Anaerobic Digestion of Laminaria japonica Waste from Industrial Production Residues in Laboratory- and Pilot-Scale
Abstract
:1. Introduction
1.1. Marine Macroalgae for Biogas Production
1.2. Anaerobic Digestion of Seaweeds
1.3. Marine Biomass from Industrial Waste Streams
1.4. Aims of the Study
2. Results
2.1. Composition of Laminaria japonica Waste LJW and Theoretical Methane Potential
| Category | Element | Standard Method | LJW | Maize | Unit |
|---|---|---|---|---|---|
| BioAbfV | Lead (Pb) | DIN 38406-E6:1981-05 | 3 | 2 | mg·kg−1 TS |
| Cadmium (Cd) | ISO 5961-E19:1995-05 | 0.5 | 0.7 | ||
| Chromium (Cr) | ISO 11885-E22:1997-11 | 14 | 0.5 | ||
| Copper (Cu) | ISO 11885-E22:1997-11 | 5 | 4.5–5 | ||
| Nickel (Ni) | ISO 11885-E22:1997-11 | 3 | 5 | ||
| Mercury (Hg) | EN 12338-E31:1998-07 | <0.04 | |||
| Zinc (Zn) | ISO 11885-E22:1997-11 | 28 | 35–56 | ||
| Macronutrients | Phosphorous (P) | ISO 11885-E22:1997-11 | 2060 | 2200 | |
| Potassium (K) | ISO 11885-E22:1997-11 | 89,900 | 17,800 | ||
| Magnesium (Mg) | ISO 11885-E22:1997-11 | 6800 | 2700 | ||
| Calcium (Ca) | ISO 11885-E22:1997-11 | 14,000 | 4500 | ||
| Sulfur (S) | ISO 11885-E22:1997-11 | 8590 | 2700 | ||
| C/N ratio | 10.5:1 | ~30:1 | |||
| Total carbon | ISO 10694:1996-08 | 21 | 43 | % TS | |
| Total nitrogen | ISO 1161:1997-05 | 20,000 | 14,000 | mg·kg−1 TS | |
| Micronutrients | Molybdenum (Mo) | ISO 11885-E22:1997-11 | 1 | 0.3 | mg·kg−1 TS |
| Iron (Fe) | ISO 11885-E22:1997-11 | 3440 | 184 | ||
| Cobalt (Co) | ISO 11885-E22:1997-11 | 1.5 | 65 | ||
| Selenium (Se) | DIN 38405-D23:1994-10 | 0.3 | |||
| Manganese (Mn) | ISO 11885-E22:1997-11 | 150 | 29 |
| Component | Share [%] | Theoretical CH4 | Unit | Standard Method | |
|---|---|---|---|---|---|
| TS | VS | ||||
| Volatile solids | 50.9 | Described in Section 4.8.1. | |||
| Carbohydrate | 39.2 | 145 | 285 | mL·g−1 | Calculated |
| Fiber | 6.4 | - | - | Weender method | |
| Protein | 11.4 | 52 | 102 | mL·g−1 | Kiejdahl method |
| Lipid | 0.3 | 2 | 4 | mL·g−1 | VDLUFA Bd. III, Kap 5.1.1 |
| Inorganic solids | 49.1 | - | - | Described in Section 4.8.1. | |
| Total | 100 | 199 | 391 | mL·g−1 | |
2.2. BMP of Acid Hydrolysis Pretreated and Untreated LJW
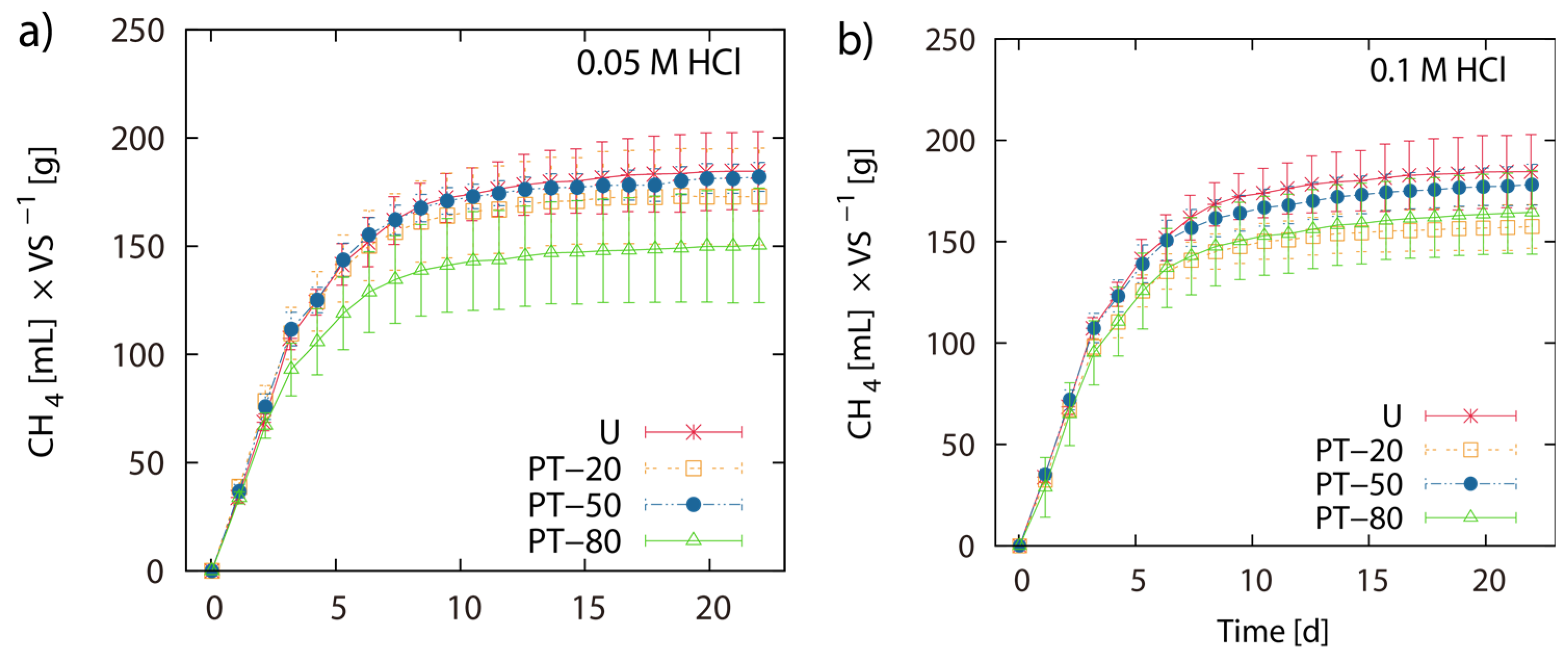
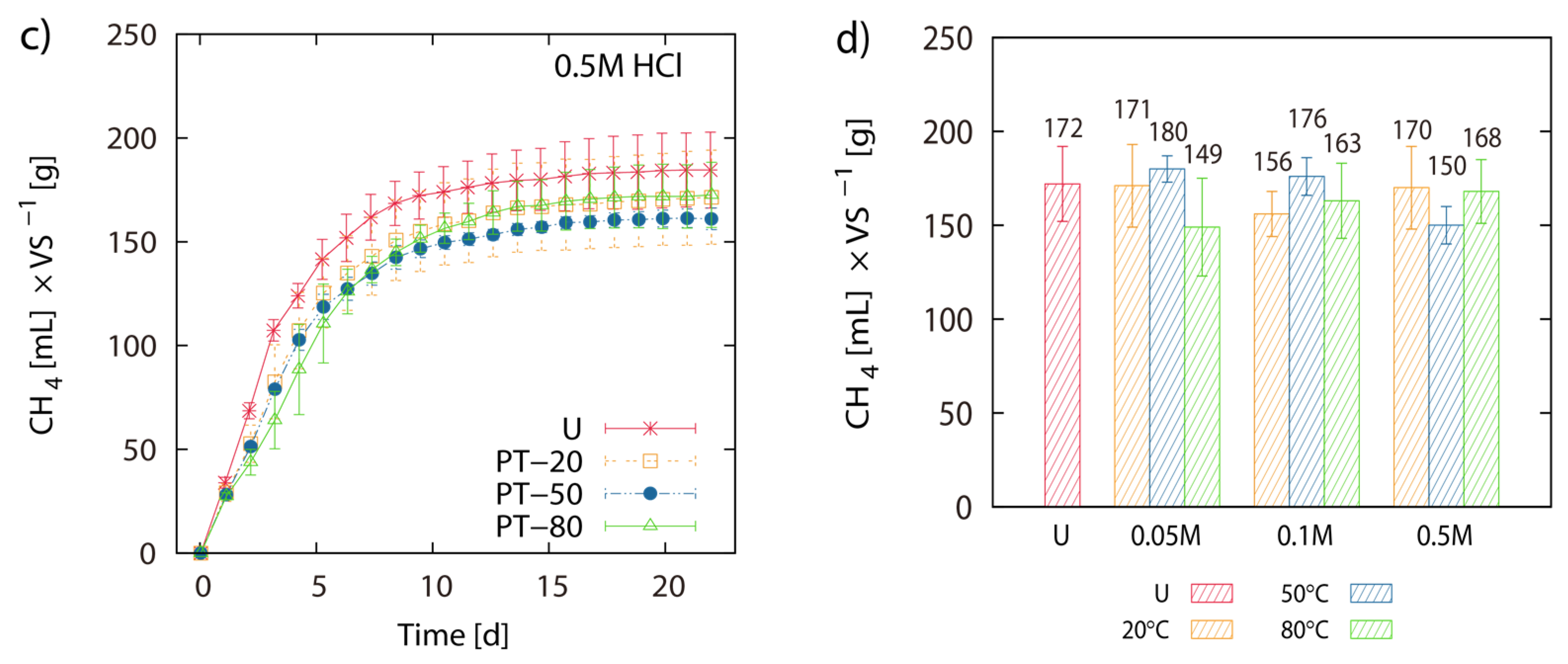


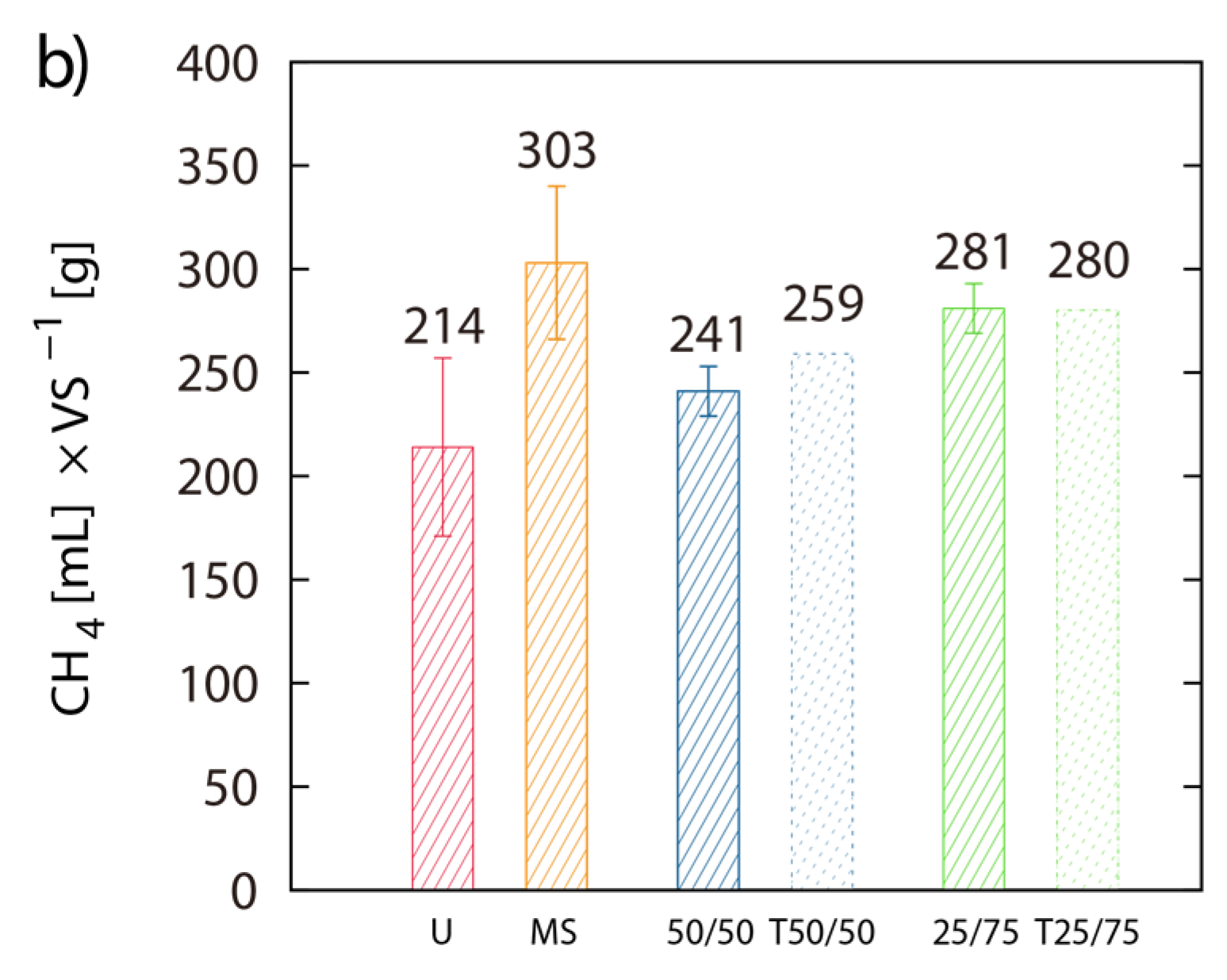
2.3. BMP of LJW and Evaluation of Methane Production for Co-Digestion with Maize Silage

2.4. Evaluation of Methane Production Dynamics
| Experiment | Substrate | K (day−1) | T50 | T70 | T90 | BMP [mL·g−1] | SD | +/− |
|---|---|---|---|---|---|---|---|---|
| E1 | U-1 | 0.2568 | 2.7 | 4.7 | 9.0 | 172 | 20 | - |
| E2 | U-2 | 0.403 | 1.7 | 3.0 | 5.7 | 178 | 25 | +3% † |
| E3 | U-3 | 0.1792 | 3.9 | 6.7 | 12.8 | 214 | 43 | +24% † |
| E1 | LJW-0.05 M-20 °C | 0.2929 | 2.4 | 4.1 | 7.9 | 171 | 22 | ±0% † |
| E1 | LJW-0.05 M-50 °C | 0.2787 | 2.5 | 4.3 | 8.3 | 180 | 7 | +5% † |
| E1 | LJW-0.05 M-80 °C | 0.2476 | 2.4 | 4.2 | 8.0 | 149 | 26 | −13% † |
| E1 | LJW-0.1 M-20 °C | 0.2836 | 2.4 | 4.2 | 8.1 | 156 | 12 | −9% † |
| E1 | LJW-0.1 M-50 °C | 0.2713 | 2.6 | 4.4 | 8.5 | 176 | 10 | +2% † |
| E1 | LJW-0.1 M-80 °C | 0.2571 | 2.7 | 4.7 | 8.0 | 163 | 23 | −5% † |
| E1 | LJW-0.5 M-20 °C | 0.2165 | 3.2 | 5.6 | 10.6 | 170 | 22 | ±0% † |
| E1 | LJW-0.5 M-50 °C | 0.2198 | 3.1 | 5.5 | 10.5 | 150 | 10 | −13% † |
| E1 | LJW-0.5 M-80 °C | 0.1712 | 4.0 | 7.0 | 13.4 | 168 | 17 | −2% † |
| E2 | LJW-HCl-80 °C-pH 1.2 | 0.29 | 2.4 | 4.1 | 7.9 | 169 | 15 | −2% † |
| E2 | LJW-FGC-80 °C-pH 1.2 | 0.2621 | 2.6 | 4.6 | 8.8 | 168 | 20 | −2% † |
| E2 | LJW-0.2 M-100 °C | 0.2643 | 2.6 | 4.6 | 8.7 | 180 | 5 | +5% † |
| E3 | MS-untreated | 0.2643 | 2.6 | 4.6 | 8.7 | 303 | 37 | - |
| E3 | MS/LJW-50/50 | 0.2047 | 3.4 | 5.9 | 11.2 | 241 | 12 | −7% ‡ |
| E3 | MS/LJW-75/25 | 0.2297 | 3.0 | 5.2 | 10.0 | 281 | 12 | ±0% ‡ |
2.5. Continuous Fermentation Studies—Laboratory-Scale
| Mode | Phase | Time (Days) | CH4 Production (mL·g−1·day−1 VS) | OLR (g·L−1·day−1) | HRT (d) | Times HRT |
|---|---|---|---|---|---|---|
| Lab-scale | P1 | 3–22 | 191 | 1.0 | 62.5 | 0.3 |
| P2 | 23–35 | 165 | 1.5 | 62.5 | 0.2 | |
| P3 | 36–40 | 124 | 2.0 | 62.5 | <0.1 | |
| P4 | 41–89 | 178 | 2.5 | 62.5 | 0.8 | |
| P5 | 90–175 | 173 | 2.5 | 40 | 2.1 | |
| Pilot-scale | P0 | ~150 | Pre-run | - | - | - |
| P1 | 4–50 | 189 | 2.0 | 32 | 1.4 |
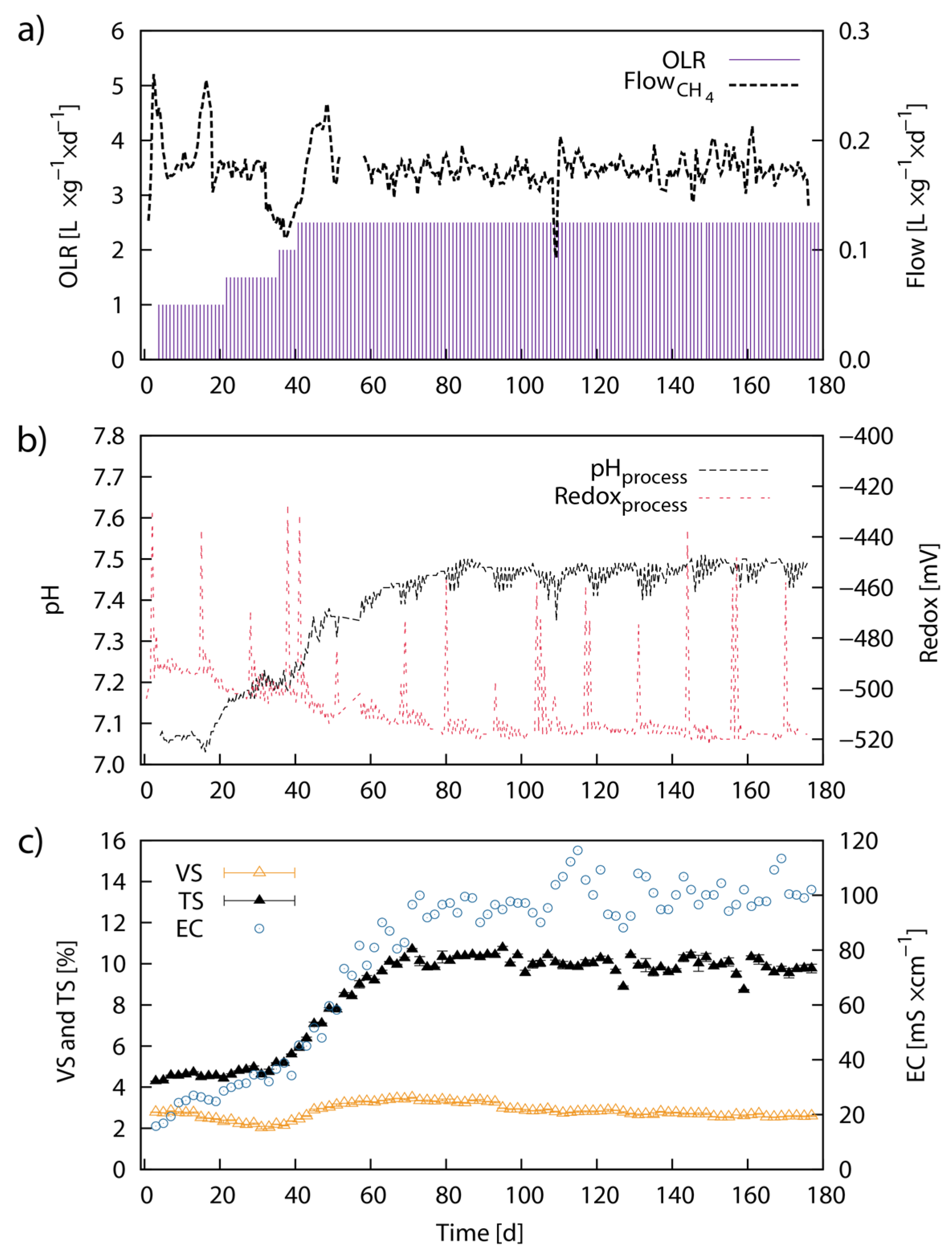

2.6. Analysis of Fermentation Residue
| Category | Element | LJWR | Digestate | DüMV | Unit |
|---|---|---|---|---|---|
| BioAbfV | Lead (Pb) | 5 | 2.9 | 150 | mg·kg−1 TS |
| Cadmium (Cd) | 1 | 0.26 | 1.5 | ||
| Chromium (Cr) | 17 | 9.0 | 300 | ||
| Copper (Cu) | 11 | 69 | 70 | ||
| Nickel (Ni) | 5 | 7.5 | 80 | ||
| Mercury (Hg) | <0.04 | 0.03 | 1 | ||
| Zinc (Zn) | 46 | 316 | 500 | ||
| Macronutrients | Phosphorous (P) | 2180 | 25,700 | 300 | |
| Potassium (K) | 238,000 | 71,400 | 500 | ||
| Magnesium (Mg) | 11,000 | 12,000 | 300 | ||
| Calcium (Ca) | 13,300 | 30,000 | 500 | ||
| Sulfur (S) | 10,300 | 4710 | 300 | ||
| C/N ratio | 4.3:1 | 6.4:1 | |||
| Total carbon | 13 | 43 | % TS | ||
| Total nitrogen | 30,000 | 67,140 | 1000 | mg·kg−1 TS | |
| Micronutrients | Molybdenum (Mo) | 1.8 | 2 | mg·kg−1 TS | |
| Iron (Fe) | 3330 | 100 | |||
| Cobalt (Co) | 2.0 | 4 | |||
| Selenium (Se) | 0.2 | ||||
| Manganese (Mn) | 170 | 200 |
2.7. Continuous Fermentation Studies—Pilot-Scale
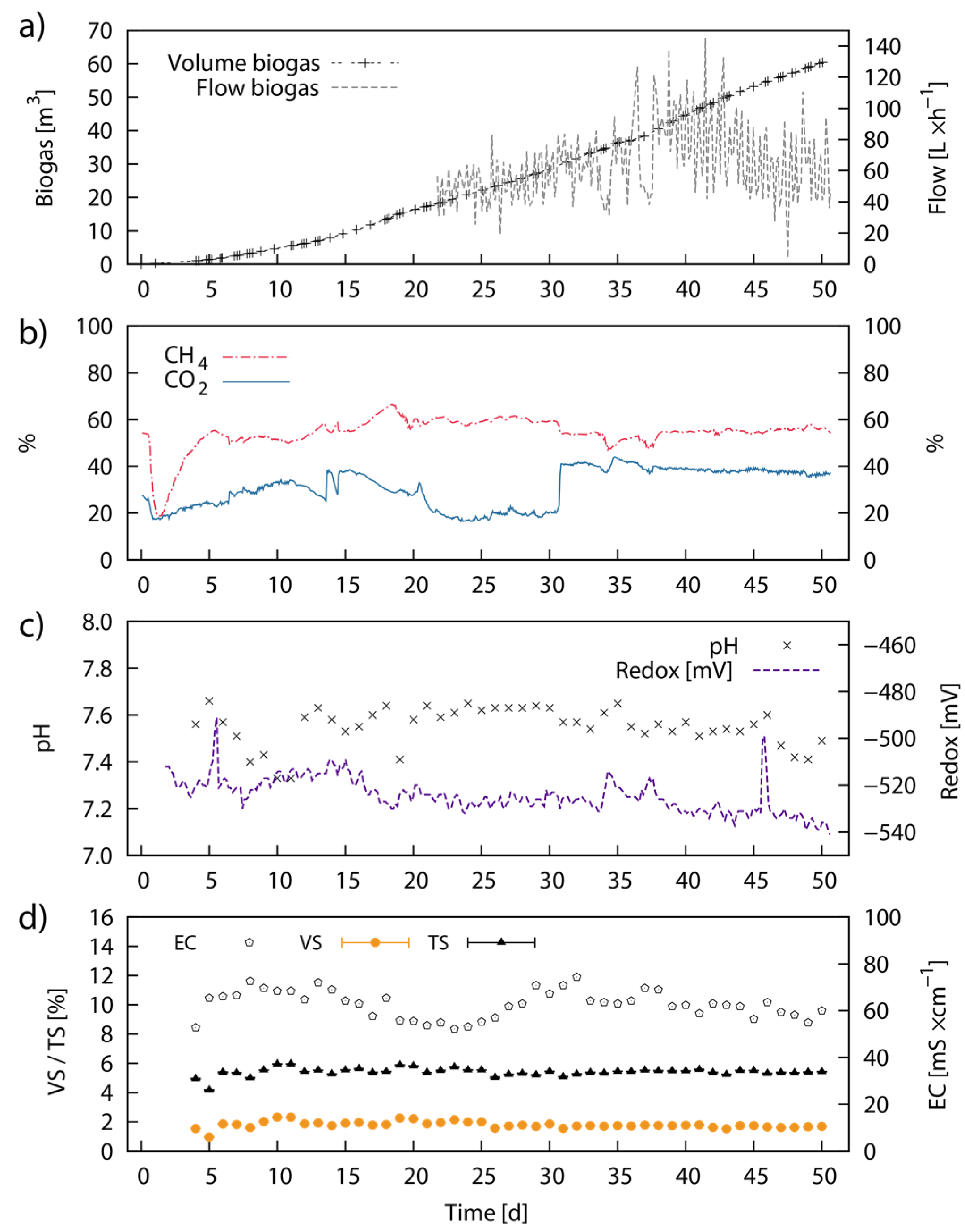
| Name | Reaction Time | Temperature | Medium | Concentration | pH after PT |
|---|---|---|---|---|---|
| U-1, U-2, U-3 | - | - | H2O | - | - |
| PT-20 | 2 h | 20 °C | HCl | 0.05 M | 4.0 |
| PT-50 | 2 h | 50 °C | HCl | 0.05 M | 4.3 |
| PT-80 | 2 h | 80 °C | HCl | 0.05 M | 4.5 |
| PT-20 | 2 h | 20 °C | HCl | 0.1 M | 2.9 |
| PT-50 | 2 h | 50 °C | HCl | 0.1 M | 2.9 |
| PT-80 | 2 h | 80 °C | HCl | 0.1 M | 3.1 |
| PT-20 | 2 h | 20 °C | HCl | 0.5 M | 0.5 |
| PT-50 | 2 h | 50 °C | HCl | 0.5 M | 0.6 |
| PT-80 | 2 h | 80 °C | HCl | 0.5 M | 0.7 |
| HCl-100 | 2 h | 100 °C | HCl | 0.2 M | 1.2 |
| HCl-80 | 2 h | 80 °C | HCl | pH 1.2 | 1.2 |
| FGC | 2 h | 80 °C | FGC | pH 1.2 | 1.9 |
3. Discussion
3.1. LJW Biomass Composition and Theoretical BMP
3.2. Effect of Acid Hydrolysis on BMP
3.3. Effect of Co-Digestion with Maize Silage on BMP
3.4. Effect of Acid Hydrolysis and Co-Digestion on Methane Formation Dynamics
3.5. Biomethane Production from LJW in Continuous Anaerobic Digestion (Laboratory-Scale)
3.6. Analysis of Fermentation Residue
3.7. Performance in Pilot-Scale Continuous Anaerobic Digestion
4. Experimental Section
4.1. Macroalgae Biomass and Maize Silage

4.2. Inoculum Sludge
4.3. Flue Gas Condensate
4.4. Acid Hydrolysis Pretreatment
4.5. BMP Tests and Batch Array
4.6. Continuous Setup CSTR 10 L
4.7. Pilot-Scale Bioreactor System
4.8. Analytical Methods and Calculations
4.8.1. Measurement of Volatile Solids and Total Solids
4.8.2. Data Treatment from Methane Production
4.8.3. Calculations for Comparison of Methane Formation Dynamics in Batch
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ölz, S.; Beerepoot, M. Deploying Renewables in Southeast Asia: Trends and Potentials. 2010. Available online: https://www.iea.org/publications/freepublications/publication/Renew_SEAsia.pdf (accessed 21 May 2015).
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- FNR. Bioenergy in Germany—Facts and Figures. 2013. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/b/a/basisdaten_9x16_2013_engl_web.pdf (accessed on 25 June 2015).
- Jung, K.A.; Lim, S.; Kim, Y.; Park, J.M. Potentials of Macroalgae as Feedstocks for Biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Scharlemann, J.P.W.; Laurance, W.F. How Green are Biofuels? Science 2008, 319, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.S. Increasing Agricultural Water use Efficiency to Meet Future Food Production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases through Emissions from Land-use Change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Beringer, T.; Lucht, W.; Schaphoff, S. Bioenergy Production Potential of Global Biomass Plantations Under Environmental and Agricultural Constraints. GCB Bioenergy 2011, 3, 299–312. [Google Scholar] [CrossRef]
- Carlsson, A.S.; Beilen, J.V.; Möller, R.; Clayton, D. Micro- and Macro-Algae: Utility for Industrial Applications. EPOBIO 2007. Available online: http://www.biofuelstp.eu/downloads/epobio_aquatic_report.pdf (accessed on 20 May 2015).
- Duarte, C.M.; Regaudie-de-Gioux, A. Thresholds of Gross Primary Production for the Metabolic Balance of Marine Planktonic Communities. Limnol. Oceanogr. 2009, 54, 1015–1022. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K. Use of Macroalgae for Marine Biomass Production and CO2 Remediation: A Review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Rojan, J.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and Macroalgal Biomass: A Renewable Source for Bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y. Marine Macroalgae: An Untapped Resource for Producing Fuels and Chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Athens, GA, US, 2011; pp. 15–47. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry. 2003, FAO Fisheries—Technical Paper 441. Available online: http://www.fao.org/3/a-y4765e.pdf (accessed on 21 May 2015).
- FAO. The State of World Fisheries and Aquaculture. 2012. Available online: http://www.fao.org/docrep/016/i2727e/i2727e.pdf (accessed on 25 June 2015).
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources—An Introduction; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 443. [Google Scholar]
- Wellinger, A.; Murphy, J.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Woodhead Publishing: Cambridge, UK, 2013; p. 512. [Google Scholar]
- Shilton, A.; Guieysse, B. Sustainable Sunlight to Biogas is Via Marginal Organics. Curr. Opin. Biotechnol. 2010, 21, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Barbot, Y.N.; Falk, H.M.; Benz, R. Thermo-Acidic Pretreatment of Marine Brown Algae Fucus Vesiculosus to Increase Methane Production—A Disposal Principle for Macroalgae Waste from Beaches. J. Appl. Phycol. 2014, 1–9. [Google Scholar] [CrossRef]
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation Potential of Macroalgae Ulva Spp. and Gracilaria Spp. and in Co-Digestion with Waste Activated Sludge. Bioresour. Technol. 2012, 114, 320–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal Variation in Laminaria Digitata and its Impact on Biochemical Conversion Routes to Biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Bird, K.T.; Chynoweth, D.P.; Jerger, D.E. Effects of Marine Algal Proximate Composition on Methane Yields. J. Appl. Phycol. 1990, 2, 207–213. [Google Scholar] [CrossRef]
- Migliore, G.; Alisi, C.; Sprocati, A.R.; Massi, E.; Ciccoli, R.; Lenzi, M.; Wang, A.; Cremisini, C. Anaerobic Digestion of Macroalgal Biomass and Sediments Sourced from the Orbetello Lagoon, Italy. Biomass Bioenergy 2012, 42, 69–77. [Google Scholar] [CrossRef]
- Vergara-Fernandez, A.; Vargas, G.; Alarcan, N.; Velasco, A. Evaluation of Marine Algae as a Source of Biogas in a Two-Stage Anaerobic Reactor System. Biomass Bioenergy 2008, 32, 338–344. [Google Scholar] [CrossRef]
- Hughes, A.D.; Maeve, S.K.; Black, K.D.; Stanley, M.S. Biogas from Macroalgae: Is it Time to Revisit the Idea? Biotechnol. Biofuels 2012, 86. [Google Scholar] [CrossRef] [PubMed]
- Østgaard, K.; Indergaard, M.; Markussen, S.; Knutsen, S.; Jensen, A. Carbohydrate Degradation and Methane Production during Fermentation of Laminaria Saccharina (Laminariales, Phaeophyceae). J. Appl. Phycol. 1993, 5, 333–342. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjak-Bræk, G. Alginates from Algae. In Polysaccharides and Polyamides in the Food Industry. Properties, Production and Patents; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production—A Feasibility Study with Recommendations for further Research. 2008. Available online: http://www.thecrownestate.co.uk/media/5765/marine_biomass_anaerobic_biogas.pdf (accessed on 25 June 2015).
- Nielsen, H.B.; Heiske, S. Anaerobic Digestion of Macroalgae: Methane Potentials, Pre-Treatment, Inhibition and Co-Digestion. Water Sci. Technol. 2011, 64, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas Production from the Brown Seaweed Saccharina Latissima: Thermal Pretreatment and Codigestion with Wheat Straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Hinks, J.; Edwards, S.; Sallis, P.J.; Caldwell, G.S. The Steady State Anaerobic Digestion of Laminaria Hyperborea—Effect of Hydraulic Residence on Biogas Production and Bacterial Community Composition. Bioresour. Technol. 2013, 143, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, F.; Blumberga, D.; Gigli, E. Biogas from Marine Macroalgae: A New Environmental Technology—Life Cycle Inventory for a further LCA. Environ. Clim. Technol. 2010, 4, 97–108. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and Microalgae: A Positive Prospect for Biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrère, H. Effect of Thermochemical Pretreatment on the Solubilization and Anaerobic Biodegradability of the Red Macroalga Palmaria Palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Edyvean, R.G.J.; Stanley, I.M.; Stanley, S.O. Biogas Production from Seaweed Waste Following Alginate Extraction. In Biodeterioration 7; Houghton, D.R., Smith, R.N., Eggins, H.O.W., Eds.; Elsevier Science Publishers Ltd.: Barking, UK, 1988; pp. 819–824. [Google Scholar]
- Kerner, K.N.; Hanssen, J.F.; Pedersen, T.A. Anaerobic Digestion of Waste Sludges from the Alginate Extraction Process. Bioresour. Technol. 1991, 37, 17–24. [Google Scholar] [CrossRef]
- Matsui, T.; Amano, T.; Koike, Y.; Saiganji, A.; Saito, H. Methane Fermentation of Seaweed Biomass. Sustainable Nonfuel Products/Production Systems from Biomass Resources (TE017). 2006. Available online: http://www3.aiche.org/Proceedings/Abstract.aspx?PaperID=73948 (accessed on 21 May 2015).
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis (PNNL-19944). 2010, PNNL-19944. Available online: http://www.pnl.gov/main/publications/external/technical_reports/PNNL-19944.pdf (accessed on 7 April 2015).
- North, W.J.; Bird, K.T.; Benson, P.H. Oceanic Farming of Macrocystis, the Problems and Non-Problems. In Seaweed Cultivation for Renewable Resources (Developments in Aquaculture and Fisheries Science); Elsevier Science Ltd.: Amsterdam, The Netherlands, 1987; pp. 1–38. [Google Scholar]
- Langlois, J.; Sassi, J.; Jard, G.; Steyer, J.; Delgenes, J.; Hélias, A. Life Cycle Assessment of Biomethane from Offshore-Cultivated Seaweed. Biofuels Bioprod. Biorefining 2012, 6, 387–404. [Google Scholar] [CrossRef]
- Yue, H.; Sun, Y.; Jing, H.; Zeng, S.; Ouyang, H. The Analysis of Laminaria Japonica Industry and International Trade Situation in China. In Proceedings of Selected Articles of 2013 World Agricultural Outlook Conference; Springer: Berlin/Heidelberg, Germany, 2013; pp. 39–51. [Google Scholar]
- Thomsen, C.; Phytolutions GmbH, Bremen, Germany. Personal Communication, 2012.
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life Cycle Assessment of Biofuel Production from Brown Seaweed in Nordic Conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Felgentreu, C. Mais Unter Trockenen Bedingungen Produzieren—Düngungsstrategien Bei Wassermangel (Teil 2). Available online: http://images.raiffeisen.com/Raicom/Images/Geno/agri-v/dateien/1-07-mais-unter-trockenen-Bedingungen.pdf (accessed on 21 May 2015).
- FNR. Guide to Biogas—From Production to Use. 2012. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/g/u/guide_biogas_engl_2012.pdf (accessed on 25 June 2015).
- VDI-4630. Fermentation of Organic Materials—Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Available online: http://www.vdi.eu/guidelines/vdi_4630-vergaerung_organischer_stoffe_substratcharakterisierung_probenahme_stoffdatenerhebung_gaerversuche/ (accessed on 10 February 2012).
- FNR. Leitfaden Biogas—Von Der Gewinnung Zur Nutzung. 2014. Available online: http://mediathek.fnr.de/media/downloadable/files/samples/l/e/leitfadenbiogas2013_web_komp.pdf (accessed on 25 June 2015).
- Demirel, B.; Scherer, P. Trace Element Requirements of Agricultural Biogas Digesters during Biological Conversion of Renewable Biomass to Methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany Macroalgae Screening: Composition and Methane Potential for Potential Alternative Sources of Energy and Products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P. Bestimmung Der Abbauraten Von Biogasanlagen. 2006. Available online: http://www.eti-brandenburg.de/fileadmin/eti_upload/downloads2008/06_Scherer.pdf (accessed on 21 May 2015).
- Chynoweth, D.P. Renewable Biomethane from Land and Ocean Energy Crops and Organic Wastes. Hortscience 2006, 40, 283–286. Available online: http://hortsci.ashspublications.org/content/40/2/283.full.pdf (accessed on 25 June 2015). [Google Scholar]
- Jung, K.; Kim, D.; Kim, H.; Shin, H. Optimization of Combined (Acid + thermal) Pretreatment for Fermentative Hydrogen Production from Laminaria Japonica using Response Surface Methodology (RSM). Int. J. Hydrog. Energy 2011, 36, 9626–9631. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.S.; Um, B.H.; Oh, K. Pretreatment of Laminaria Japonica for Bioethanol Production with Extremely Low Acid Concentration. Renew. Energy 2013, 54, 196–200. [Google Scholar] [CrossRef]
- Aida, T.M.; Yamagata, T.; Watanabe, M.; Smith, R.L., Jr. Depolymerization of Sodium Alginate Under Hydrothermal Conditions. Carbohydr. Polym. 2010, 80, 296–302. [Google Scholar] [CrossRef]
- Sarker, S.; Møller, H.B.; Bruhn, A. Influence of Variable Feeding on Mesophilic and Thermophilic Co-Digestion of Laminaria Digitata and Cattle Manure. Energy Convers. Manag. 2014, 87, 513–520. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas Productivity by Co-Digesting Taihu Blue Algae with Corn Straw as an External Carbon Source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wiese, J.; König, R. Einsatz Von Mess- Und Automatisierungstechnik Auf Modernen Biogasanlagen—Ergebnisse Großtechnischer Anwendungen. DVGW Energie Wasser Praxis 2008, 11, 16–21. [Google Scholar]
- Hanssen, J.F.; Indergaard, M.; Østgaard, K.; Bævre, O.A.; Pedersen, T.A.; Jensen, A. Anaerobic Digestion of Laminaria Spp. and Ascophyllum Nodosum and Application of End Products. Biomass 1987, 14, 1–13. [Google Scholar] [CrossRef]
- Menardo, S.; Gioelli, F.; Balsari, P. The Methane Yield of Digestate: Effect of Organic Loading Rate, Hydraulic Retention Time, and Plant Feeding. Bioresour. Technol. 2011, 102, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing Amendment Properties of Digestate by Studying the Organic Matter Composition and the Degree of Biological Stability during the Anaerobic Digestion of the Organic Fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review. In Biogas; Kumar, S., Ed.; InTech Europe: Rijeka, Croatia, 2012; p. 295. [Google Scholar]
- Vodegel, S.; Kristin, A.; Thomsen, C. Strandalgen Als Energierohstoff—Eine Verwertungsmöglichkeit? Müll Abfall 2011, 7, 319–321. [Google Scholar]
- Hobson, P.; Wheatley, A. Anaerobic Digestion—Modern Theory and Practice; Elsevier Applied Science: London, UK, 1993; p. 269. [Google Scholar]
- Matsui, T.; Koike, Y. Methane Fermentation of a Mixture of Seaweed and Milk at a Pilot-Scale Plant. J. Biosci. Bioeng. 2010, 110, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C. Potential Errors in the Quantitative Evaluation of Biogas Production in Anaerobic Digestion Processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The Potential of Algae Blooms to Produce Renewable Gaseous Fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbot, Y.N.; Thomsen, C.; Thomsen, L.; Benz, R. Anaerobic Digestion of Laminaria japonica Waste from Industrial Production Residues in Laboratory- and Pilot-Scale. Mar. Drugs 2015, 13, 5947-5975. https://doi.org/10.3390/md13095947
Barbot YN, Thomsen C, Thomsen L, Benz R. Anaerobic Digestion of Laminaria japonica Waste from Industrial Production Residues in Laboratory- and Pilot-Scale. Marine Drugs. 2015; 13(9):5947-5975. https://doi.org/10.3390/md13095947
Chicago/Turabian StyleBarbot, Yann Nicolas, Claudia Thomsen, Laurenz Thomsen, and Roland Benz. 2015. "Anaerobic Digestion of Laminaria japonica Waste from Industrial Production Residues in Laboratory- and Pilot-Scale" Marine Drugs 13, no. 9: 5947-5975. https://doi.org/10.3390/md13095947






