A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi
Abstract
:1. Introduction
2. Diversity of Deep-Sea Fungi
3. Bioactive Metabolites of Deep-Sea Fungi
3.1. Anticancer
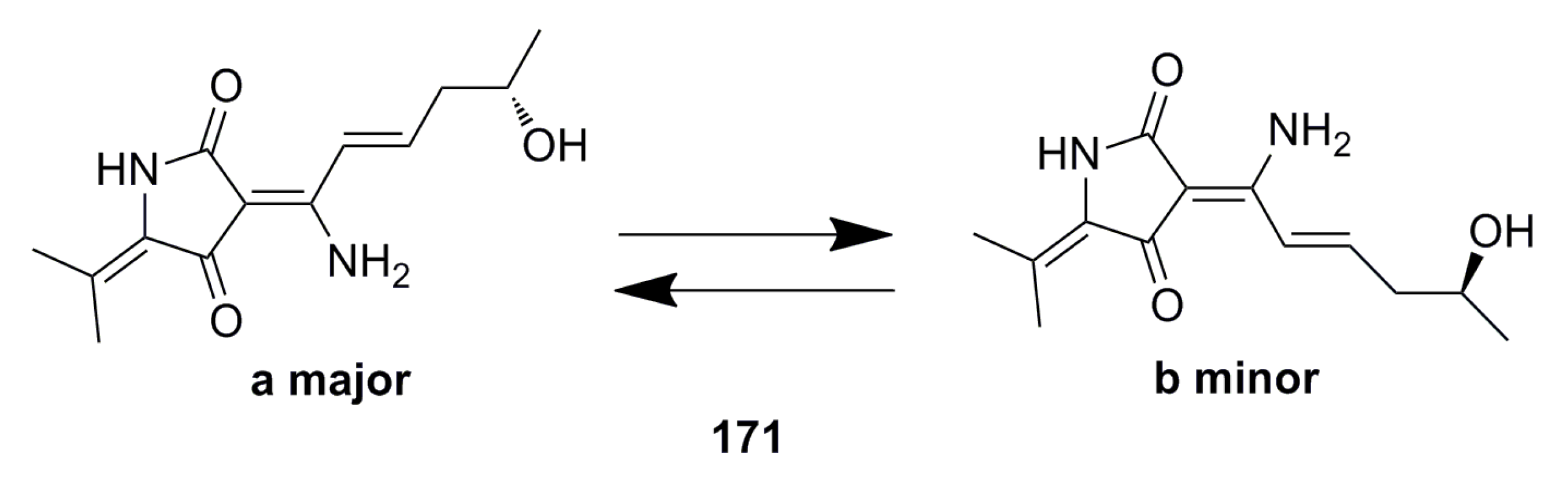
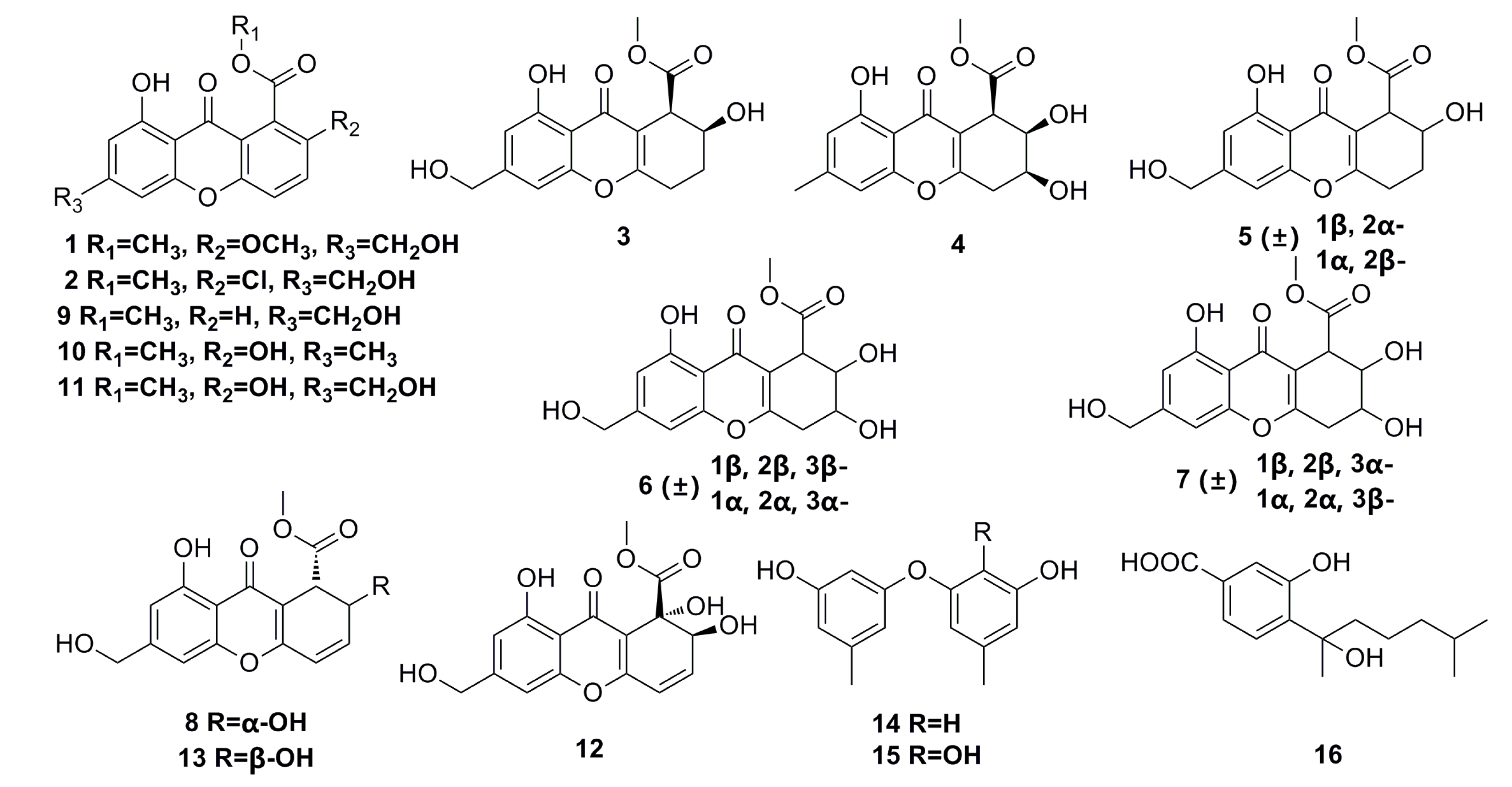
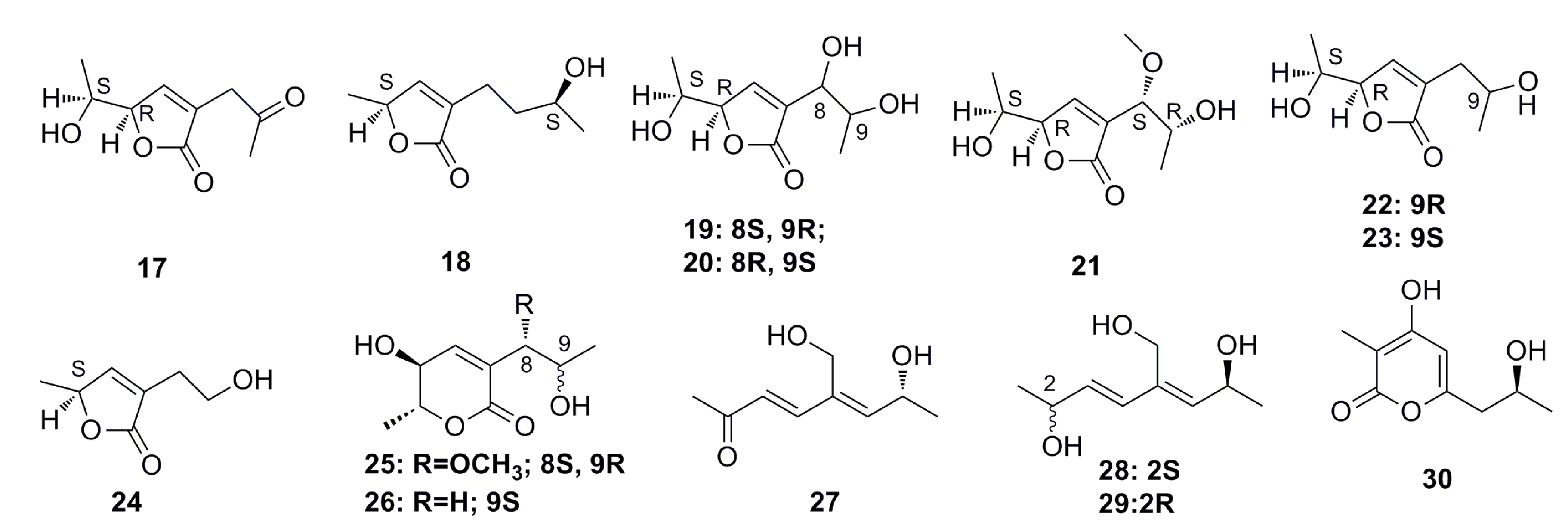
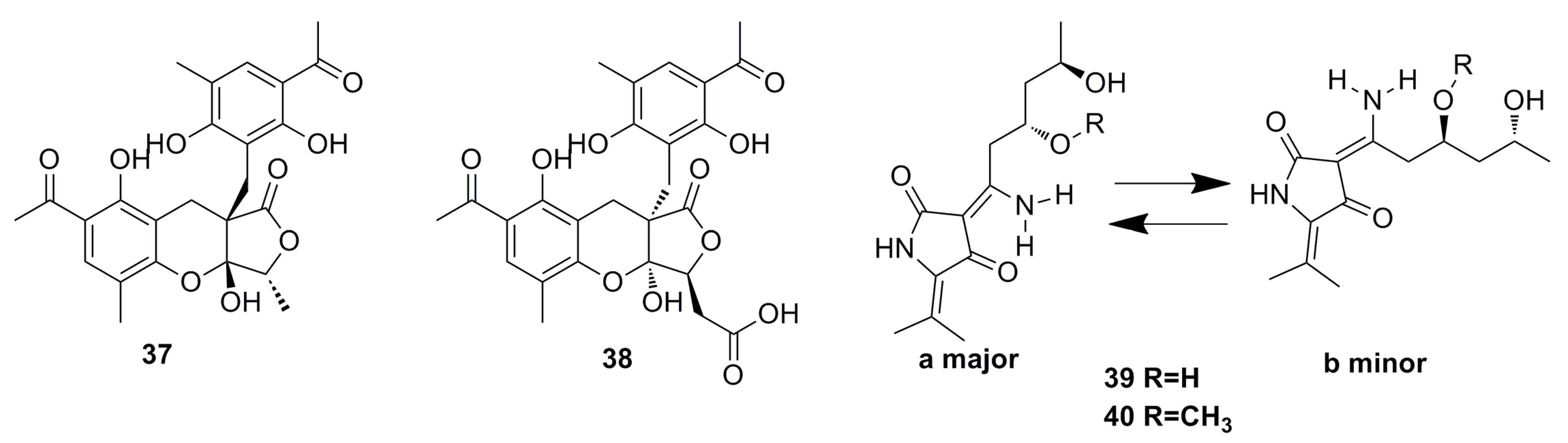
3.1.1. Polyketides Compounds

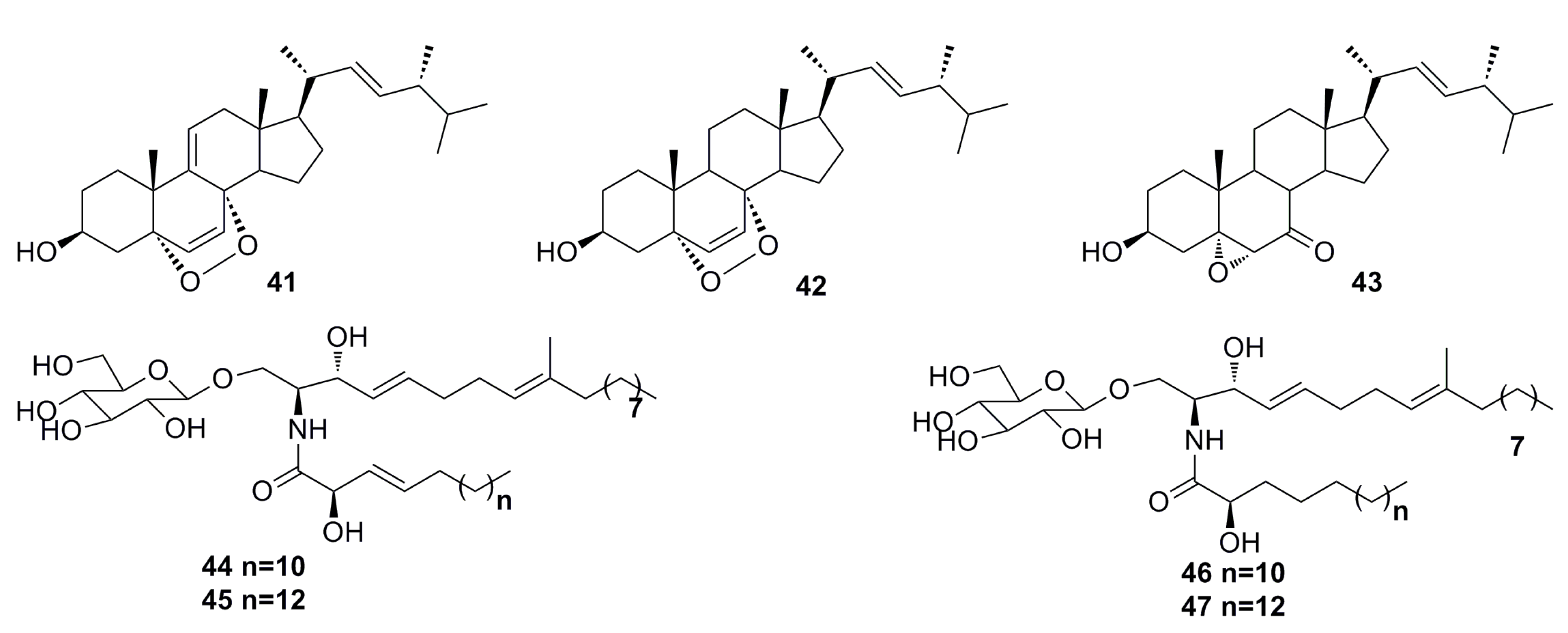
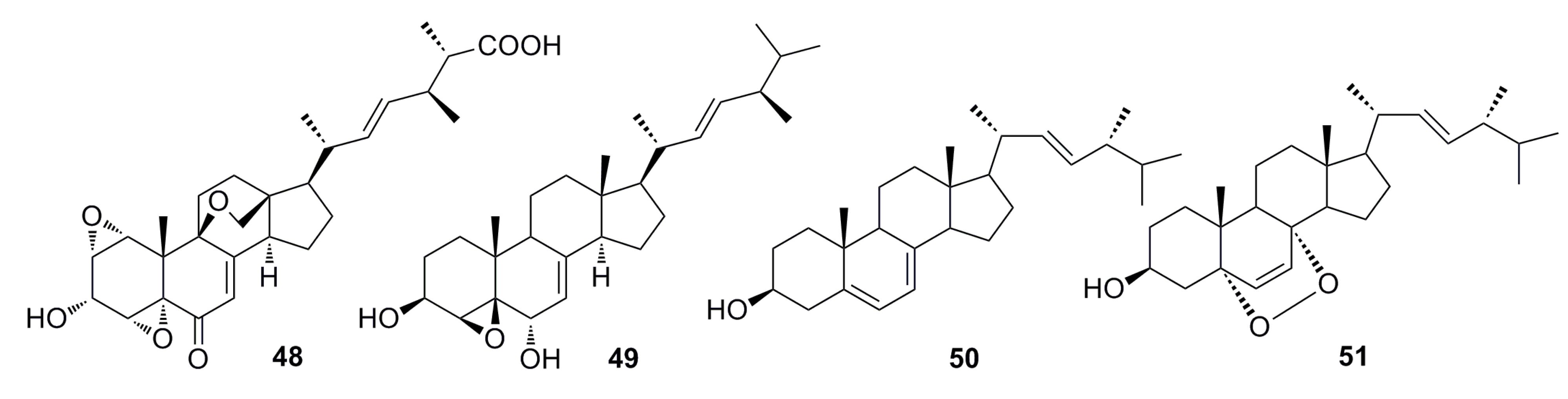
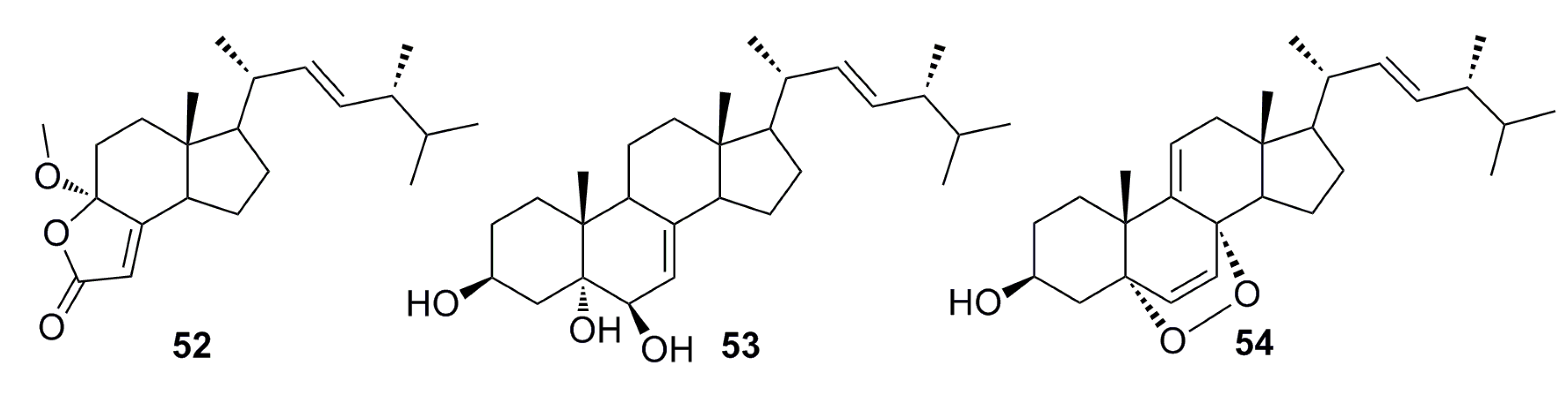
3.1.2. Steroid Derivatives
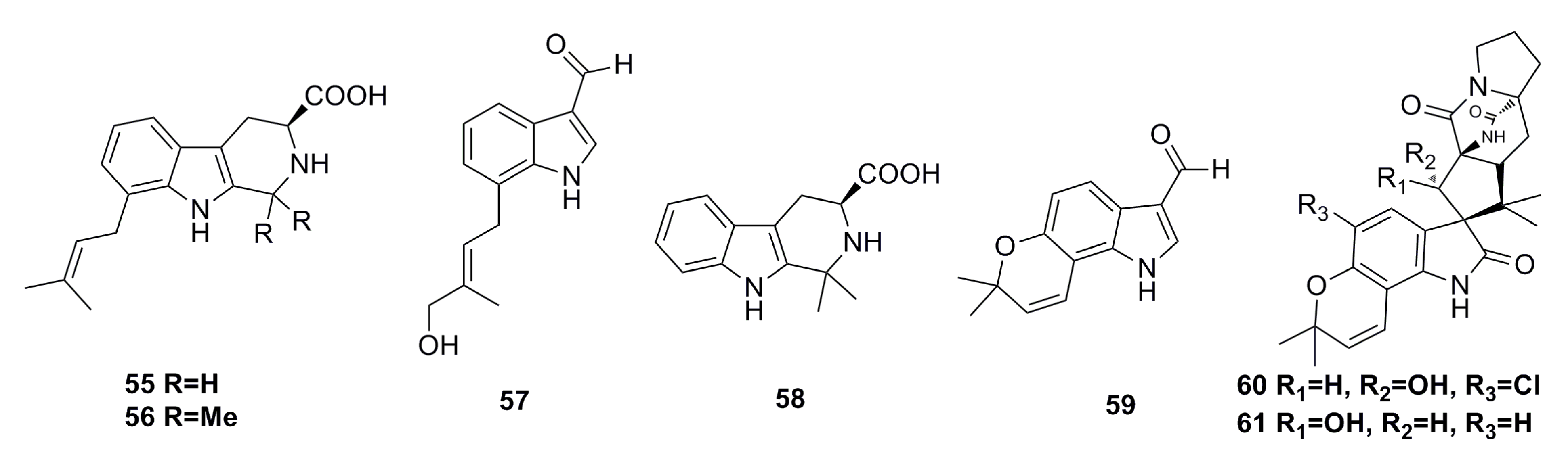
3.1.3. Indole Derivatives
3.1.4. Sesquiterpenoids
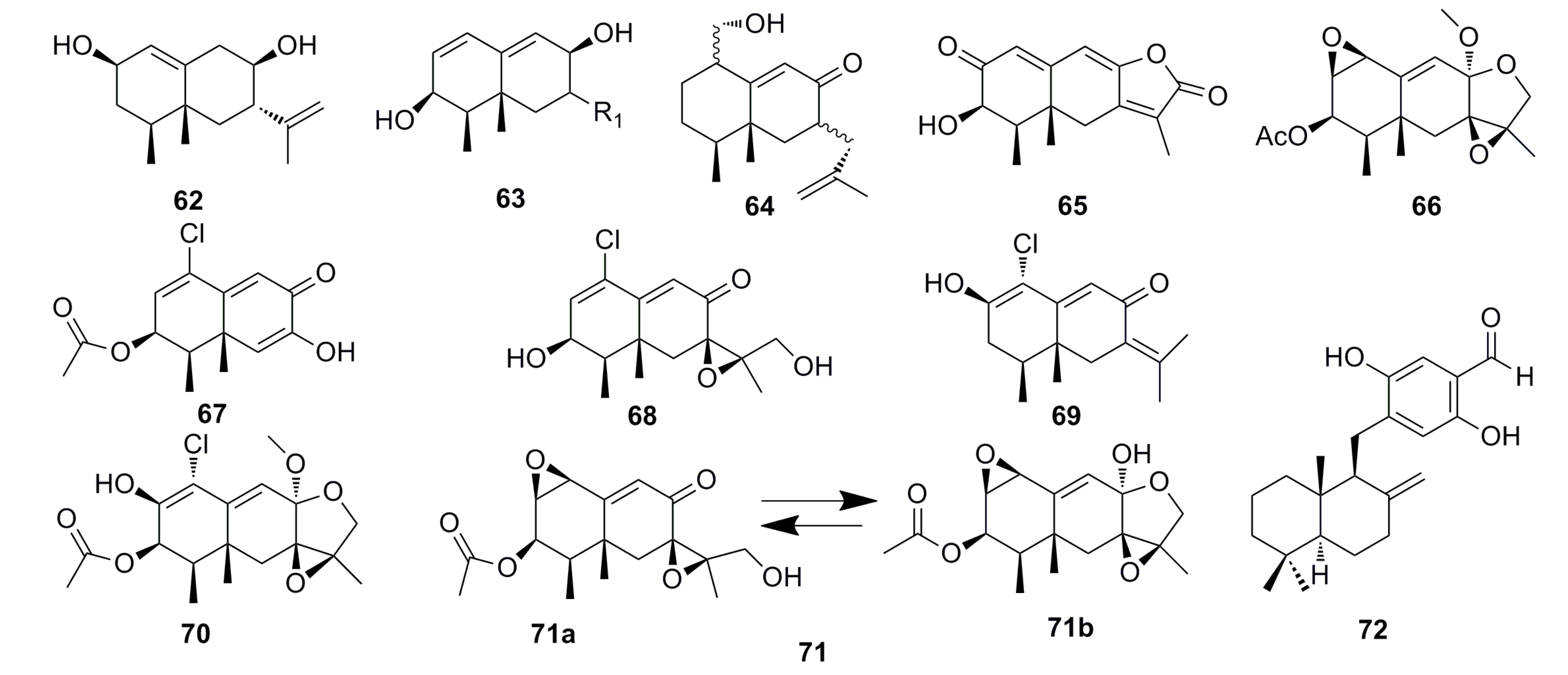
3.1.5. Alkaloid Compounds
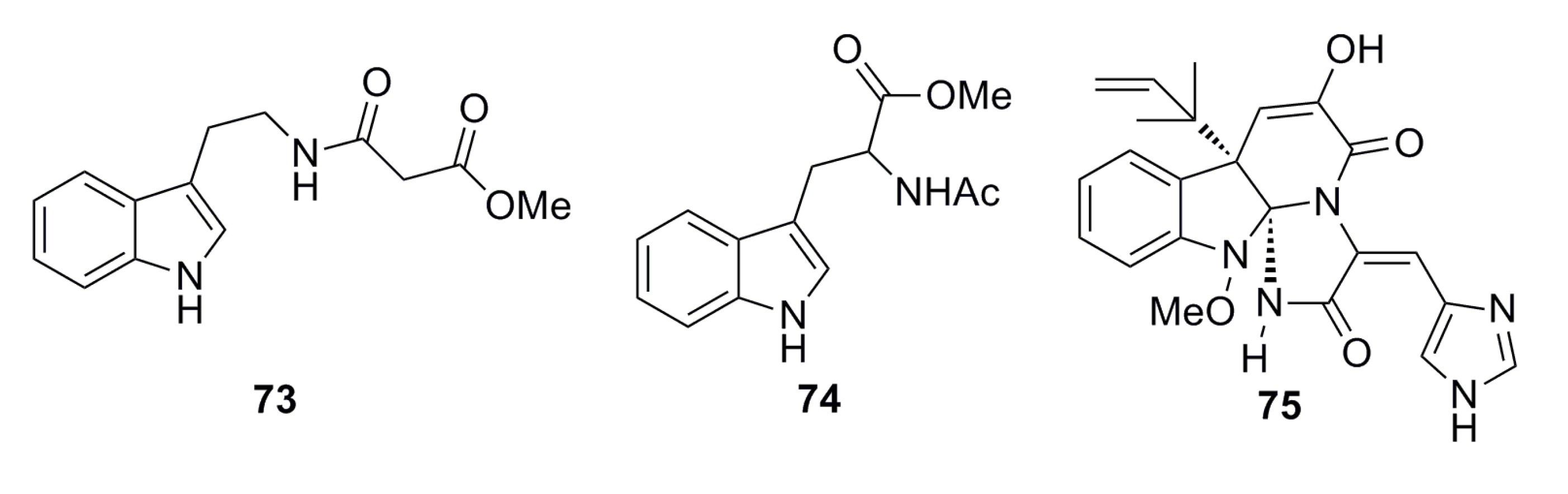
3.1.6. Aromatic Compounds
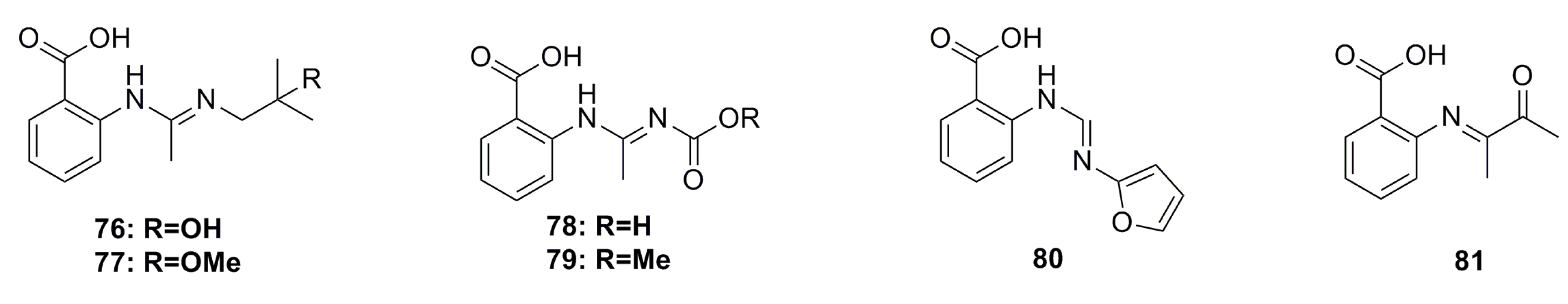
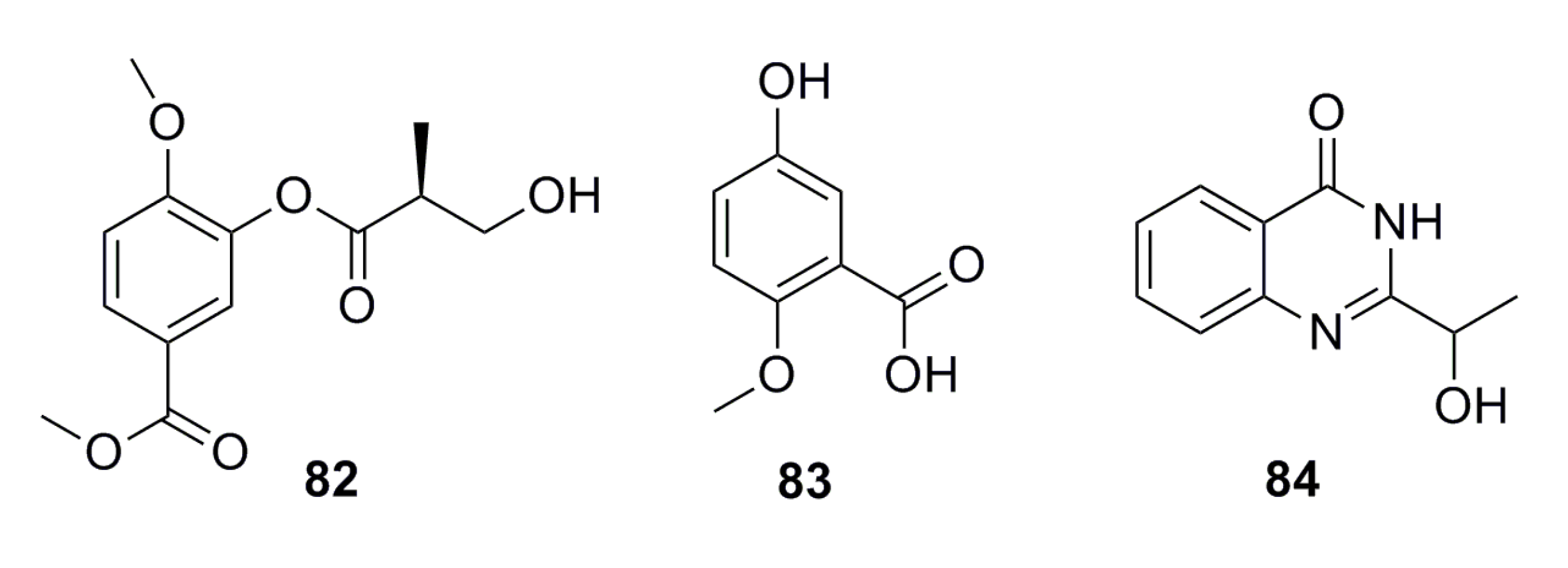
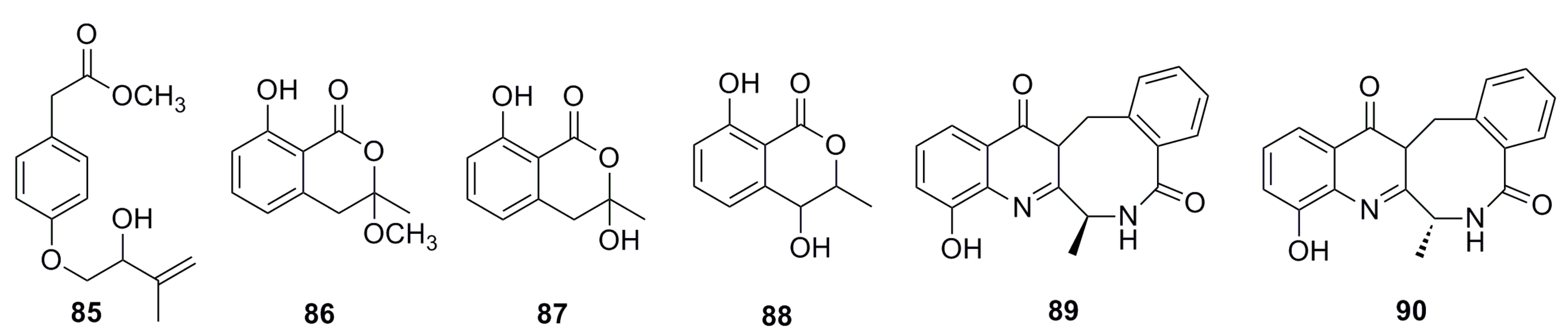
3.1.7. Fatty Acids
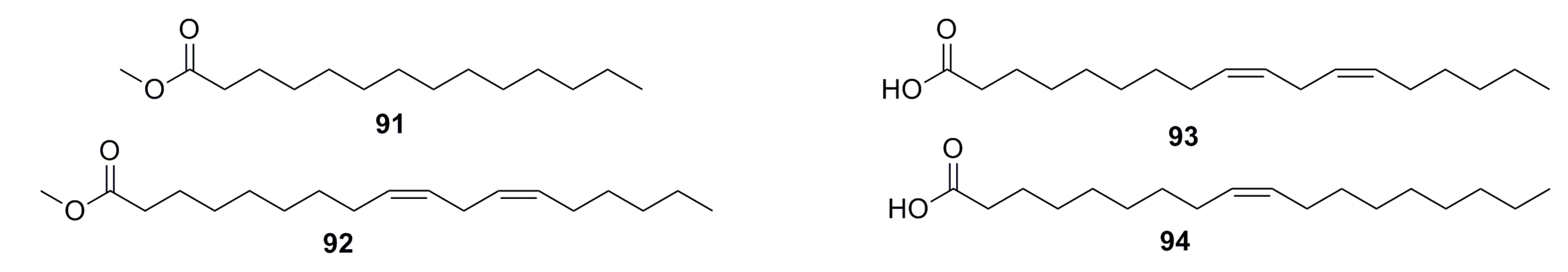
3.1.8. Pyrone Analogues
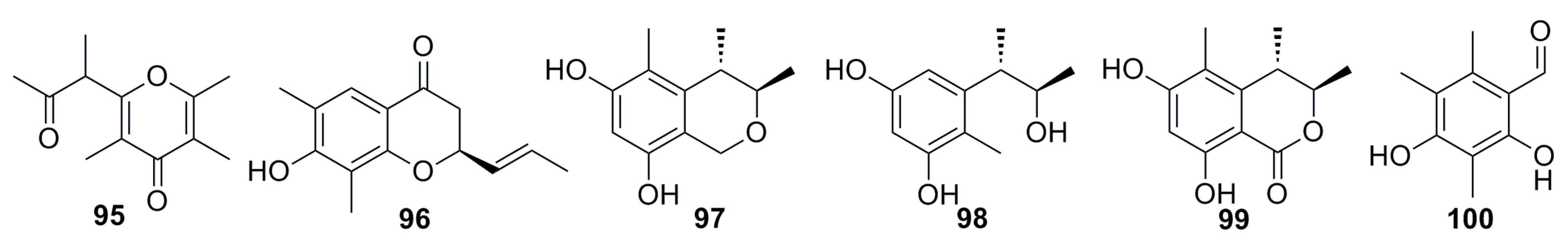
3.1.9. Sorbicillin Derivative

3.1.10. Breviane Derivative
3.1.11. Compounds Containing Amino Acid Structure
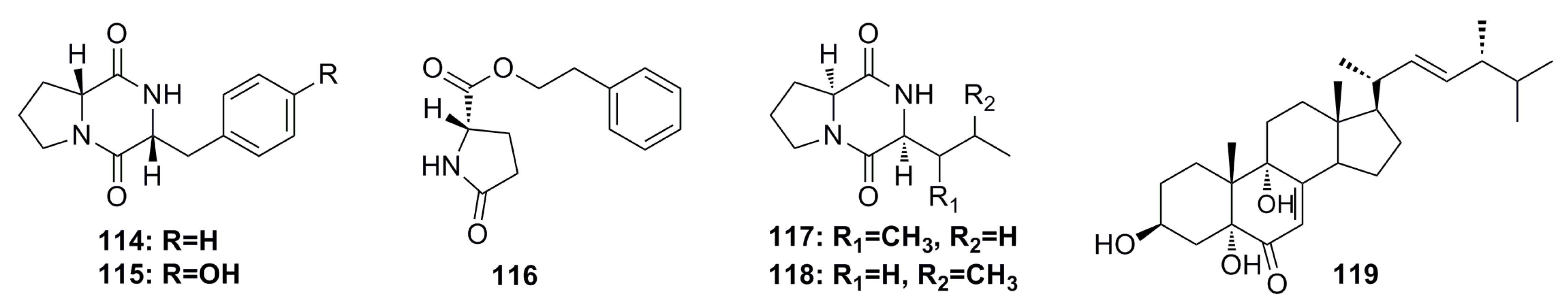
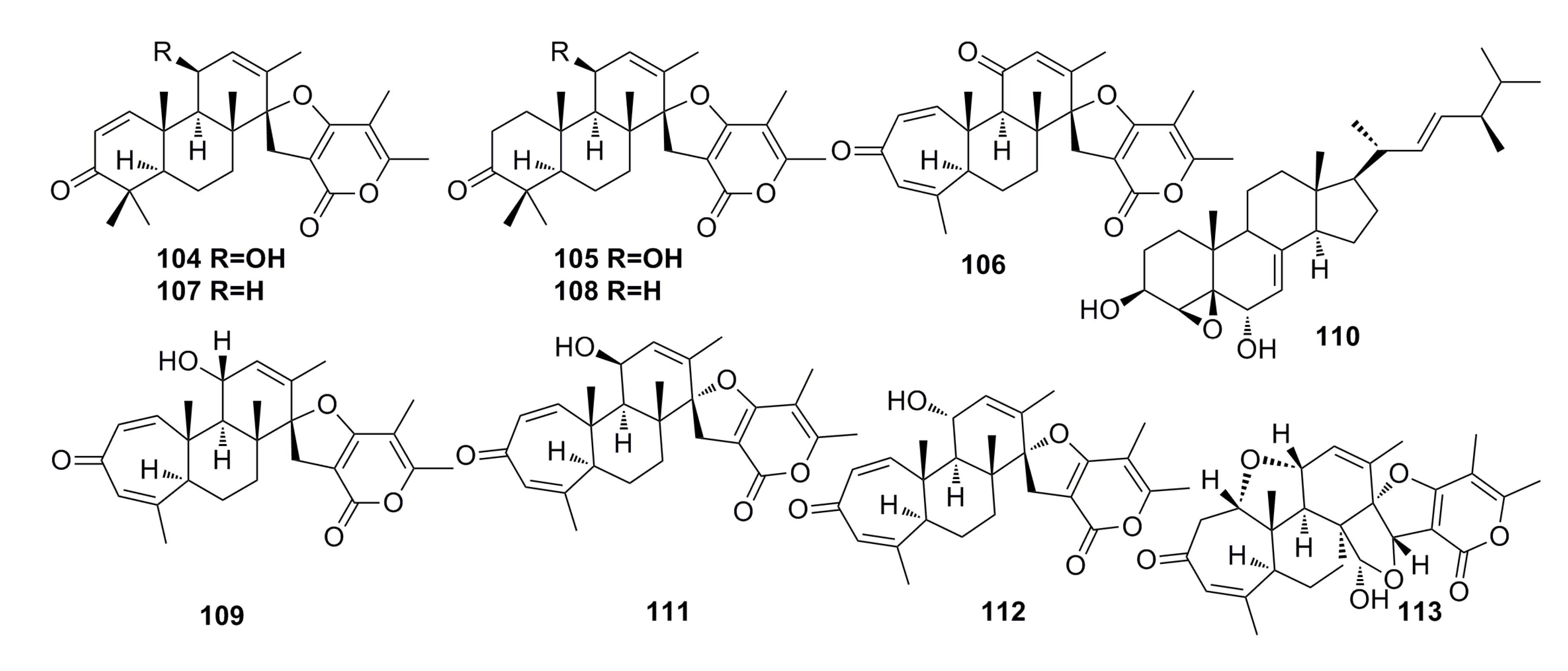
3.1.12. Other Compounds
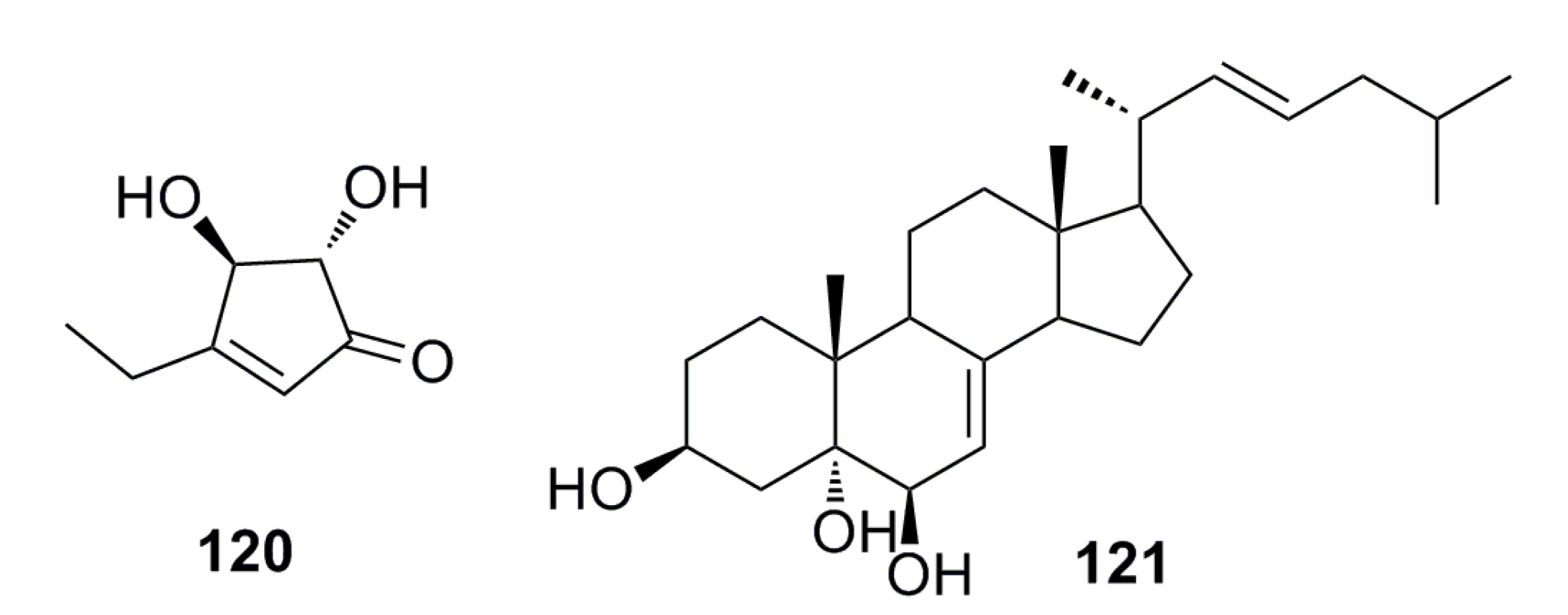
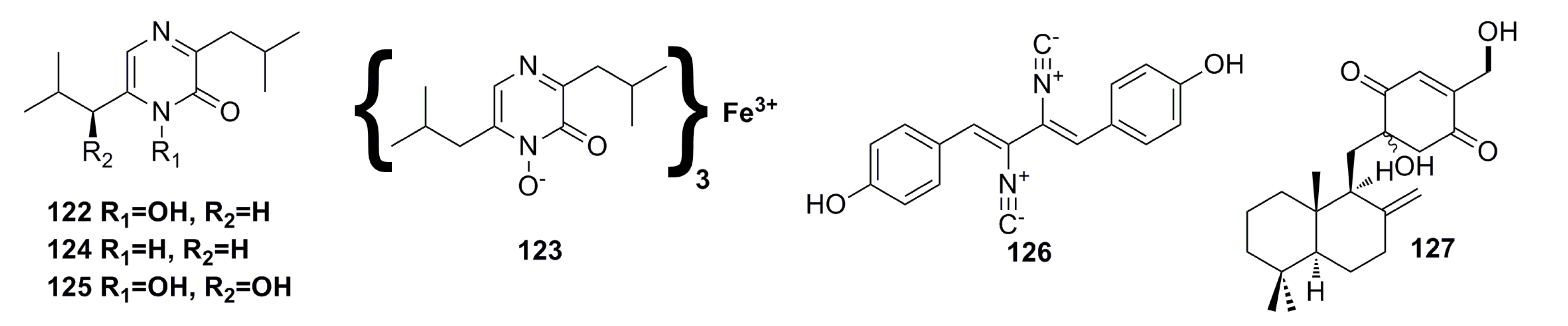
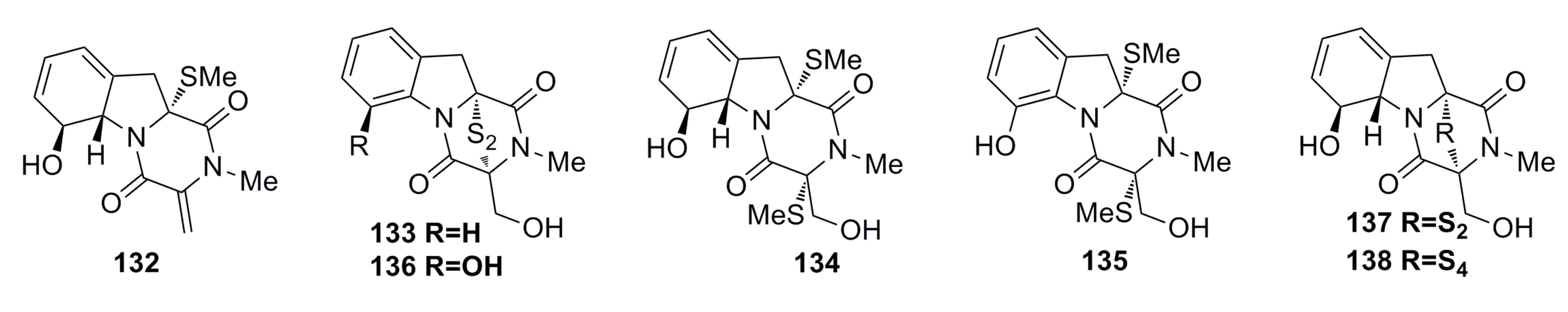
3.2. Antibacterial
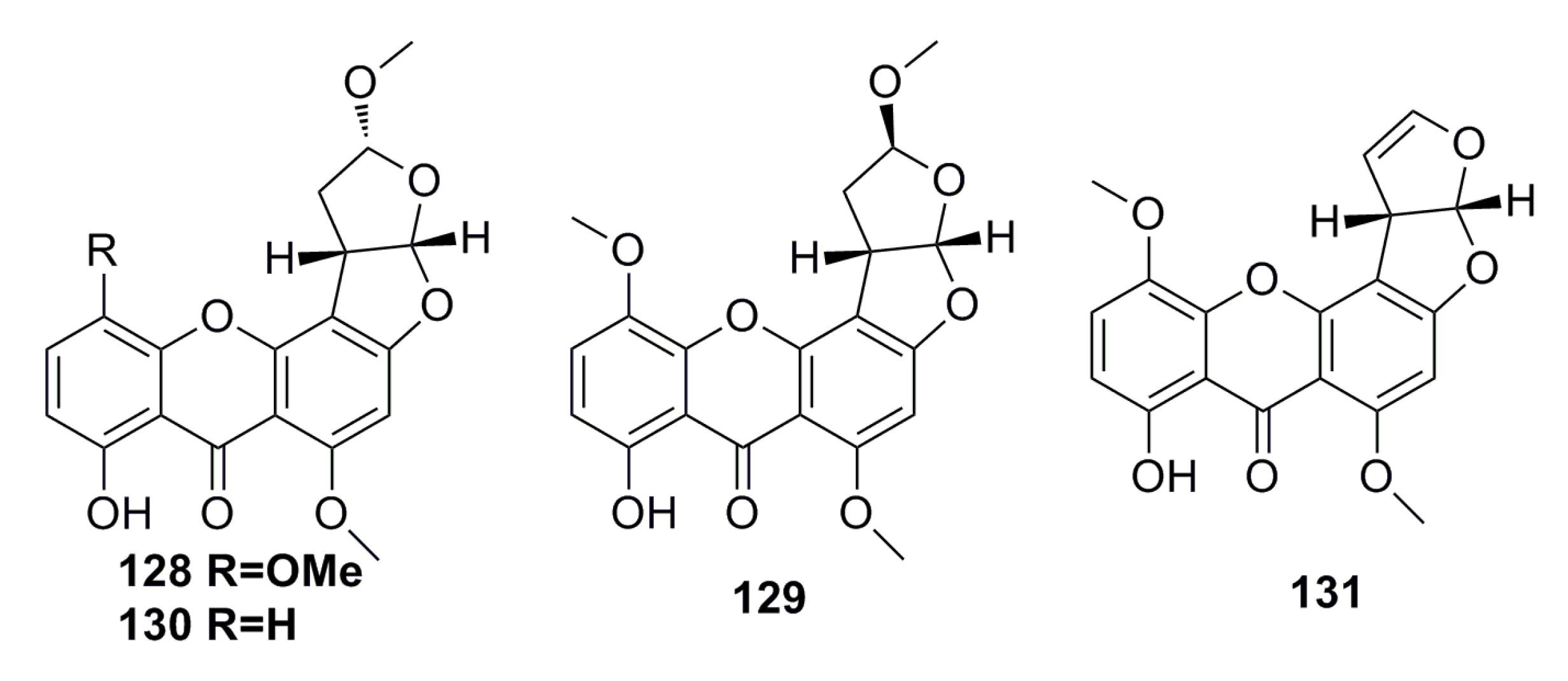
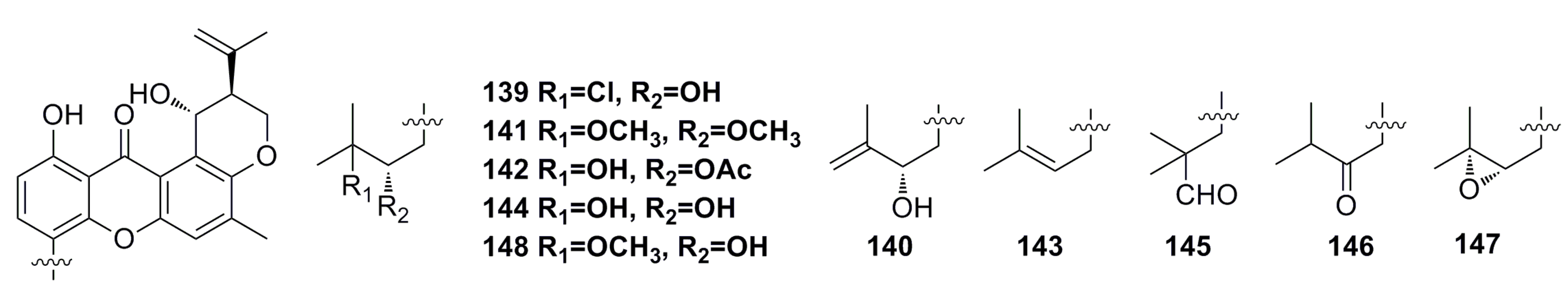
3.2.1. Prenylxanthones
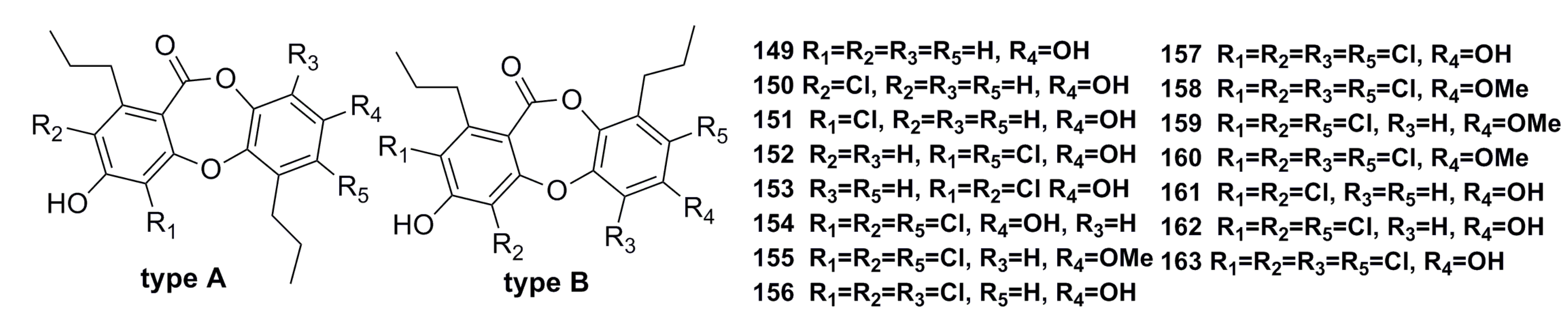
3.2.2. Depsidone-Based Analogues
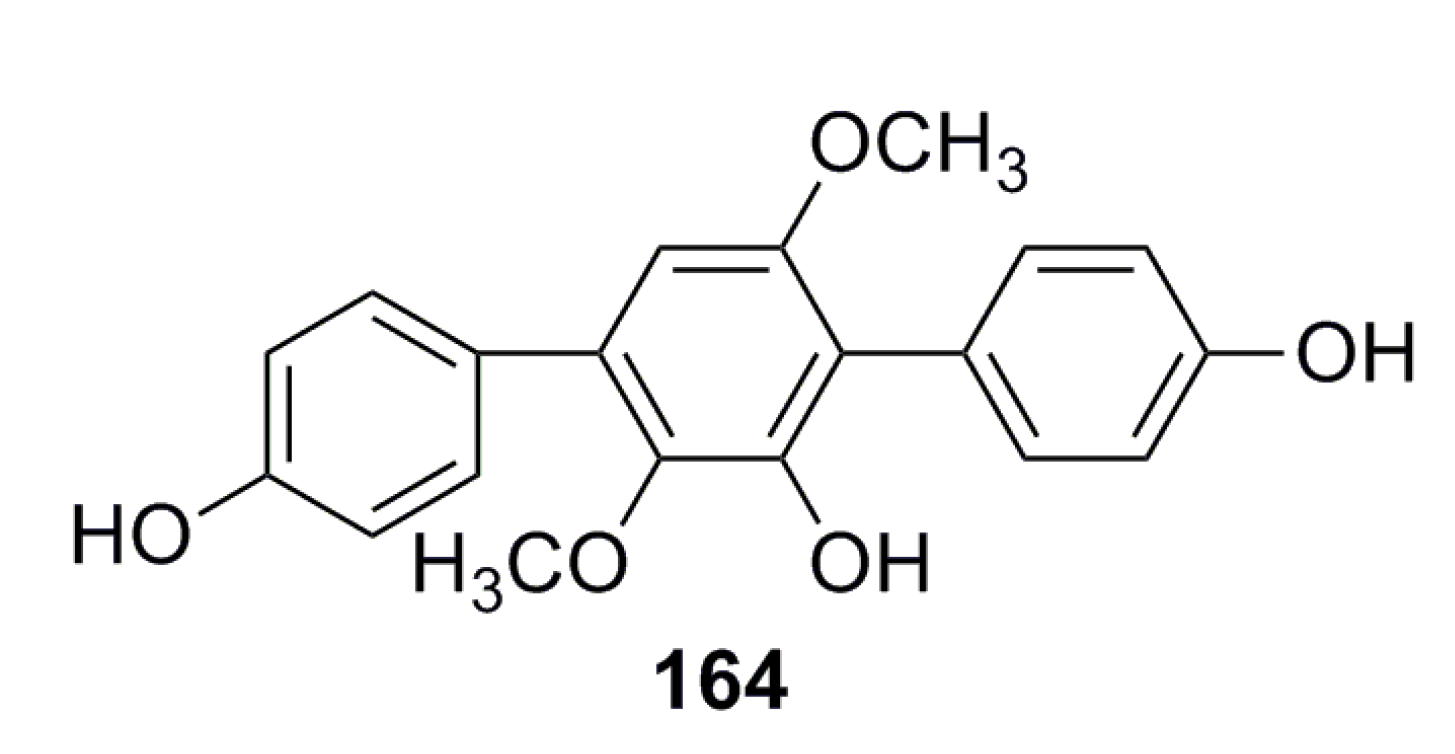
3.2.3. Triple Benzene Compound
3.2.4. Citromycetin Analogue
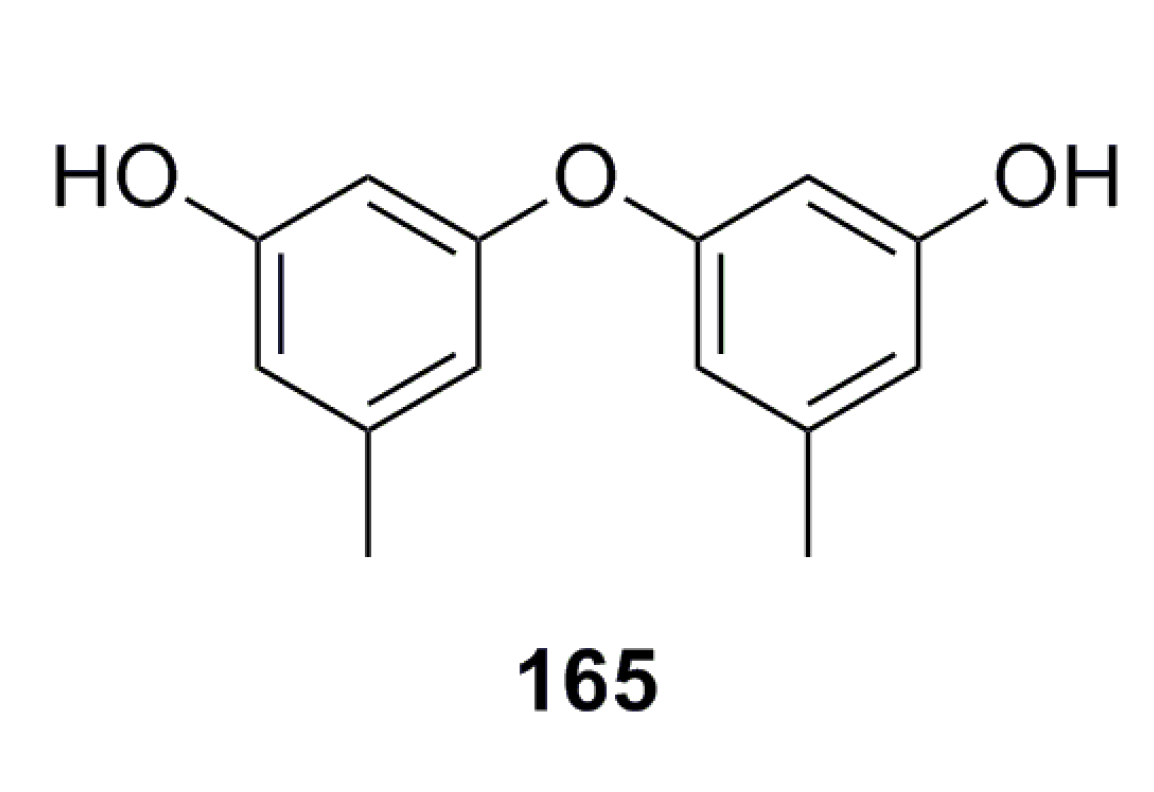
3.2.5. Other Compounds
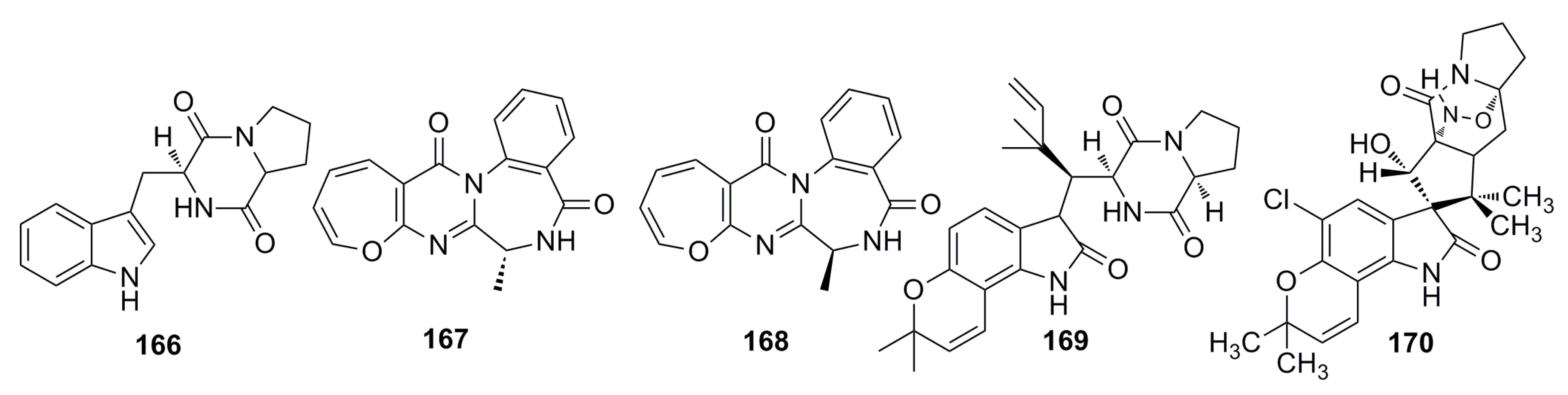
3.4. Antioxidant and Antifouling
3.4.1. Diketopiperazine Derivatives

3.4.2. Hydroxyphenylacetic Acid

3.5. Antifungal

3.6. Compounds with Other Bioactivities

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Park, Y.C.; Gunasekera, S.P.; Lopez, J.V.; McCarthy, P.J.; Wright, A.E. Metabolites from the marine-derived fungus Chromocleista sp. isolated from a deep-water sediment sample collected in the Gulf of Mexico. J. Nat. Prod. 2006, 69, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Swathi, J.; Narendra, K.; Sowjanya, K.M.; Satya, A.K. Evaluation of biologically active molecules isolated from obligate marine fungi. Mintage J. Pharm. Med. Sci. 2013, 2, 45–47. [Google Scholar]
- Mahé, S.; Rédou, V.; Calvez, T.L.; Vandenkoornhuyse, P.; Burgaud, G. Fungi in deep-sea environments and metagenomics. In The Ecological Genomics of Fungi; Martin, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 325–354. [Google Scholar]
- Hua, W.; Chen, X.; Cui, X. Preliminary study of deep-sea fungi and their bioactive secondary metabolites. In Proceedings of the Eleventh National Symposia of Chinese Medicine and Natural Products, Shenyang, China, 19–20 October 2011; p. 46. (In Chinese)
- Yu, L. Identification and biological activity evaluation of three microbial strains isolated from deep-sea sediments. Master’s Thesis, The Third Institute of Oceanography, State Oceanic Administration, Xiamen, 2011. [Google Scholar]
- Nagano, Y.; Nagahama, T. Fungal diversity in deep-sea extreme environments. Fungal Ecol. 2012, 5, 463–471. [Google Scholar] [CrossRef]
- Roth, F.J., Jr.; Orpurt, P.A.; Ahearn, D.G. Occurrence and distribution of fungi in a subtropical marine environment. Can. J. Bot. 1964, 42, 375–383. [Google Scholar] [CrossRef]
- Fredimoses, M.; Zhou, X.; Lin, X.; Tian, X.; Ai, W.; Wang, J.; Liao, S.; Liu, J.; Yang, B.; Yang, X. New prenylxanthones from the deep-sea derived fungus Emericella sp. SCSIO 05240. Mar. Drugs 2014, 12, 3190–3202. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sun, X.; Yu, G.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Cladosins A–E, hybrid polyketides from a deep-sea-derived fungus, Cladosporium sphaerospermum. J. Nat. Prod. 2014, 77, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Review: Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Review: Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Thaler, A.D.; van Dover, C.L.; Vilgalys, R. Ascomycete phylotypes recovered from a Gulf of Mexico methane seep are identical to an uncultured deep-sea fungal clade from the Pacific. Fungal Ecol. 2012, 5, 270–273. [Google Scholar] [CrossRef]
- DeLong, E.F.; Pace, N.R. Environmental diversity of bacteria and archaea. Syst. Biol. 2001, 50, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. 2006, 103, 12115–12120. [Google Scholar] [CrossRef] [PubMed]
- Luna, G.M.; Stumm, K.; Pusceddu, A.; Danovaro, R. Archaeal diversity in deep-sea sediments estimated by means of different terminal-restriction fragment length polymorphisms (T-RFLP) protocols. Curr. Mircrobiol. 2009, 59, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Nagahama, T.; Hatada, Y.; Nunoura, T.; Takami, H.; Miyazaki, J.; Takai, K.; Horikoshi, K. Fungal diversity in deep-sea sediments-the presence of novel fungal groups. Fungal Ecol. 2010, 3, 316–325. [Google Scholar] [CrossRef]
- Singh, P.; Raghukumar, C.; Verma, P.; Shouche, Y. Assessment of fungal diversity in deep-sea sediments by multiple primer approach. World J. Microbiol. Biotechnol. 2012, 28, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Inoue, A.; Fuji, F.; Horikoshi, K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol. Lett. 1997, 152, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, C.; Raghukumar, S.; Sheelu, G.; Gupta, S.M.; Nath, B.N.; Rao, B.R. Buried in time: Culturable fungi in a deep-sea sediment core from the Chagos Trench, Indian Ocean. Deep Sea Res. Oceanogr. Res. Pap. 2004, 51, 1759–1768. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, X.; Qi, S. Diverse deep-sea fungi from the South China Sea and their antimicrobial activity. Curr. Microbiol. 2013, 67, 525–530. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dai, H.; Chen, Z.; Liu, G.; He, Z.; Song, F.; Yang, X.; Fu, H.; Zhang, L.; Chen, X. Trichoderone, a novel cytotoxic cyclopentenone and cholesta-7,22-diene-3β,5α,6β-triol, with new activities from the marine-derived fungus Trichoderma sp. J. Ind. Microbiol. Biotechnol. 2010, 37, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Cao, L.; Tan, H.; Fang, S.; Huang, Y.; Zhou, S. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J. 2007, 1, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Peng, J.; Ma, C.; Nong, X.; Bao, J.; Zhang, G.; Qi, S. Antifouling potentials of eight deep-sea-derived fungi from the South China Sea. J. Ind. Microbiol. Biotechnol. 2014, 41, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lin, A.; Gu, Q.; Zhu, T.; Li, D. Four new chloro-eremophilane sesquiterpenes from an antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 2013, 11, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, H.; Zhu, T.; Li, J.; Gu, Q.; Li, D. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745–9749. [Google Scholar] [CrossRef]
- Stock, A.; Breiner, H.; Pachiadaki, M.; Edgcomb, V.; Filker, S.; La Cono, V.; Yakimov, M.M.; Stoeck, T. Microbial eukaryote life in the new hypersaline deep-sea basin Thetis. Extremophiles 2012, 16, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Pang, K.; Luo, Z. High fungal diversity and abundance recovered in the deep-sea sediments of the Pacific Ocean. Microb. Ecol. 2014, 68, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, N.G.; Kopytina, N.I. The first marine filamentous fungi discovered in the bottom sediments of the oxic/anoxic interface and in the bathyal zone of the Black Sea. Turkish J. Fish. Aquat. Sci. 2014, 14, 497–505. [Google Scholar]
- Wu, J.; Gao, W.; Johnson, R.H.; Zhang, W.; Meldrum, D.R. Integrated metagenomic and metatranscriptomic analyses of microbial communities in the meso-and bathypelagic realm of North Pacific Ocean. Mar. Drugs 2013, 11, 3777–3801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, G.; Xu, X.; Nong, X.; Qi, S. Insights into deep-sea sediment fungal communities from the East Indian Ocean using targeted environmental sequencing combined with traditional cultivation. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Borse, B.D.; Borse, K.N.; Pawar, N.S.; Tuwar, A.R. Marine fungi from India-XII. A revised check list. Indian J. Geo-Mar. Sci. 2013, 42, 110–119. [Google Scholar]
- Xu, W.; Li, G.; Huang, X.; Luo, Z. Fungal diversity study in the deep sea sediments of three oceans by culture-dependent approach. J. Appl. Oceanogr. 2015, 34, 103–110. (In Chinese) [Google Scholar]
- Hao, W. Fungal diversity in The Atlantic Ocean and the South China Sea deep-sea sediments and screening of the low-temperature enzyme producing strains from the extreme environment. Master’s Thesis, Xiamen University, Xiamen, China, 2014. [Google Scholar]
- Bhadury, P.; Bik, H.; Lambshead, J.D.; Austen, M.C.; Smerdon, G.R.; Rogers, A.D. Molecular diversity of fungal phylotypes co-amplified alongside nematodes from coastal and deep-sea marine environments. PLoS ONE 2011. [Google Scholar] [CrossRef]
- Takishita, K.; Tsuchiya, M.; Reimer, J.D.; Maruyama, T. Molecular evidence demonstrating the basidiomycetous fungus Cryptococcus curvatus is the dominant microbial eukaryote in sediment at the Kuroshima Knoll methane seep. Extremophiles 2006, 10, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, T.; Takahashi, E.; Nagano, Y.; Abdel Wahab, M.A.; Miyazaki, M. Molecular evidence that deep-branching fungi are major fungal components in deep-sea methane cold-seep sediments. Environ. Microbiol. 2011, 13, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal diversity in deep-sea hydrothermal ecosystems. Appl. Environ. Microb. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Jebaraj, C.S.; Raghukumar, C.; Behnke, A.; Stoeck, T. Fungal diversity in oxygen-depleted regions of the Arabian Sea revealed by targeted environmental sequencing combined with cultivation. FEMS Microbiol. Ecol. 2010, 71, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Farce, A.; Loge, C.; Gallet, S.; Lebegue, N.; Carato, P.; Chavatte, P.; Berthelot, P.; Lesieur, D. Docking study of ligands into the colchicine binding site of tubulin. J. Enzym. Inhib. Med. Chem. 2004, 19, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Geng, R.; Zhou, C. Benzene and imidazoles drug research progress. Chin. J. New Drugs 2009, 18, 1505–1514. [Google Scholar]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic polyketides from the deep-sea-derived fungus Engyodontium album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Cui, C.; Hua, W.; Zhu, T.; Gu, Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Peng, J.; Zhu, T.; Gu, Q.; Keyzers, R.A.; Li, D. Sorbicillamines A–E, nitrogen-containing sorbicillinoids from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2013, 76, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, G.; Zhu, T.; Gu, Q.; Li, D. Cladosins F and G, two new hybrid polyketides from the deep-sea-derived Cladosporium sphaerospermum 2005-01-E3. J. Asian Nat. Prod. Res. 2014, 17, 1–5. [Google Scholar]
- Cui, X.; Li, C.; Wu, C.; Hua, W. Metabolites of Paecilomyces lilacinus ZBY-1 from deep-sea water and their antitumor activity. J. Int. Pharm. Res. 2013, 40, 177–186. (In Chinese) [Google Scholar]
- Li, Y.; Ye, D.; Shao, Z.; Cui, C.; Che, Y. A sterol and spiroditerpenoids from a Penicillium sp. isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, X.; Wang, F.; Liu, K.; Yang, B.; Yang, X.; Peng, Y.; Liu, J.; Ren, Z.; Liu, Y. A new cytotoxic sesquiterpene quinone produced by Penicillium sp. F00120 isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Sun, S.; Cai, S. Studies on the active secondary metabolites from the deep-sea-derived fungus Aspergillus CXCTD-06-6a. Chin. J. Mar. Drugs 2011, 30, 1–6. (In Chinese) [Google Scholar]
- Tian, L.; Cai, S.; Li, D.; Lin, Z.; Zhu, T.; Fang, Y.; Liu, P.; Gu, Q.; Zhu, W. Two new metabolites with cytotoxicities from deep-sea fungus, Aspergillus sydowi YH11-2. Arch. Pharm. Res. 2007, 30, 1051–1054. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X.; Meng, L.; Li, C.; Gao, S.; Huang, C.; Wang, B. Chemical profile of the secondary metabolites produced by a deep-sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Oceanol. Limnol. 2012, 30, 305–314. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; An, C.; Wang, B. Prenylated indole alkaloid derivatives from marine sediment-derived fungus Penicillium paneum SD-44. Helv. Chim. Acta 2014, 97, 1440–1444. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, X.; Tu, Z.; Xu, X.; Qi, S. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. J. Nat. Prod. 2013, 76, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Wu, G.; Gu, Q.; Zhu, T.; Li, D. New eremophilane-type sesquiterpenes from an Antarctic deep-sea derived fungus, Penicillium sp. PR19 N-1. Arch. Pharm. Res. 2014, 37, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, D.H.; Cai, S.X.; Wang, F.P.; Xiao, X.; Gu, Q.Q. A new cytotoxic metabolite from a deep sea derived fungus, Phialocephala sp. Acta Pharm. Sin. 2010, 45, 1275–1278. [Google Scholar]
- Li, C.; Li, X.; Gao, S.; Lu, Y.; Wang, B. Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44. Mar. Drugs 2013, 11, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Hua, W. Metabolites of Aspergillus sp. 16-02-1 isolated from a deep sea sediment and preliminary test of their antitumor and antifungal activities. Chin. J. Mar. Drugs 2013, 32, 1–10. (In Chinese) [Google Scholar]
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Xian, J.; Liu, Y. Westerdijkin A, a new hydroxyphenylacetic acid derivative from deep sea fungus Aspergillus westerdijkiae SCSIO 05233. Nat. Prod. Res. 2015, 29, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, C.; Wu, C.; Hua, W. Secondary metabolites of Paecilomyces lilacinus ZBY-1 and their antitumor activity. J. Int. Pharm. Res. 2013, 40, 765–771. (In Chinese) [Google Scholar]
- Li, D.; Wang, F.; Xiao, X.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Trisorbicillinone A, a novel sorbicillin trimer, from a deep sea fungus, Phialocephala sp. FL30r. Tetrahedron Lett. 2007, 48, 5235–5238. [Google Scholar] [CrossRef]
- Li, D.; Wang, F.; Cai, S.; Zeng, X.; Xiao, X.; Gu, Q.; Zhu, W. Two new bisorbicillinoids isolated from a deep-sea fungus, Phialocephala sp. FL30r. J. Antibiot. 2007, 60, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Chen, X.; Lu, X.; Shao, Z.; Zhang, H.; Che, Y. Breviane spiroditerpenoids from an extreme-tolerant Penicillium sp. isolated from a deep sea sediment sample. J. Nat. Prod. 2009, 72, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C.; Li, C.; Hua, W.; Wu, C.; Zhu, T.; Gu, Q. Activation of dormant secondary metabolite production by introducing neomycin resistance into the deep-sea fungus, Aspergillus versicolor ZBY-3. Mar. Drugs 2014, 12, 4326–4352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Shang, Z.; Jiao, B.; Yuan, B.; Sun, W.; Wang, B.; Miao, M.; Huang, C. SD118-xanthocillin X (1), a novel marine agent extracted from Penicillium commune, induces autophagy through the inhibition of the MEK/ERK pathway. Mar. Drugs 2012, 10, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhu, T.; Du, L.; Zhao, B.; Li, D.; Gu, Q. Sterigmatocystins from the deep-sea-derived fungus Aspergillus versicolor. J. Antibiot. 2011, 64, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2011, 75, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xia, J.; Wang, W. Isolation and identification of two terphenyl compounds from Aspergillus candidus metabolites. J. Xiamen Univ. 2013, 52, 670–674. (In Chinese) [Google Scholar]
- Tian, Y.; Lin, X.; Liu, J.; Kaliyaperumal, K.; Ai, W.; Ju, Z.; Yang, B.; Wang, J.; Yang, X.; Liu, Y. Ascomycotin A, a new citromycetin analogue produced by Ascomycota sp. Ind19F07 isolated from deep sea sediment. Nat. Prod. Res. 2014, 29, 1–7. [Google Scholar]
- Kong, X.; Cai, S.; Zhu, T.; Gu, Q.; Li, D.; Luan, Y. Secondary metabolites of a deep sea derived fungus Aspergillus versicolor CXCTD-06-6a and their bioactivity. J. Ocean. Univ. China 2014, 13, 691–695. [Google Scholar] [CrossRef]
- Liu, H.; Kong, M.; Zheng, Y. Studies on secondary metabolites produced by Aspergillus sp. SCSIOW3 isolated from deep sea and their anti-Aβ peptide aggregation activity. Chin. J. Mar. Drugs 2014, 33, 71–74. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi. Mar. Drugs 2015, 13, 4594-4616. https://doi.org/10.3390/md13084594
Wang Y-T, Xue Y-R, Liu C-H. A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi. Marine Drugs. 2015; 13(8):4594-4616. https://doi.org/10.3390/md13084594
Chicago/Turabian StyleWang, Yan-Ting, Ya-Rong Xue, and Chang-Hong Liu. 2015. "A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi" Marine Drugs 13, no. 8: 4594-4616. https://doi.org/10.3390/md13084594