1. Introduction
Fucoidan is a class of biopolymers that has a sulfated homo- or hetero-polysaccharide backbone. It is particularly found in the fibrilar part of the cell wall and intercellular spaces, especially of the brown seaweeds (Phaeophyta) [
1]. The exact function of fucoidan is still not completely understood and needs more investigation, but it is assumed that it prevents the desiccation of the thallus tissues, especially when the algae is subjected to lower tide levels or in the higher temperature of summer [
2]. It also acts as cross-linker between cellulose and hemicellulose and participates in the cellular structural integrity [
3].
l-Fucose represents the major repeating monomer, that is linked by different glycosidic linkages (e.g., alternating α-1,3 and α-1,4
l-fucopyranosyls in fucoidan derived from
F. vesiculosus) to form a straight or branched-chain polysaccharide skeletons. Other sugar monomers such as galactose, mannose, glucose, and xylose, as well as traces of uronic acids, could be present, in addition to sulfate and acetate ester groups, which bind in a species-dependent pattern [
4,
5].
The first extraction and isolation was carried out by Kylin approximately 100 years ago in 1913 [
6]. Since then fucoidan research has been dramatically increased, especially during the last four decades, due to its promising and diverse pharmacological activities (e.g., antiviral, antithrombotic and anticoagulant, anti-inflammatory, cytotoxic, and their effects against various renal and hepatic diseases) [
7].
Fucoidan is freely soluble in solvents of higher dielectric constants like water, due to isolated shielded opposite groups [
8], and that is why hot water extraction, either in acidic or alkaline conditions, is usually the method of choice for its extraction. However, solvents of lower dielectric constants, like ethanol, are usually used for precipitation and isolation from other co-extracted natural compounds.
Different methods (e.g., hot acidic or alkaline, enzyme-, microwave- or ultrasound-assisted aqueous extraction) were developed to optimize the fucoidan extraction process. Nevertheless, time-consuming and expensive purification techniques are still applied in order to obtain a purified grade from its crude extracts. Among applied purification techniques are anion exchange and gel-permeation chromatography, depending on its higher anionic charge density and molecular weights.
Anion exchange chromatography has several disadvantages such as adsorption of other acidic compounds in alkaline pH such as alginate or polyphenols or even to free hydrolyzed sulfate ester groups of fucoidan, which elute afterwards in desorption step with intact fucoidan and distort the accurate determination of sulfur content (S%). In addition, positively-charged quaternary ammonium groups of resin beads could be leaked to give another possibility for contamination and higher nitrogen content (N%). The resin usually needs a tedious loading regeneration step after each cycle to regain its functional groups and capacity. Major structural features of fucoidan, such as molecular weight, monomeric composition, sulfate ester content, and position, are affected during extraction and purification processes and resulted in inaccurate characters [
9]. Additionally, seasonal and geographical factors are also involved in such structural conflicts [
10].
Recently, a purification protocol was developed based on dye affinity chromatography and proved its ability to capture intact fucoidan selectively from synthetic and crude extracts by immobilized thiazine dyes e.g., toluidine blue and thionine acetate. This protocol was performed through several steps of washing of the derivatized beads with different solvents to remove non-specific binding compounds before the elution step to obtain a high purified grade [
11].
The present research aims to apply this new protocol using different conditions e.g., pH and simultaneous extraction and purification to obtain several fucoidan fractions from the brown macroalgae Fucus vesiculosus, in addition to their evaluation using physicochemical characters and monomeric composition, as well as biological activities, such as anticoagulant and antiviral activities.
3. Experimental Sections
3.1. Chemicals
Sepabeads® EC-EA (S-grade) was a gift from Resindion s.r.l. (Binasco MI, Italy). Particle size range of the carrier was 100–300 µm with a median pore diameter of 10–20 nm. Reagents used to measure the anticoagulant activity were purchased from Siemens Healthcare Diagnostics Products GmbH (Marburg, Germany). All other chemicals including reference fucoidan isolated from F. vesiculosus (≥95% pure) were purchased from Sigma Aldrich® (St. Louis, MO, USA).
3.2. Instrumentations
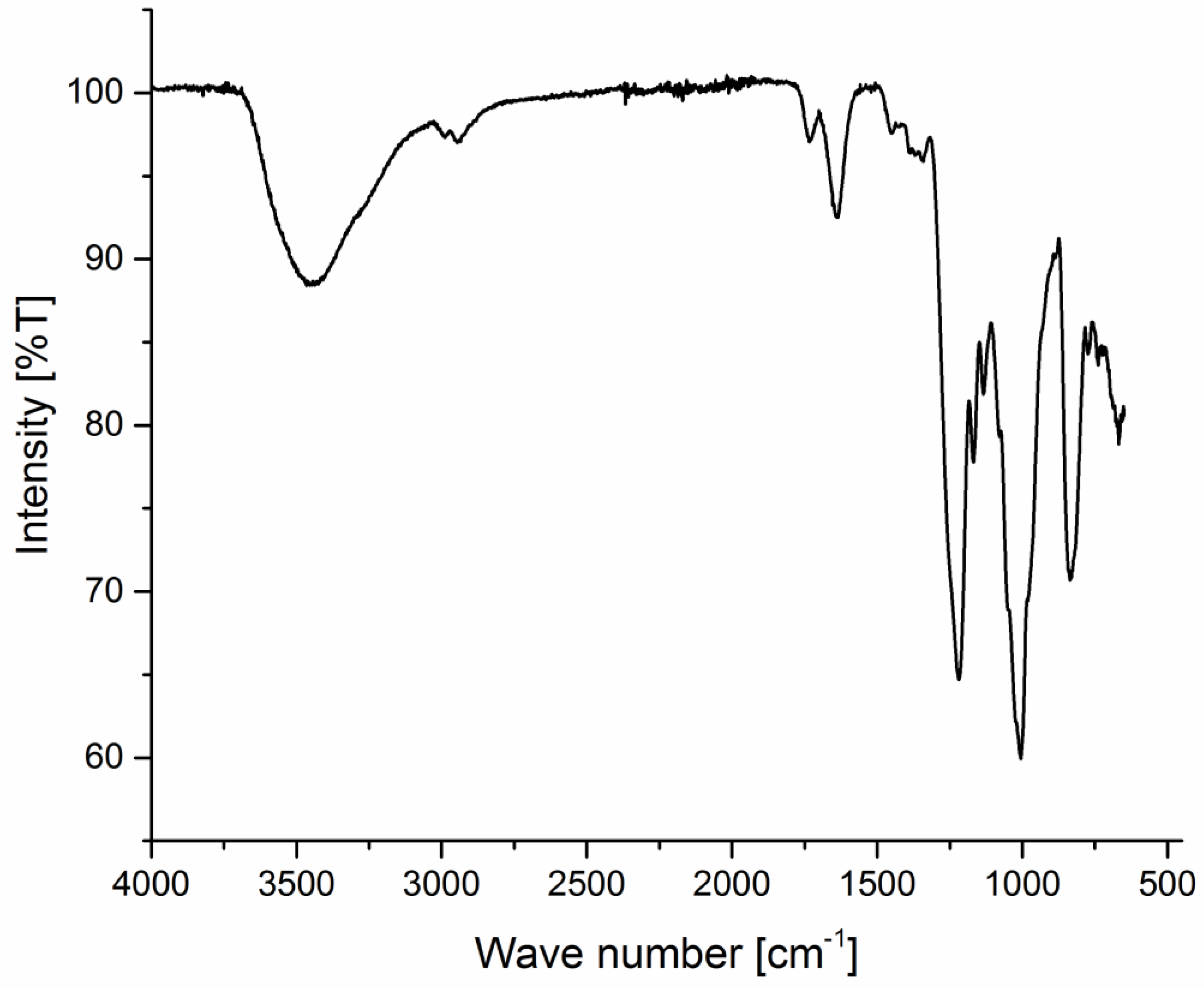
HPLC pump L 7100 with an auto sampler AS-2000 A (both Merck-Hitachi, Darmstadt, Germany), a GPC_MCX column 8 × 30 mm (PSS, Mainz, Germany) and Shodex® RI-101 detector (Shimadzu Corporation, Kyoto, Japan) were used for molecular weight averages measurement. Spectrum 100 FT-IR, (Perkin Elmer, Waltham, MA, USA) was used to identify and characterize fucoidan fractions in comparison with reference fucoidan. An overhead shaker Multi Bio RS-24 and a thermoshaker TS-100 (Biosan, Riga, Latvia) were used to stir suspensions during adsorption and elution incubation periods of purification process, respectively. Elemental analysis was performed using Vario Micro cube WLD Board V 2.0.11 (Elementar Analysensysteme GmbH, Hanau, Germany). P-2000 digital polarimeter (Jasco, Gross-Ulmstadt, Germany) supplied with a sodium spectral lamp and adjusted at λ 589 nm for specific optical rotation measurement. DigiMelt-MPA 160, SRS (Scientific Instruments GmbH, Gilching bei München, Germany) for melting point determination. A conductivity meter set (Qcond 2200, VWR International GmbH, Darmstadt, Germany) was used for conductivity measurement. A BCS® System (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) was programmed to perform the different coagulation protocol and measure the coagulation times. Fluorescence measurements were performed using a Spectra-Photometer V630 (Jasco, Gross-Ulmstadt, Germany) to assess the antiviral activity.
3.3. Extraction and Preparation of the Crude Extract
3.3.1. Algal Preparation and Pretreatment
Fresh algal biomass of F. vesiculosus was harvested from the North Sea at the region of south beaches of Wilhelmshaven (Germany, 53°31.236N, 8°13.849E) in July 2007. The algal biomass was washed with tap, and then deionized, water, air-dried for two days, then in the oven at 50 °C until constant weight, and milled afterwards. 100 g dried algal powder, before the extraction step, were handled by several pretreatment steps successively under continuous stirring overnight at room temperature with 1 L of acetone, hexane:isopropanol mixture (3:2), 80% (v/v) ethanol, ethanol:water:formaldehyde (80:15:5) at pH 2, and finally washed again with 1 L 80% (v/v) ethanol. After each step, the suspension was centrifuged (4000 rpm, 10 min), and the supernatant is decanted. The pretreated algal powder was then dried again at 50 °C and stored at room temperature in a well-closed plastic container.
3.3.2. Extraction
Two methods for fucoidan extraction were applied:
Procedure A: The first method was performed by exhaustion of 10 g of pretreated powder with 100 mL 1% (
w/
v) aqueous CaCl
2 for 6 h at 70 °C under reflux with continuous stirring at pH 2, as described previously by Hahn T.
et al. [
11]. After centrifugation (4500 rpm, 15 min), the algal biomass was removed and the supernatant was neutralized by 0.2 M ammonium carbonate. Crude fucoidan was then isolated via precipitation by ethanol with a final concentration of 70% (
v/
v), cooling overnight at 4 °C, centrifugation, and then drying of the precipitate at 50 °C.
Procedure B: The other method administered 20 mM maleic acid buffer (MAB) at pH 1 as a solvent for the 1% (w/v) CaCl2 solution. The pretreated algal powder was afterwards extracted using the same conditions previously mentioned in Procedure A. The pH was adjusted regularly every 2 h at 1 by 1 M HCl, as necessary. Centrifugation (4500 rpm, 15 min) was also used to separate the supernatant from the algal biomass. The supernatant was stored afterwards in the refrigerator untl the next step of purification.
3.3.3. Immobilization of Toluidine Blue, Purification, and Recovery of Fucoidan Fractions
According to immobilization protocol described by HahnT,
et al. [
11], an aqueous 2 mM toluidine blue O (TB) solution was prepared and immobilized on the Sepabeads
® EC-EA to produce the derivatized beads.
The first two fucoidan fractions were obtained by reconstitution of crude fucoidan powder obtained by procedure A in a concentration of 2.5 mg·mL−1 in two different buffer solutions: 20 mM MAB pH 1 and 20 mM MES pH 6 and purified to obtain fucoidan_1 and fucoidan_6, respectively. Procedures for purification and recovery were performed by incubation of 50 mg of the derivatized beads with 1.5 mL of the stock solutions in a 2 mL reaction vessel at room temperature for 40 h and placed in an overhead shaker adjusted at 60 rpm. The beads were then washed with water and 0.1 M NaCl in 20 mM MAB at pH 2, successively. 1.5 mL of 3 M NaCl dissolved in 30% (v/v) ethanol in a 20 mM MES buffer pH 6 was used as an eluent with shaking (800 rpm) at 50 °C. The eluates were afterwards dialyzed through a MWCO 3.5 kD membrane tube for salt removal and lyophilized at the end.
The third fraction, fucoidan_M, was isolated by incubation of 75 mg of derivatized beads with 1.5 mL of crude extract obtained by procedure B for 48 h. The other steps for washing and recovery were performed as described previously with the other two fractions.
3.3.4 Evaluation of Beads Leakage
Dextran solution of a concentration 2.5 mg·mL−1 from Leuconostoc sp. (Mr ~500 kDa) was prepared in the same eluent of fucoidan (3 M NaCl in 30% ethanol in 20 mM MES pH 6) and incubated with the derivatized beads at conditions mimic to that applied in fucoidan elution phase for 12 h. The solution obtained after incubation was afterwards dialyzed, lyophilized, and elementally analyzed in comparison with non-treated dextran powder.
3.4 Physicochemical Investigations
3.4.1 Molecular Weight Averages
Molecular weight measurements were performed using an isocratic HPLC pump L 7100 with an auto sampler AS-2000 A. Samples were dissolved in eluent in a concentration of 4 mg·mL−1 and mixed with an equal volume of 1 mg·mL−1 ethylene glycol solution as a flow marker. Separation was performed at 25 °C using a GPC_MCX column 8 × 30 mm, which was previously calibrated with dextran sulfate (analytical standard grade for GPC) of different molecular sizes (25–670 kDa). The injection volume was 10 µL and eluent was 0.05 M phosphate buffer at pH 9.1 with a volumetric flow rate of 1 mL·min−1. The sample detections were performed using Shodex® RI-101 detector.
3.4.2. Melting Point
Two mg of each fucoidan fraction was placed in a capillary tube and placed in the melting point apparatus. Temperature increment was 2 °C·min−1 and three temperature points, at which powders started to melt, changed their color, and finally decomposed with charring, were observed and recorded.
3.4.3. Specific Optical Rotation
An aqueous 0.4% w/v solution of each fucoidan was prepared and the specific optical rotation was measured at 22 °C.
3.5. Monomeric Composition
Monomeric composition of the different fractions of fucoidan was determined according to protocols developed by Rühmann,
et al. [
27]. Briefly, 1 g·L
−1 aqueous solution of the three fucoidan fractions were prepared and incubated with 2 M trifluroacetic acid for 90 min at 121 °C. The monomers fucose, xylose, and galactose of hydrolyzed polysaccharides were detected by UV–IS, while glucose and uronic acid dimers were detected by MS.
3.6. Biological Investigations
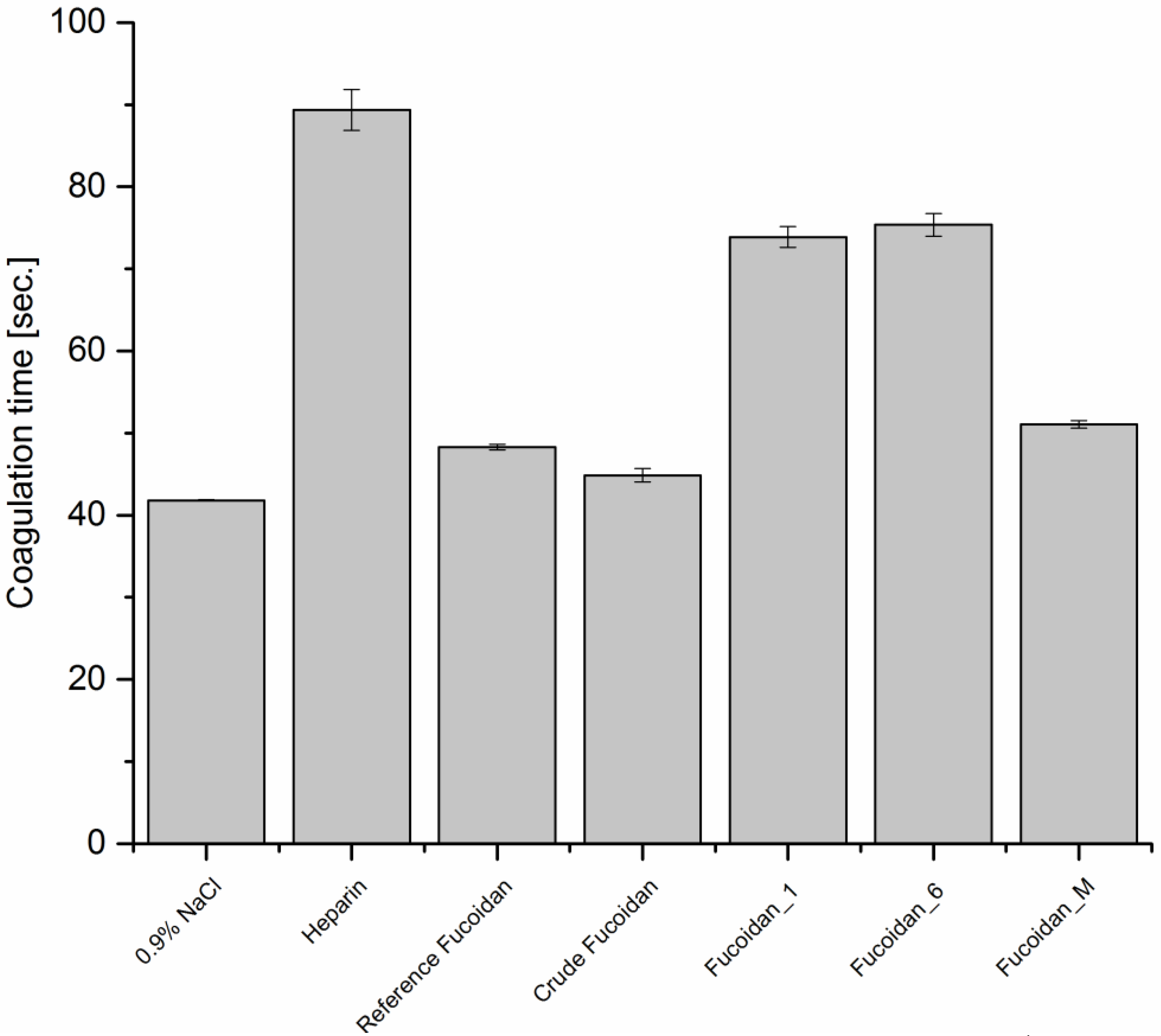
3.6.1. Anticoagulant Activity
Activated Partial Thromboplastin Time (APTT)
APTT was determined for all fucoidan fractions, according to the protocol described by Anderson
et al. [
28] with few modifications. All the samples were prepared in an isotonic solution of 0.9%
w/
v NaCl. The device was programmed to mix 0.6 mL of platelet-poor plasma with 0.3 mL of 10 µg·mL
−1 fucoidan solution. Each mixture was then incubated for 60 s at 37 °C before the addition of 0.5 mL pre-warmed APPT reagent and then incubated again for 5 min at 37 °C. Afterwards, 0.6 mL of a pre-warmed 0.25 M CaCl
2 solution was added and the time for clot formation was recorded. These steps were repeated three times. Heparin in a concentration of 5 µg·mL
−1 and 0.9%
w/
v NaCl were used as positive and negative control, respectively.
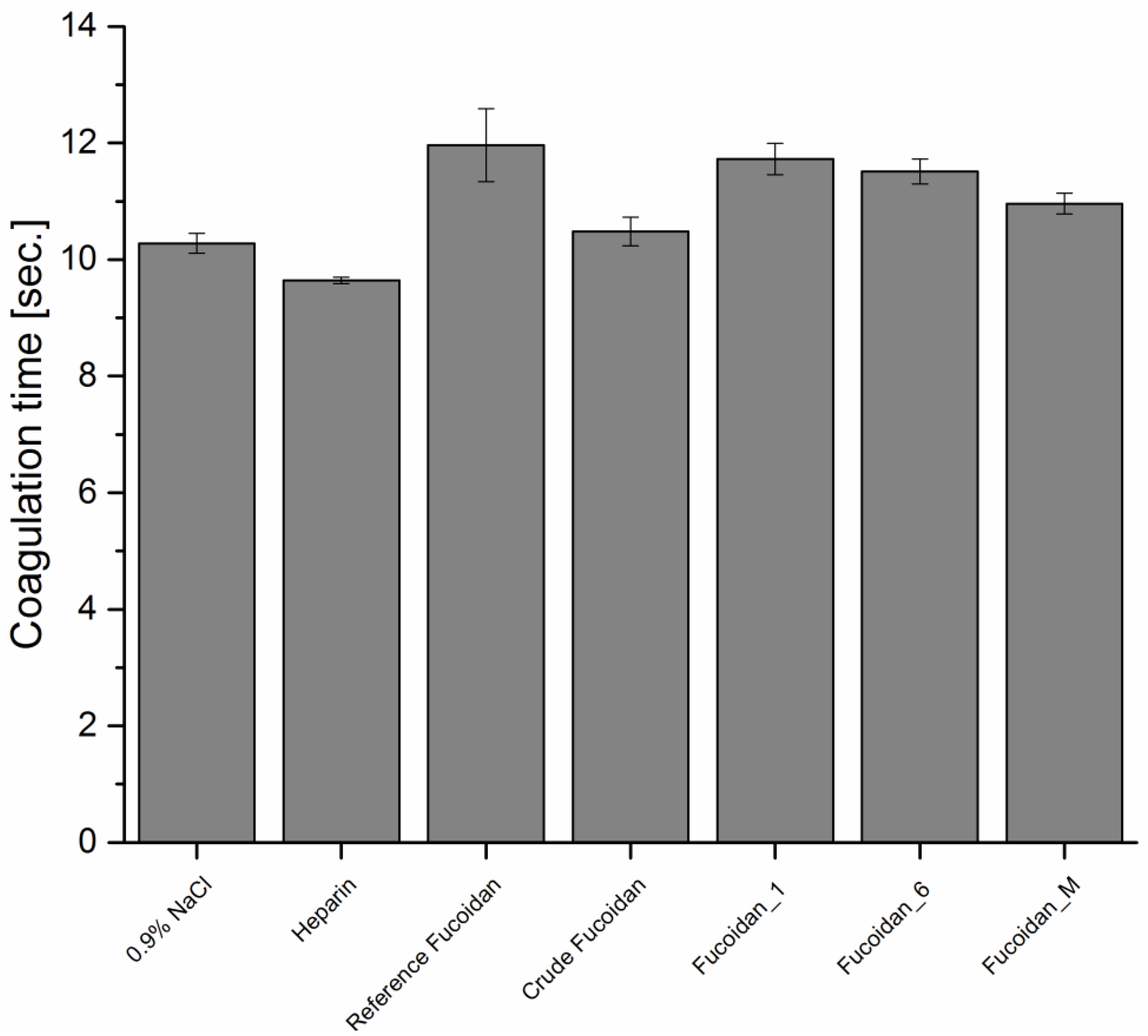
Prothrombin Time (PT)
PT was also determined using the protocol of Quick [
29] with some modifications. The device was also programmed to perform the same procedure mentioned previously in APTT determination, but each poor-platelets plasma and fucoidan solution mixture was incubated for 3 min at 37 °C. Afterwards, 0.6 mL pre-warmed PT reagent was added and the time for clot formation was observed and repeated three times. Heparin and NaCl were also used as positive and negative control, respectively.
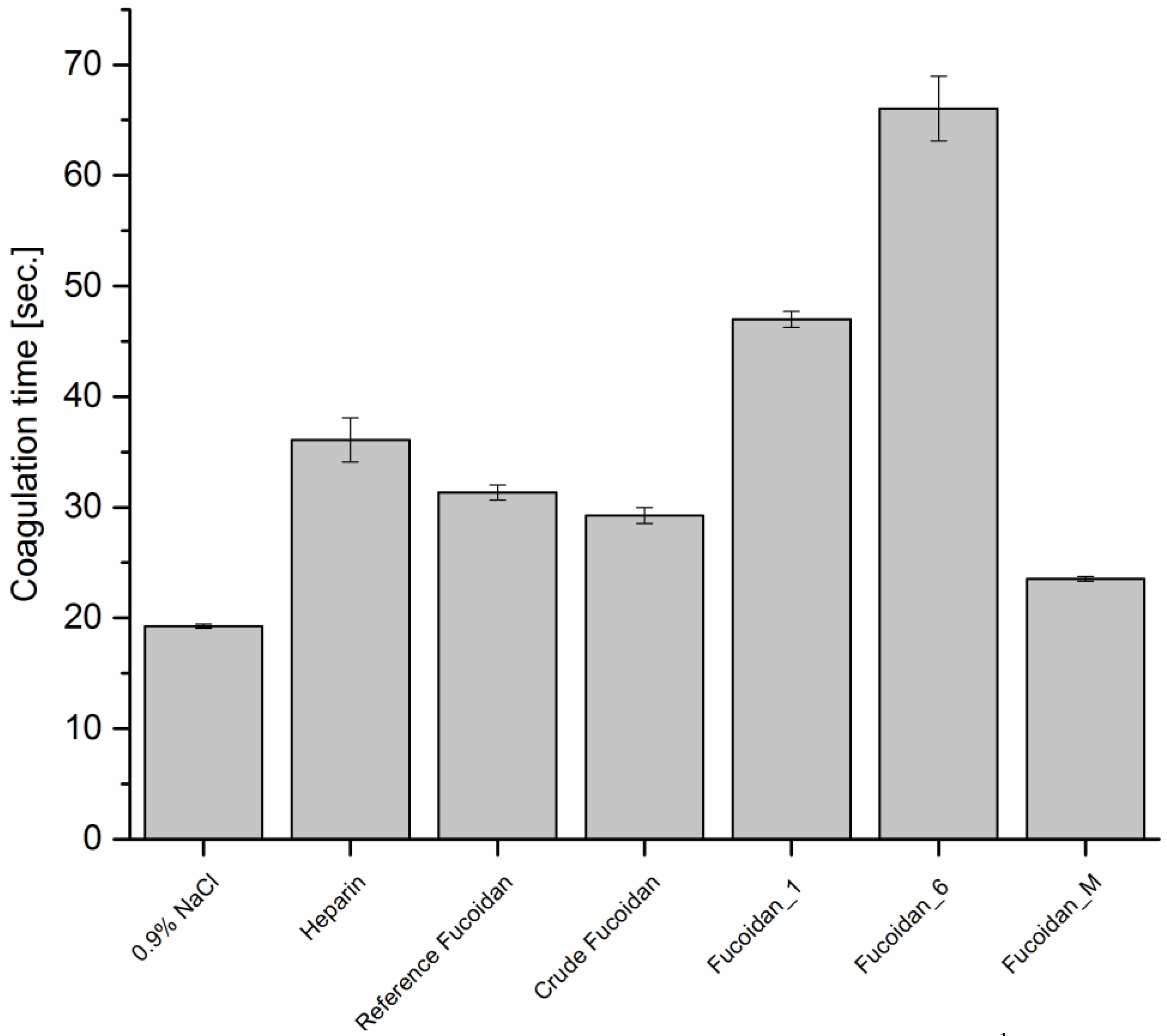
Thrombin Time (TT)
TT was determined applying protocol described by Denson and Bonnnar [
30]. In a ratio of 3:1, 0.6 mL of platelet-poor plasma was mixed with 0.2 mL of fucoidan solutions at a concentration of 10 µg·mL
−1. Each mixture was then incubated for 3 min at 37 °C before the addition of 0.2 mL pre-warmed TT reagent and the time for clot formation was recorded with three repetitions. Heparin (5 µg·mL
−1) and NaCl (0.9%
w/
v) were used as positive and negative control, respectively.
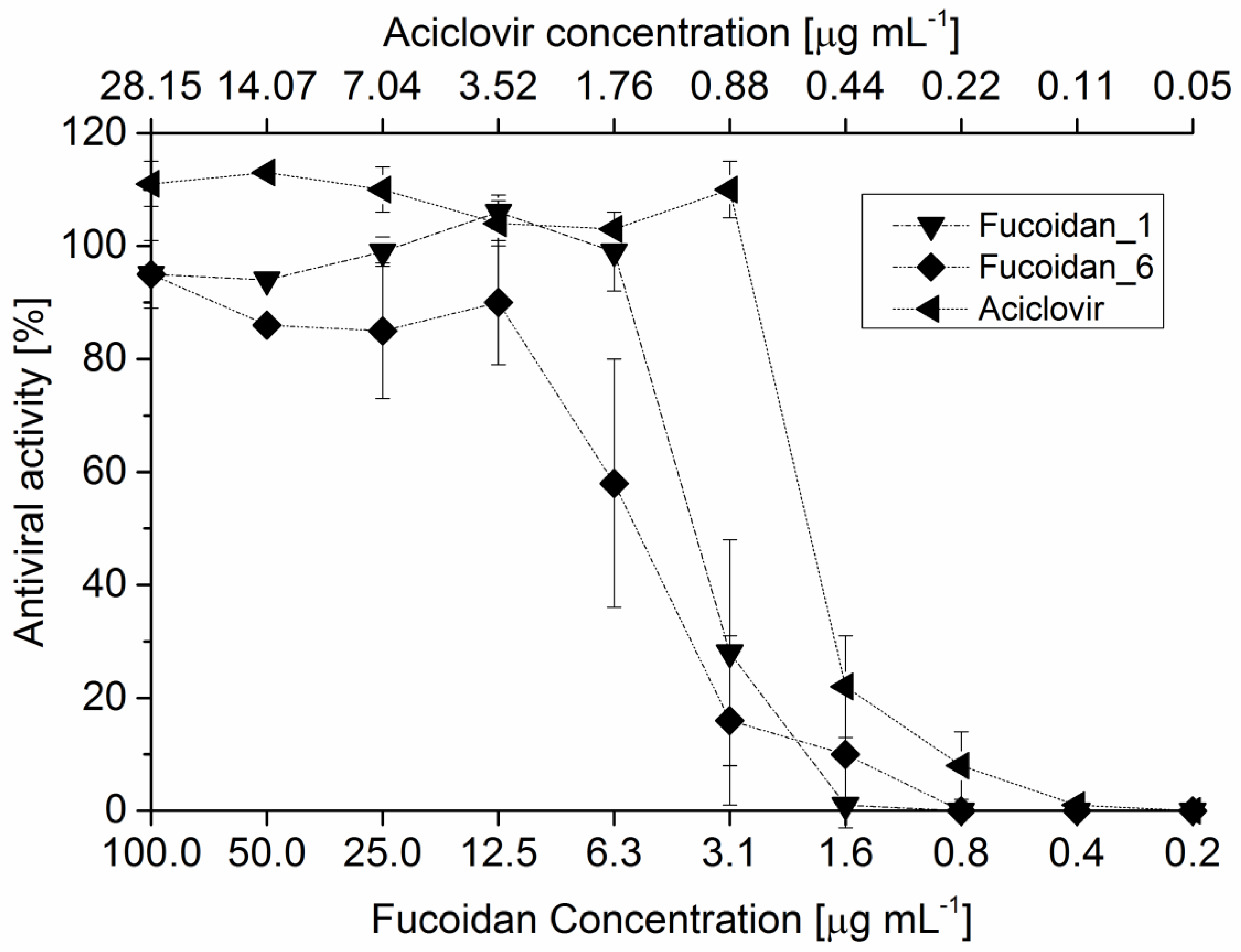
3.7. Antiviral Assay
The antiviral activity was carried out against a representative of the double strand DNA (
dsDNA) viruses: Herpes Simplex virus-type 1 (HSV-1). The screening assay was based on Tissue Culture Infection Dose 50 (TCID50) for antivirals and modified by Kleymann and Werling [
31]. A stock solution of 5 mg·mL
−1 of the three different fucoidan fractions were dissolved in phosphate saline buffer (PBS) at pH 7.4, while acyclovir as 10 mM in DMSO. The 50% inhibitory concentration (IC
50) was determined using a two-fold serial dilution (10 different concentrations) in the range of 0.2–100 and 0.054–28 µg·mL
−1 for fucoidan solutions and acyclovir, respectively. Each well contained 10,000 cells of Vero B4 cells (50 µL of a solution with 200,000 cells/mL provided in Roswell Park Memorial Institute medium (RPMI) containing 10% fetal calf serum (FCS) and penicillin/streptomycin (PS)), 50 µL pathogen with 5 to 500 CFU of HSV1 (strain HF ATCC-VR-260), the final volume of each well was then completed to 200 µL by the culture medium. Negative controls are performed by mixing 50 µL of pathogen, 50 µL cell suspensions, and 100 µL medium. After the respective incubation period, the wells of the microplate were washed with phosphate-buffered saline (PBS, 200 μL) and then filled with 200 μL PBS containing 10 μg·mL
−1 fluorescein diacetate (FDA), which is widely used to count viable cells and analyzes their vitality. Viable cells are able to convert the dye enzymatically by a cellular esterase activity releasing the fluorophore from the quenched dye of the non-fluorescent FDA. After 45 min of incubation at room temperature, fluorescence (RFU, relative fluorescence units) was measured using 485 nm for excitation and 538 nm for emission.
4. Conclusions
Physicochemical and biological evaluation of different fucoidan fractions purified by dye affinity chromatography is presented for the first time. These fractions were achieved by a recently-developed dye affinity chromatography technique, which is based on a specific formation of a charge transfer complex between thiazine dyes and sulfated polysaccharides. In addition, development of this technique helped downstream processing of extraction and purification simultaneously through selective capture of fucoidan from its crude extract, obtaining a new fraction of fucoidan called fucoidan_M. Pharmacological comparison between the different fucoidan fractions showed the importance of chain length, molecular weight, and structure comfortability for the anticoagulant activity, while sulfate ester groups position and content for the anti-herpetic activity.
Production of different fucoidan fractions with these qualities is a promising step on the way to standardize a method for fucoidan extraction, reveal new secrets of fucoidan structure-activity relationship (SAR) and elucidate its native and diverse chemical structures. It also helps to provide the market with a protocol for a reproducible commercial production of fucoidan.