL) | [59] | ||
| Laurencia dendroidea (Hudson) J.V. Lamouroux | Lipophilic extracts | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 17.9–34.2 μg/mL (106.2–131.7 μg/mL) | [60] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 8.7–10.8 μg/mL (106.2–131.7 μg/mL) | [60] | ||
| Dichloromethane/methanol extract | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 30.1–97.2 μg/mL (187.0–240.0 μg/mL) | [60] | |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 16.8–22.4 μg/mL (187.0–240.0 μg/mL) | [60] | ||
| Laurencia microcladia Kützing | Organic extracts | L. mexicana | Promastigote | In vitro: Microscopic counting | 16.3 μg/mL (119.8 μg/mL) | [46] |
| Lomentaria articulata (Hudson) Lyngb | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 60.0 μg/mL (>90.0 μg/mL) | [54] |
| Mastocarpus stellatus (Stackhouse) Guiry | Isopropyl alcohol- Trichloromethane/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 44.1 μg/mL (>90.0 μg/mL) | [54] |
| Melanothamnus afaqhusainii M. Shameel | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 32.6–32.5 μg/mL (ND) | [25,55] |
| Ochtodes secundiramea (Montagne) M. Howe | Acetone extract | L. braziliensis | Promastigote | In vitro: MTT assay | 99.7% inhibition at 50.0 μg/mL (>50.0 μg/mL) | [29] |
| Osmundaria obtusiloba (C. Agardh) R.E. Norris | Ethanol extract | L. amazonensis | Promastigote | In vitro: MTT assay | 24.5 μg/mL (240.0 μg/mL) | [47] |
| L. amazonensis | In vivo | Active at 5.0 and 20.0 mg/kg | [47] | |||
| Ethyl acetate-Hexane extract | L. amazonensis | Promastigote | In vitro: MTT assay | 22.0 μg/mL (198.0 μg/mL) | [47] | |
| Osmundea hybrida (A.P. de Candolle) K.W. Nam | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 49.2 μg/mL (>90.0 μg/mL) | [54] |
| Osmundea pinnatifida (Hudson) Stackhouse | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 6.25 μg/mL (ND) | [55] |
| Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 32.7 μg/mL (>90.0 μg/mL) | [54] | |
| Palisada flagellifera (J. Agardh) K.W. Nam | Dichloromethane/methanol extract | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 30.7 μg/mL (198.0 μg/mL) | [59] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 34.5 μg/mL (198.0 μg/mL) | [59] | ||
| Palisada perforata (Bory) K.W. Nam | Dichloromethane/methanol extract | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 36.1–46.7 μg/mL (267.0 μg/mL) | [59] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 29.7–34.5 μg/mL (267.0 μg/mL) | [59] | ||
| Plocamium cartilagineum (Linnaeus) P.S. Dixon | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 21.2 μg/mL (>90.0 μg/mL) | [54] |
| Polyides rotundus (Hudson) Gaillon | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 57.3 μg/mL (>90.0 μg/mL) | [54] |
| Porphyra linearis Grev | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 55.5 μg/mL (>90.0 μg/mL) | [54] |
| Scinaia Fascicularis (Børgesen) Huisman | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 59.6 μg/mL (ND) | [55] |
| Scinaia hatei Børgesen | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 14.1 μg/mL (ND) | [25,55] |
| Scinaia indica Børgesen | Ethanol extract | L. major | Promastigote | In vitro: Microscopic counting | 59.6 μg/mL (ND) | [25] |
| Chlorophyceae | ||||||
| Anadyomene saldanhae A.B. Joly and E.C. Oliveira | Acetone extract | L. braziliensis | Promastigote | In vitro: MTT assay | 87.9% inhibition at 50.0 μg/mL (294.2 μg/mL) | [29] |
| L. braziliensis | Intracellular amastigote | In vitro: Microscopic counting | 23.9 μg/mL (294.2 μg/mL) | [29] | ||
| Caulerpa racemosa (Forsskål) J. Agardh | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 22.66 μg/mL (>90.0 μg/mL) | [45] |
| Ethyl acetate-Hexane extract | L. amazonensis | Promastigote | In vitro: MTT assay | 47.5–154 μg/mL (48.0–115.0 μg/mL) | [47] | |
| Caulerpa cupressoides (Vahl) C. Agardh | Acetone extract | L. braziliensis | Promastigote | In vitro: MTT assay | 51.7% inhibition at 50.0 μg/mL (>50.0 μg/mL) | [29] |
| Caulerpa sertularioides (S.G. Gmelin) M. Howe | Hot water extract | L. major | Promastigote | In vitro: MTT assay | 85.0 μg/mL (ND) | [50] |
| Cladophora rupestris (L.) Kützing | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 20.2 μg/mL (>90.0 μg/mL) | [61] |
| Codium bursa (L.) C. Agardh | Methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 31.71 μg/mL (>90.0 μg/mL) | [45] |
| Codium fragile (Sur.) Hariot ssp. Tomentosoides (van Goor) Silva | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 16.6 μg/mL (>90.0 μg/mL) | [61] |
| Halimeda opuntia (L.) Lamouroux | Aqueous | L. amazonensis | Promastigote | In vitro: Phosphatase assay | 83.5 μg/mL (526.4 μg/mL) | [57] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 70.7 μg/mL (526.4 μg/mL) | [57] | ||
| Ulva intestinalis Linnaeus | Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 14.9 μg/mL (>90.0 μg/mL) | [61] |
| Ulva lactuca Linnaeus | Ethanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 5.9 μg/mL (>90.0 μg/mL) | [48] |
| Isopropyl alcohol- Chloroform/methanol extract | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 12.0 μg/mL (>90.0 μg/mL) | [61] | |
| Chemical Type | Chemical Classe | Isolated Compounds | Species of Marine Algae; Phylum | Leishmania Species | Parasites Stage | Method of Activity Evaluation | Activity (IC50 or % of Inhibition) Cytotoxicity (CC50) | References |
|---|---|---|---|---|---|---|---|---|
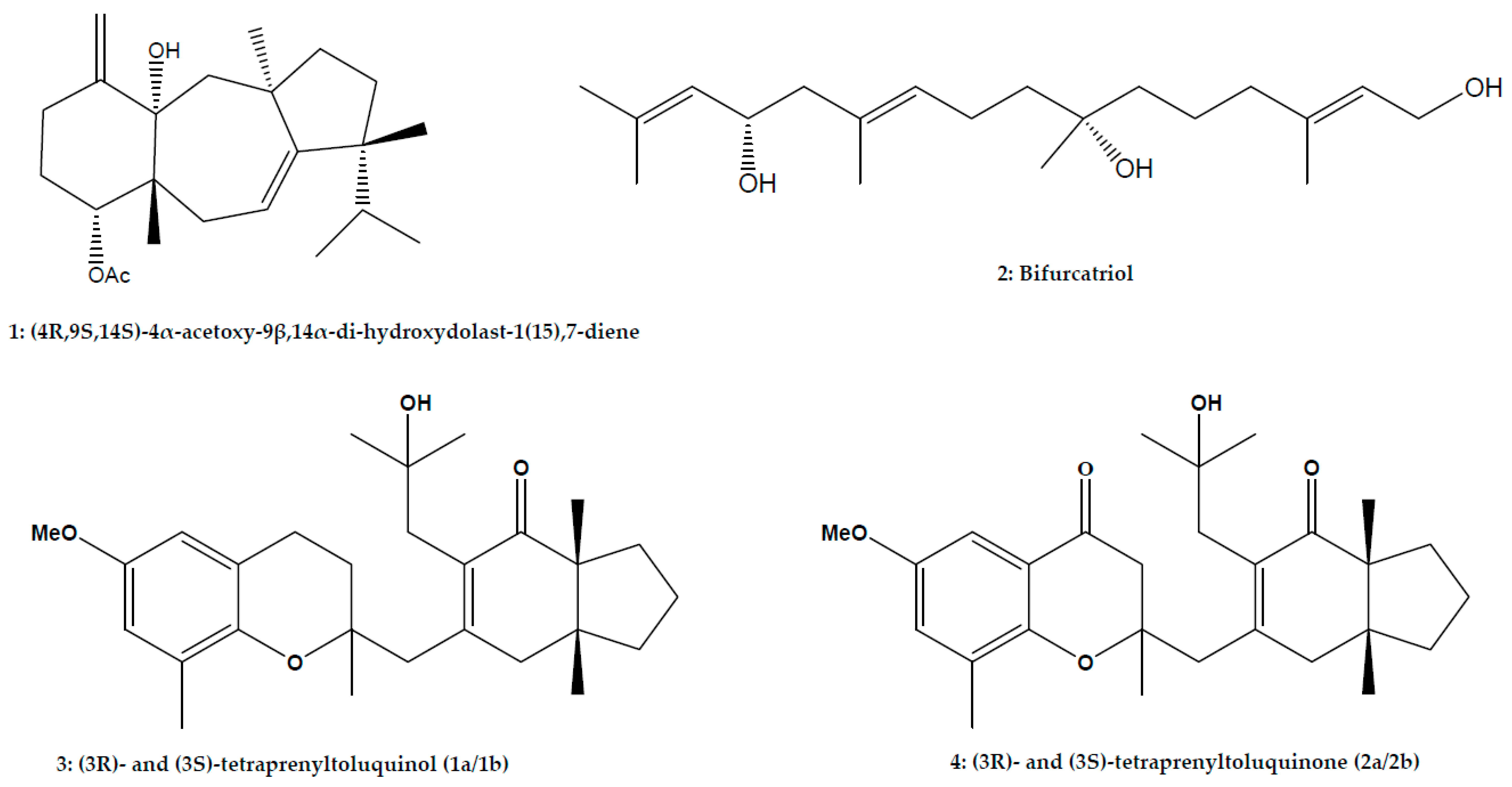
| Diterpene | Dolastane | (4R,9S,14S)-4α-acetoxy-9β, 14α-dihydroxydolast-1(15), 7-diene | Canistrocarpus cervicornis (Kützing) De Paula and De Clerck; Phaeophyceae | L. amazonensis | Promastigote | In vitro: Microscopic counting | 2.0 μg/mL (186.0 μg/mL) | [43] |
| L. amazonensis | Axenic amastigote | In vitro: Microscopic counting | 12.0 μg/mL (186.0 μg/mL) | [43] | ||||
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 4.0 μg/mL (186.0 μg/mL) | [43] | ||||
| Meroditerpenoid | (3R)- and (3S)-tetraprenyltoluquinol (1a/1b) (3R)- and (3S)-tetraprenyltoluquinone (2a/2b) | Cystoseira baccata (S.G. Gmelin) P.C.; Phaeophyceae | L. infantum | Promastigote | In vitro: MTT assay | 44.9 μM (126.6 μM) | [44] | |
| L. infantum | Intracellular amastigote | In vitro: MTT assay | 25.0 μM (126.6 μM) | [44] | ||||
| L. infantum | Promastigote | In vitro: MTT assay | 94.4 μM (84.5 μM) | [44] | ||||
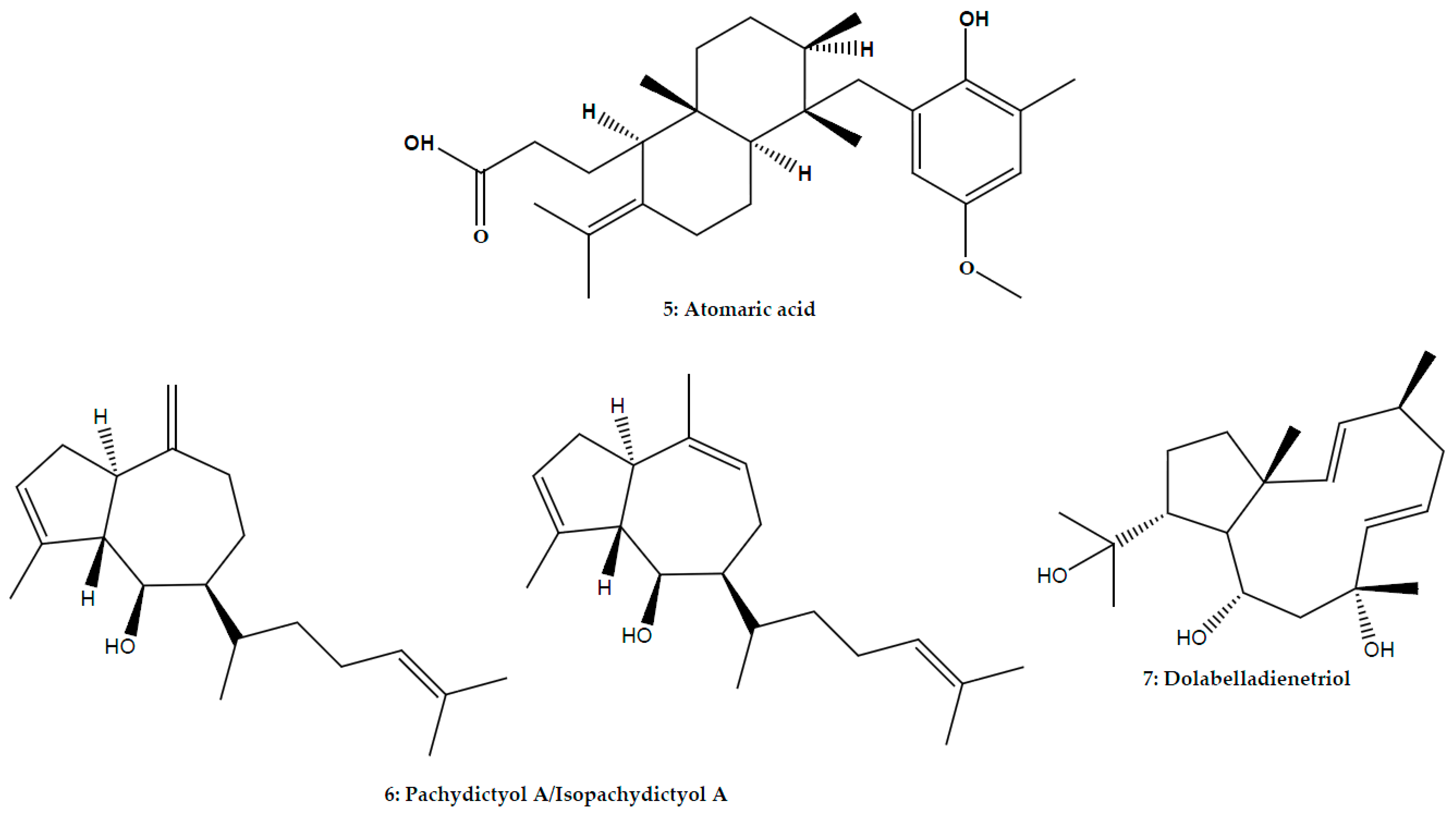
| Atomaric acid | Stypopodium zonale (J.V. Lamouroux) Papenfuss; Phaeophyceae | L. amazonensis | Promastigote | In vitro: Microscopic counting | 86.0% inhibition at 50.0 μM (169.5 μM) | [51] | ||
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 20.2 μM (169.5 μM) | [51] | ||||
| Hydroazulene | Mixture of isomers pachydictyol A/isopachydictyol A | Dictyota menstrualis (Hoyt) Schnetter, Hörning and Weber-Peuker; Phaeophyceae | L. amazonensis | Promastigote | In vitro: MTT assay | 23.5 μg/mL (30.0 μg/mL) | [47] | |
| Dolabellane | Dolabelladienetriol | Dictyota pfaffii Schnetter; Phaeophyceae | L. amazonensis | Promastigote | In vitro: Microscopic counting | 95.5% Inhibition at 100.0 μM (>100.0 μM) | [14] | |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 43.9 μM (>100.0 μM) | [14] | ||||
| Leishmania/HIV-1 co-infection | Intracellular amastigote/HIV-1 | In vitro: Microscopic counting | 56.0% inhibition at 50.0 μM (>100.0 μM) | [14] | ||||
| Linear diterpene | Bifurcatriol | Bifurcaria bifurcata R.Ross; Phaeophyceae | L. donovani | Axenic amastigote | In vitro: Resazurin assay | 18.8 μg/mL (56.6 μg/mL) | [74] | |
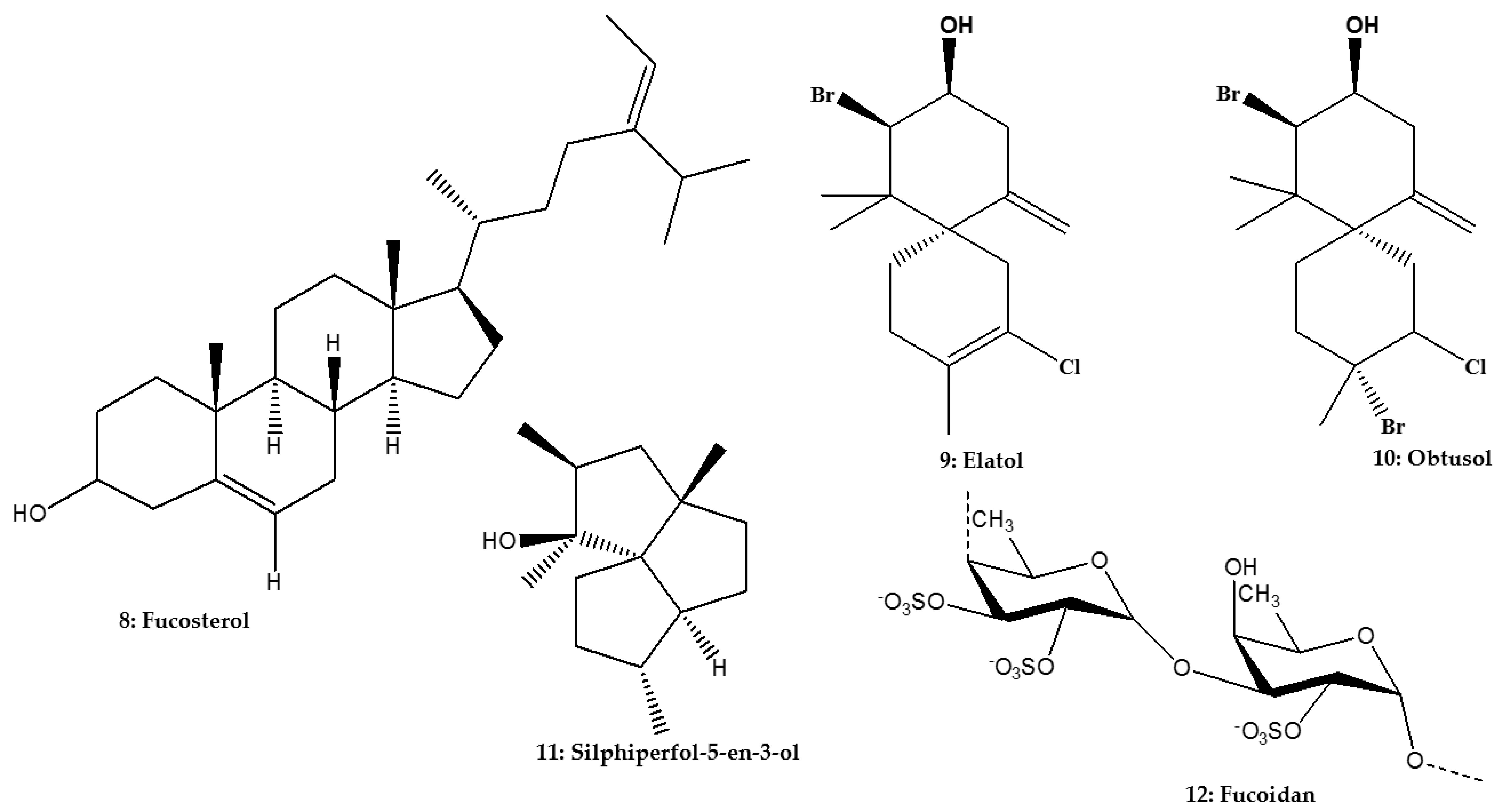
| Triterpene | Phytosterol | Fucosterol | Lessonia vadosa Searles; Phaeophyceae | L. infantum | Promastigote | In vitro: Resazurin assay | 45.0 μM (>100.0 μM) | [75] |
| L. infantum | Intracellular amastigote | In vitro: Resazurin assay | 10.30 μΜ (>100.0 μM) | [75] | ||||
| L. amazonensis | Promastigote | In vitro: Resazurin assay | 55.0 μM (>100.0 μM) | [75] | ||||
| L. amazonensis | Intracellular amastigote | In vitro: Resazurin assay | 7.89 μM (>100.0 μM) | [75] | ||||
| Sesquiterpene | Chamigrene | Elatol | Laurencia dendroidea (Hudson) J.V. Lamouroux; Rhodophyta | L. amazonensis | Promastigote | In vitro: Microscopic counting and Green fluorescent protein assay | 4.0 μM; 9.7 μg/mL (1.4 μM; 112.9–120.0 μg/mL) | [26,60] |
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 0.45 μM; 4.5 μg/mL (1.4 μM; 112.9–120.0 μg/mL) | [26,60] | ||||
| Obtusol | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 6.2 μg/mL (133.5–139.3 μg/mL) | [60] | |||
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 3.9 μg/mL (133.5–139.3 μg/mL) | [60] | ||||
| Triquinane | Silphiperfol-5-en-3-ol | L. amazonensis | Promastigote | In vitro: Green fluorescent protein assay | 43.8 μg/mL (160.2–172.8 μg/mL) | [60] | ||
| L. amazonensis | Intracellular amastigote | In vitro: Microscopic counting | 48.7 μg/mL (160.2–172.8 μg/mL) | [60] | ||||
| Sulfated polysaccharide | Fucan | Fucoidan | Fucus vesiculosus (Linnaeus); Phaeophyceae | L. donovani | Intracellular amastigote | In vitro: Microscopic counting | 93.0% inhibition at 50.0 μg/mL | [76] |
| L. donovani | In vivo | 100.0% supression at 200.0 mg/kg/day (ND) | [76] | |||||
| ND | Sulfated polysaccharide (NI) | Botryoclada occidentalis (Børgesen) Kylin; Rhodophyta | L. amazonensis | Promastigote | In vitro: MTT assay | 63.7 μg/mL (27.3 μg/mL) | [77] | |
| Caulerpa racemosa (Forsskål) J. Agardh; Chlorophyta | L. amazonensis | Promastigote | In vitro: MTT assay | 34.5 μg/mL (49.3 μg/mL) | [77] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchokouaha Yamthe, L.R.; Appiah-Opong, R.; Tsouh Fokou, P.V.; Tsabang, N.; Fekam Boyom, F.; Nyarko, A.K.; Wilson, M.D. Marine Algae as Source of Novel Antileishmanial Drugs: A Review. Mar. Drugs 2017, 15, 323. https://doi.org/10.3390/md15110323
Tchokouaha Yamthe LR, Appiah-Opong R, Tsouh Fokou PV, Tsabang N, Fekam Boyom F, Nyarko AK, Wilson MD. Marine Algae as Source of Novel Antileishmanial Drugs: A Review. Marine Drugs. 2017; 15(11):323. https://doi.org/10.3390/md15110323
Chicago/Turabian StyleTchokouaha Yamthe, Lauve Rachel, Regina Appiah-Opong, Patrick Valere Tsouh Fokou, Nole Tsabang, Fabrice Fekam Boyom, Alexander Kwadwo Nyarko, and Michael David Wilson. 2017. "Marine Algae as Source of Novel Antileishmanial Drugs: A Review" Marine Drugs 15, no. 11: 323. https://doi.org/10.3390/md15110323