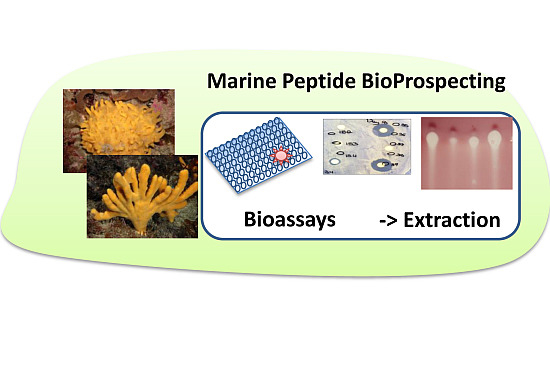
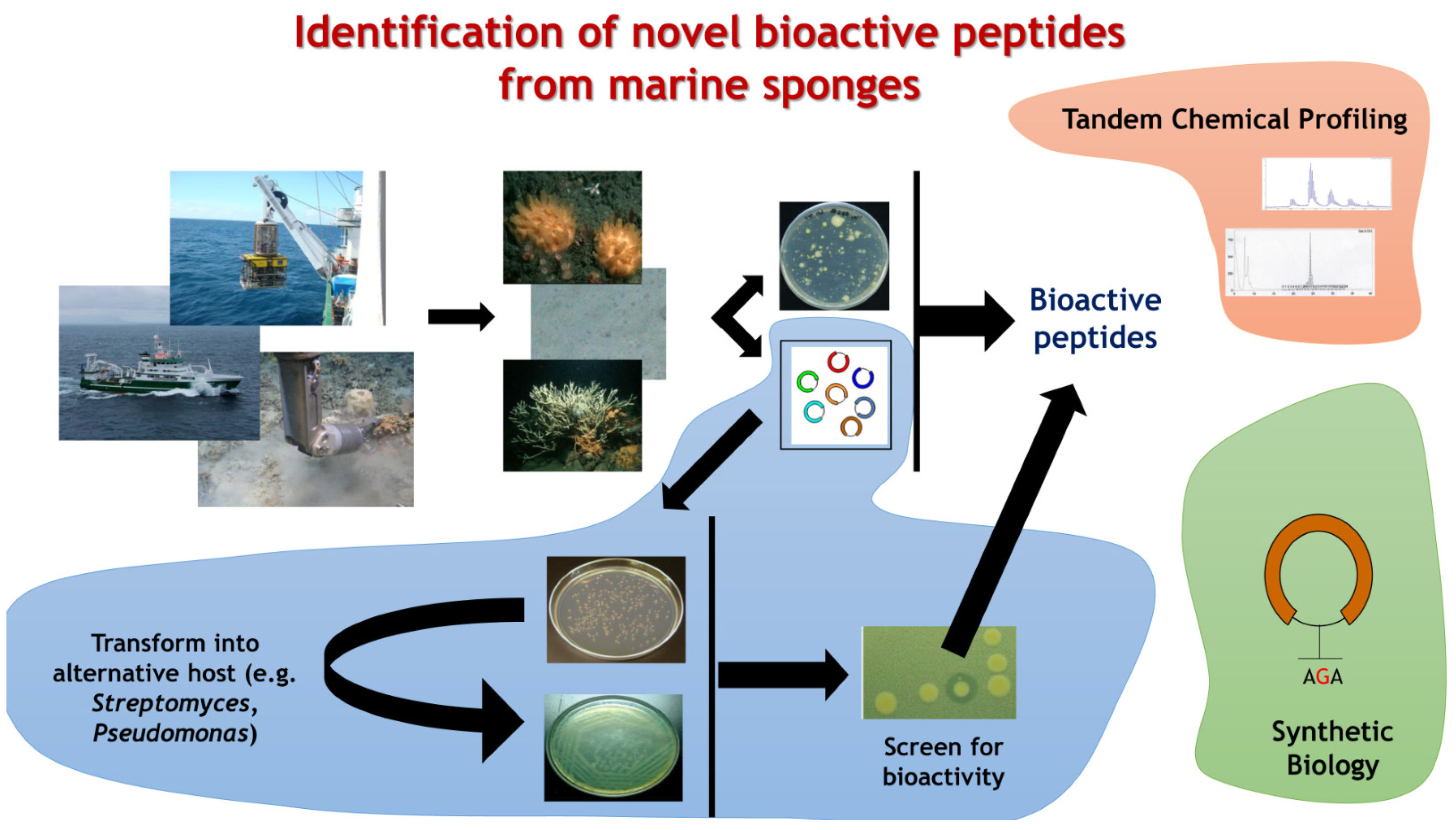
Emerging Concepts Promising New Horizons for Marine Biodiscovery and Synthetic Biology
Abstract
:1. Introduction
| Marine Sector | Application |
|---|---|
| Functional Ingredients | Algae and biofuels |
| Seafood byproducts | |
| Food and Nutrition | Food safety and quality |
| Aquaculture | |
| Bioprospecting | |
| Nutrition | |
| Product stability | |
| Seafood and health | |
| Nutraceuticals | |
| New Horizons | Photography |
| Textiles | |
| Leather | |
| Electronics | |
| Cosmetics | Biofilms |
| Antioxidants | |
| Dispersants | |
| Emulsifiers | |
| Anti-ageing |
2. Bioactive Marine Peptides
2.1. Antimicrobials/Lantibiotics
2.2. Nanoantibiotics

2.3. Peptidomimetics
2.4. Peptide Nucleic Acids
3. Marine Peptides and Cell-Cell Communication
3.1. Signaling in the Marine Ecosystem
3.2. Peptides and Microbial Culturability
4. Next Generation Marine Peptides: Anti-Biofilm and Anti-Virulence Bioactivity
4.1. Anti-QS Compounds
| Bacterial Species | Isolated From | Peptide Inhibitor | Target QS System and Phenotypes | Source |
|---|---|---|---|---|
| P. aeruginosa | Marine Antarctic sponge | DKP, Cyclic dipeptide: Cyclo l-Pro-l-tyr | Interferes with AHL-QS system | [94] |
| Inhibits bioluminescence by V. harveyi | ||||
| Inhibits V. fischeri luxR | ||||
| Bacillus sp. D28 | Marine sediment | DKP, Cyclic dipeptide: Cyclo l-Pro-l-tyr | Interferes with AHL-QS system | [79] |
| Inhibits bioluminescence by V. harveyi | ||||
| Inhibits V. fischeri luxR | ||||
| Streptomyces NIO 10068 | Marine sponge | Linear dipeptides: Pro-Gly | Interferes with AHL-QS system | [98] |
| Inhibition against P. aeruginosa: Swarming motility, pyocyanin production, biofilm formation, rhamnolipid production, LasA protease production | ||||
| Inhibits violacein production by C. violaceum | ||||
| N-amido-α-Pro | Belong to the active fraction, but its effect against QS system was not demonstrated | [98] | ||
| Photobacterium halotolerans | Mussel surface | Cyclodepsipeptides: Solonamide B | Interferes with the AIP-QS system | [78,99,100] |
| Increases spa expression | ||||
| Reduces the expression of hla and rnaIII | ||||
| Interferes with the binding of S. aureus AIPs | ||||
| Ngercheumicin F,G,H, and I | Interferes with the AIP-QS system | [77] | ||
| Modulate the expression of QS regulated virulence genes: Increases spa expression | ||||
| Reduces the expression of hla and rnaIII |
4.2. Anti-Biofilm Compounds
5. Mining in the Informatics Era
5.1. Metagenomes and Genomes
| Metagenomic Challenges | |
|---|---|
| When and where to sample | With the dynamic nature of population flux already reported, where the isolation of novel bioactive natural products is the ultimate goal, the source of the metagenomic DNA is a central consideration. |
| Ability to isolate DNA from these samples | Something that has proven a major bottleneck to complete coverage of library construction and associated screens. The diversity of organisms present, the extent to which they will yield their DNA using conventional or adapted isolation protocols, the differences in abundance between the dominant potentially uninteresting species and the rare potentially lucrative organisms, all present a major headache that needs to be overcome if we are to maximally exploit this technology. |
| Size and complexity of current metagenomic datasets | This presents an additional challenge to researchers with computational advances now urgently required to meet the explosion in available data. Automated genome mining tools and eventually pattern recognition based algorithms are required to deal with the large datasets emerging from these studies. This is crucial in overcoming the oversampling of abundant organisms with loss of information from the lower abundant species. |
| Inability to taxonomically link bioactivities to producing organisms | Perhaps an obvious limitation arising from the heterogeneous nature of microbial communities and the fragment sizes that classically populate metagenomic libraries. |
| Expression of eDNA in heterologous hosts | Even if bottlenecks in sequencing and bioinformatics are overcome, heterologous expression, although possible, is fraught with limitations, including codon usage, rare tRNAs, promoter recognition, toxicity, yield and stability. |
| Cryptic or silent bioactive gene clusters | Activating silent or inactive biosynthetic clusters remains a major challenge, with significant bioactive potential ‘locked in’ within the marine microbial community. |
| Standardization of cluster metadata | The exponential increase in sequence data requires the urgent development of unified standards for the cross-community annotation and description of biosynthetic gene clusters. |
5.2. Single Cell Genomics
5.3. Chemi-Informatics
5.4. Tandem Chemical Profiling
5.5. The Advent of Synthetic Biology
6. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Func. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekaraa, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Func. Foods 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Schofield, M.M.; Chlipala, G.E.; Schultz, P.J.; Yim, I.; Newmister, S.A.; Nusca, T.D.; Scaglione, J.B.; Hanna, P.C.; Tamayo-Castillo, G.; et al. Baulamycins A and B, broad-spectrum antibiotics identified as inhibitors of siderophore biosynthesis in Staphylococcus aureus and Bacillus anthracis. J. Am. Chem. Soc. 2014, 136, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jacob, M.R.; Rao, R.R.; Wang, Y.H.; Agarwal, A.K.; Newman, D.J.; Khan, I.A.; Clark, A.M.; Li, X.C. Antifungal cyclic peptides from the marine sponge. Res. Rep. Med. Chem. 2012, 2, 7–14. [Google Scholar] [PubMed]
- Zheng, L.H.; Wang, Y.J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Harrington, C.; Dobson, A.D.; O’Gara, F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs 2014, 12, 3516–3559. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.A.; Feng, Z.; Brady, S.F. Biocatalysts and small molecule products from metagenomic studies. Curr. Opin. Chem. Biol. 2012, 16, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Flemer, B.; Jackson, S.A.; Lejon, D.P.; Morrissey, J.P.; O’Gara, F.; Dobson, A.D. Marine metagenomics: New tools for the study and exploitation of marine microbial metabolism. Mar. Drugs 2010, 8, 608–628. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tang, S.L. Marine microbial metagenomics: From individual to the environment. Int. J. Mol. Sci. 2014, 15, 8878–8892. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Piel, J. Metagenomic approaches for exploiting uncultivated bacteria as a resource for novel biosynthetic enzymology. Chem. Biol. 2013, 20, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Bruns, A.; Cypionka, H.; Overmann, J. Cyclic Amp and Acyl homoserine lactones increase the cultivation efficiency of heterotrophic bacteria from the central Baltic Sea. Appl. Environ. Microbiol. 2002, 68, 3978–3987. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.S. The phenomenon of microbial uncultivability. Curr. Opin. Microbiol. 2013, 16, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Bell, T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 2012, 22, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kwon, K.K.; Kang, S.G.; Cha, S.S.; Kim, S.J.; Lee, J.H. Approaches for novel enzyme discovery from marine environments. Curr. Opin. Biotechnol. 2010, 21, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short peptide induces an “uncultivable” microorganism to grow in vitro. Appl. Environ. Microbiol. 2008, 74, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Kim, J. Cultivation of unculturable soil bacteria. Trends Biotechnol. 2012, 30, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Puspita, I.D.; Kamagata, Y.; Tanaka, M.; Asano, K.; Nakatsu, C.H. Are uncultivated bacteria really uncultivable? Microbes Environ. 2012, 27, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Pustam, A.; Smith, C.; Deering, C.; Grosicki, K.M.; Leng, T.Y.; Lin, S.; Yang, J.; Pink, D.; Gill, T.; Graham, L.; et al. Interactions of protamine with the marine bacterium, Pseudoalteromonas sp. NCIMB 2021. Lett. Appl. Microbiol. 2014, 58, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for culture of “unculturable” bacteria. FEMS Microbiol. Lett. 2010, 309, 1–7. [Google Scholar] [PubMed]
- Lazcano-Perez, F.; Roman-Gonzalez, S.A.; Sanchez-Puig, N.; Arreguin-Espinosa, R. Bioactive peptides from marine organisms: A short overview. Prot. Pept. Lett. 2012, 19, 700–707. [Google Scholar] [CrossRef]
- Kennedy, J.; Baker, P.; Piper, C.; Cotter, P.D.; Walsh, M.; Mooij, M.J.; Bourke, M.B.; Rea, M.C.; O’Connor, P.M.; Ross, R.P.; et al. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from irish waters. Mar. Biotechnol. 2009, 11, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; McCullough, B.; Clarke, D.J.; Langley, R.J.; Pechenick, T.; Hill, A.; Campopiano, D.J.; Barran, P.E.; Dorin, J.R.; Govan, J.R. Covalent dimer species of β-defensin Defr1 display potent antimicrobial activity against multidrug-resistant bacterial pathogens. Antimicrob. Agents Chemother. 2007, 51, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Karnik, S.; Barai, R.S.; Jayaraman, V.K.; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010, 38, D774–D780. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of Gram-positive bacteria. Microbiol. Rev. 1995, 59, 171–200. [Google Scholar] [PubMed]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Line, J.E.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S.; Siragusa, G.R.; et al. Isolation and purification of enterocin E-760 with broad antimicrobial activity against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2008, 52, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Shokri, D.; Zaghian, S.; Khodabakhsh, F.; Fazeli, H.; Mobasherizadeh, S.; Ataei, B. Antimicrobial activity of a UV-stable bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium strain DSH20 against vancomycin-resistant enterococcus (VRE) strains. J. Microbiol. Immunol. Infect. 2014, 47, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Cotter, P.D.; Ross, R.P.; Hill, C. Discovery of medically significant lantibiotics. Curr. Drug Discov. Technol. 2009, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Worobo, R.W. Chemical and genetic characterization of bacteriocins: Antimicrobial peptides for food safety. J. Sci. Food Agric. 2014, 94, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Pham, T.T.; Nguyen, T.H.; Nguyen, T.T.; Hoj, L. Screening of marine bacteria with bacteriocin-like activities and probiotic potential for ornate spiny lobster (Panulirus ornatus) juveniles. Fish Shellfish Immunol. 2014, 40, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Haft, D.H.; Basu, M.K. Biological systems discovery in silico: Radical S-adenosylmethionine protein families and their target peptides for posttranslational modification. J. Bacteriol. 2011, 193, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; O’Sullivan, O.; Rea, M.C.; Cotter, P.D.; Ross, R.P.; Hill, C. Genome mining for radical SAM protein determinants reveals multiple sactibiotic-like gene clusters. PLoS ONE 2011, 6, e20852. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sareen, D. Novel lant-associated lantibiotic clusters identified by genome database mining. PLoS ONE 2014, 9, e91352. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, A.J.; Montalban-Lopez, M.; Kuipers, O.P. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.M.; van der Donk, W.A. Lantibiotics: Peptides of diverse structure and function. Annu. Rev. Microbiol. 2007, 61, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; O’Connor, P.M.; Draper, L.A.; Lawton, E.M.; Deegan, L.H.; Hill, C.; Ross, R.P. Posttranslational conversion of l-serines to d-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc. Natl. Acad. Sci. USA 2005, 102, 18584–18589. [Google Scholar] [CrossRef] [PubMed]
- Phelan, R.W.; Barret, M.; Cotter, P.D.; O’Connor, P.M.; Chen, R.; Morrissey, J.P.; Dobson, A.D.; O’Gara, F.; Barbosa, T.M. Subtilomycin: A new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge Haliclona simulans. Mar. Drugs 2013, 11, 1878–1898. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, C.Z.; Zhu, Y.G.; Wang, P.X.; Qi, Q.D.; Fu, J.J.; Peng, D.H.; Ruan, L.F.; Sun, M. ApnI, a transmembrane protein responsible for subtilomycin immunity, unveils a novel model for lantibiotic immunity. Appl. Environ. Microbiol. 2014, 80, 6303–6315. [Google Scholar] [CrossRef] [PubMed]
- Saude, A.C.; Ombredane, A.S.; Silva, O.N.; Barbosa, J.A.; Moreno, S.E.; Araujo, A.C.; Falcao, R.; Silva, L.P.; Dias, S.C.; Franco, O.L. Clavanin bacterial sepsis control using a novel methacrylate nanocarrier. Int. J. Nanomed. 2014, 9, 5055–5069. [Google Scholar]
- Almeida, J.P.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Controll. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Vukomanovic, M.; Logar, M.; Skapina, S.D.; Suvorova, D. Hydroxyapatite/gold/arginine: Designing the structure to create antibacterial activity. J. Mater. Chem. B 2014, 2, 1557–1564. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Liddington, R.C. Structural basis of protein-protein interactions. Methods Mol. Biol. 2004, 261, 3–14. [Google Scholar] [PubMed]
- Brady, R.M.; Baell, J.B.; Norton, R.S. Strategies for the development of conotoxins as new therapeutic leads. Mar. Drugs 2013, 11, 2293–2313. [Google Scholar] [CrossRef] [PubMed]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, P. Peptides and peptidomimetics in medicinal chemistry. In Medicinal Chemistry and Drug Design; Ekinci, D., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Som, A.; Navasa, N.; Percher, A.; Scott, R.W.; Tew, G.N.; Anguita, J. Identification of synthetic host defense peptide mimics that exert dual antimicrobial and anti-inflammatory activities. Clin. Vaccine Immunol. 2012, 19, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avan, I.; Hall, C.D.; Katritzky, A.R. Peptidomimetics via modifications of amino acids and peptide bonds. Chem. Soc. Rev. 2014, 43, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.L.; Regatieri, C.V.; Lima, M.A.; Paredes-Gamero, E.J.; Brito, A.S.; Chavante, S.F.; Belfort, R., Jr.; Farah, M.E.; Nader, H.B. A heparin mimetic isolated from a marine shrimp suppresses neovascularization. J. Thromb. Haemost. 2010, 8, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Li, R.; Sung, S.S.; Wu, H.; Liu, Y.; Manieri, W.; Krishnegowda, G.; Awwad, A.; Dewey, A.; Liu, X.; et al. Discovery of marinopyrrole a (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. J. Biol. Chem. 2012, 287, 10224–10235. [Google Scholar] [CrossRef]
- Billard, C. BH3 mimetics: Status of the field and new developments. Mol. Cancer Ther. 2013, 12, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Good, L.; Nielsen, P.E. Peptide nucleic acid (PNA) antisense effects in Escherichia coli. Curr. Issues Mol. Biol. 1999, 1, 111–116. [Google Scholar] [PubMed]
- Worden, A.Z.; Chisholm, S.W.; Binder, B.J. In situ hybridization of Prochlorococcus and Synechococcus (marine cyanobacteria) spp. with rRNA-targeted peptide nucleic acid probes. Appl. Environ. Microbiol. 2000, 66, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Wynendaele, E.; Bronselaer, A.; Nielandt, J.; D’Hondt, M.; Stalmans, S.; Bracke, N.; Verbeke, F.; van de Wiele, C.; de Tre, G.; de Spiegeleer, B. Quorumpeps database: Chemical space, microbial origin and functionality of quorum sensing peptides. Nucleic Acids Res. 2013, 41, D655–D659. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; You, Y.; Yan, H.; Meng, J.; Xue, X.; Hou, Z.; Zhou, Y.; Ma, X.; Sang, G.; Luo, X. Antisense inhibition of gene expression and growth in Gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials 2012, 33, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, A.; Nielsen, P.E. Potent antibacterial antisense peptide-peptide nucleic acid conjugates against Pseudomonas aeruginosa. Nucleic Acid Ther. 2012, 22, 323–334. [Google Scholar] [PubMed]
- Good, L.; Awasthi, S.K.; Dryselius, R.; Larsson, O.; Nielsen, P.E. Bactericidal antisense effects of peptide-PNA conjugates. Nat. Biotechnol. 2001, 19, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kurupati, P.; Tan, K.S.; Kumarasinghe, G.; Poh, C.L. Inhibition of gene expression and growth by antisense peptide nucleic acids in a multiresistant β-lactamase-producing Klebsiella pneumoniae strain. Antimicrob. Agents Chemother. 2007, 51, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Nekhotiaeva, N.; Awasthi, S.K.; Nielsen, P.E.; Good, L. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol. Ther. 2004, 10, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Patenge, N.; Pappesch, R.; Krawack, F.; Walda, C.; Mraheil, M.A.; Jacob, A.; Hain, T.; Kreikemeyer, B. Inhibition of growth and gene expression by PNA-peptide conjugates in Streptococcus pyogenes. Mol. Ther. Nucleic Acids 2013, 2, e132. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, P.; Alexander, J.C.; Seleem, M.N.; Jain, N.; Sriranganathan, N.; Wattam, A.R.; Setubal, J.C.; Boyle, S.M. Peptide nucleic acids inhibit growth of Brucella suis in pure culture and in infected murine macrophages. Int. J. Antimicrob. Agents 2013, 41, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, M.; Ohashi, A.; Imachi, H. Peptide nucleic acids (PNAs) antisense effect to bacterial growth and their application potentiality in biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Liou, J.S.; Chen, Y.J.; Huang, Y.W.; Lee, H.J. Delivery of nucleic acids, proteins, and nanoparticles by arginine-rich cell-penetrating peptides in rotifers. Mar. Biotechnol. 2013, 15, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.C.; Federle, M.J. Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol. Rev. 2014, 38, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Grossart, H.P.; Schlingloff, A.; Kiorboe, T. Possible quorum sensing in marine snow bacteria: Production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 2002, 68, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.M.; Cicirelli, E.M.; Kan, J.; Chen, F.; Fuqua, C.; Hill, R.T. Diversity and quorum-sensing signal production of Proteobacteria associated with marine sponges. Environ. Microbiol. 2008, 10, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kjaerulff, L.; Nielsen, A.; Mansson, M.; Gram, L.; Larsen, T.O.; Ingmer, H.; Gotfredsen, C.H. Identification of four new agr quorum sensing-interfering cyclodepsipeptides from a marine Photobacterium. Mar. Drugs 2013, 11, 5051–5062. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Mansson, M.; Bojer, M.S.; Gram, L.; Larsen, T.O.; Novick, R.P.; Frees, D.; Frokiaer, H.; Ingmer, H. Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS ONE 2014, 9, e84992. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, M.E.; Donovan, K.A.; Forschner-Dancause, S.R.; Rowley, D.C. Gram-positive marine bacteria as a potential resource for the discovery of quorum sensing inhibitors. Mar. Biotechnol. 2011, 13, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Decho, A.W.; Browne, K.A.; Zimmer-Faust, R.K. Chemical cues: Why basic peptides are signal molecules in marine environments. Limnol. Oceanogr. 1998, 43, 1410–1417. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Woods, A.; Cotter, R.J.; Sun, Z. Resuscitation of dormant Mycobacterium tuberculosis by phospholipids or specific peptides. Biochem. Biophys. Res. Commun. 2001, 284, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Mizrahi, V. Resuscitation-promoting factors as lytic enzymes for bacterial growth and signaling. FEMS Immunol. Med. Microbiol. 2010, 58, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.; Reen, F.J.; Baysse, C.; Mooij, M.J.; O’Gara, F. Subinhibitory concentrations of the cationic antimicrobial peptide colistin induce the pseudomonas quinolone signal in Pseudomonas aeruginosa. Microbiology 2009, 155, 2826–2837. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Kuchma, S.L.; O’Toole, G.A. Keeping their options open: Acute versus persistent infections. J. Bacteriol. 2006, 188, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A.; Guthmiller, J.M.; Taylor, C.E. Human polymicrobial infections. Lancet 2005, 365, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.M.; Mack, J.L.; Hall, P.R.; Alsup, A.A.; Alexander, S.M.; Sully, E.K.; Sawires, Y.S.; Cheung, A.L.; Otto, M.; Gresham, H.D. Apolipoprotein B is an innate barrier against invasive Staphylococcus aureus infection. Cell Host Microbe 2008, 4, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Sussmuth, R.; Vuong, C.; Jung, G.; Gotz, F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999, 450, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Giacometti, A.; Cirioni, O.; Gov, Y.; Ghiselli, R.; Mocchegiani, F.; Viticchi, C.; del Prete, M.S.; Saba, V.; Scalise, G.; et al. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 2003, 187, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Fernandes, M.M.; Tzanov, T. Current advances on bacterial pathogenesis inhibition and treatment strategies. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 4. [Google Scholar]
- Giacometti, A.; Cirioni, O.; Gov, Y.; Ghiselli, R.; Del Prete, M.S.; Mocchegiani, F.; Saba, V.; Orlando, F.; Scalise, G.; Balaban, N.; et al. RNA III inhibiting peptide inhibits in vivo biofilm formation by drug-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Kiran, M.D.; Adikesavan, N.V.; Cirioni, O.; Giacometti, A.; Silvestri, C.; Scalise, G.; Ghiselli, R.; Saba, V.; Orlando, F.; Shoham, M.; et al. Discovery of a quorum-sensing inhibitor of drug-resistant Staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008, 73, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Vanderah, D.J.; Hollenbeak, K.H.; Enwall, C.E.; Gopichand, Y.; Sengupta, P.K.; Hossain, M.B.; van der Helm, D. Metabolites from the marine sponge Tedania ignis. A new atisanediol and several known diketopiperazines. J. Organ. Chem. 1983, 48, 3941–3945. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an Antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.C.; Cardellina, J.H., 2nd; Singleton, F.L. A marine micrococcus produces metabolites ascribed to the sponge Tedania ignis. Experientia 1988, 44, 1021. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Mitova, M.; Tommonaro, G. Marine bacteria associated with sponge as source of cyclic peptides. Biomol. Eng. 2003, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Ram Chhabra, S.; de Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D.; et al. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol. Microbiol. 1999, 33, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Naik, D.N.; Wahidullah, S.; Meena, R.M. Attenuation of Pseudomonas aeruginosa virulence by marine invertebrate-derived Streptomyces sp. Lett. Appl. Microbiol. 2013, 56, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Mansson, M.; Gram, L.; Larsen, T.O. Production of bioactive secondary metabolites by marine Vibrionaceae. Mar. Drugs 2011, 9, 1440–1468. [Google Scholar] [CrossRef] [PubMed]
- Mansson, M.; Nielsen, A.; Kjaerulff, L.; Gotfredsen, C.H.; Wietz, M.; Ingmer, H.; Gram, L.; Larsen, T.O. Inhibition of virulence gene expression in Staphylococcus aureus by novel depsipeptides from a marine Photobacterium. Mar. Drugs 2011, 9, 2537–2552. [Google Scholar] [CrossRef] [PubMed]
- Desriac, F.; Jegou, C.; Balnois, E.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Antimicrobial peptides from marine Proteobacteria. Mar. Drugs 2013, 11, 3632–3660. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Russell, R.; Moreira, C.G.; Adebesin, A.M.; Wang, C.; Williams, N.S.; Taussig, R.; Stewart, D.; Zimmern, P.; Lu, B.; et al. QseC inhibitors as an antivirulence approach for Gram-negative pathogens. mBio 2014, 5, e02165. [Google Scholar] [CrossRef] [PubMed]
- Karavolos, M.H.; Winzer, K.; Williams, P.; Khan, C.M. Pathogen espionage: Multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol. Microbiol. 2013, 87, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Parrilli, E.; Sannino, F.; Barbato, G.; Tutino, M.L.; Artini, M.; Selan, L. Anti-biofilm activity of the antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res. Microbiol. 2013, 164, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sayem, S.M.; Manzo, E.; Ciavatta, L.; Tramice, A.; Cordone, A.; Zanfardino, A.; de Felice, M.; Varcamonti, M. Anti-biofilm activity of an exopolysaccharide from a sponge-associated strain of Bacillus licheniformis. Microb. Cell Fact. 2011, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, R.; Nithyanand, P.; Rathna, J.; Pandian, S.K. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 2009, 57, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Mukherjee, S.; Sen, R. Antiadhesive action of a marine microbial surfactant. Colloids Surf. B Biointerfaces 2009, 71, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Dheilly, A.; Soum-Soutera, E.; Klein, G.L.; Bazire, A.; Compere, C.; Haras, D.; Dufour, A. Antibiofilm activity of the marine bacterium Pseudoalteromonas sp. Strain 3J6. Appl. Environ. Microbiol. 2010, 76, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Paillard, C.; Dufour, A.; Bazire, A. Antibiofilm activity of the marine bacterium Pseudoalteromonas sp. 3J6 against Vibrio tapetis, the causative agent of brown ring disease. Probiotics Antimicrob. Proteins 2015, 7, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Jansson, J.; Field, D.; Fierer, N.; Desai, N.; Fuhrman, J.A.; Hugenholtz, P.; van der Lelie, D.; Meyer, F.; Stevens, R.; et al. Unlocking the potential of metagenomics through replicated experimental design. Nat. Biotechnol. 2012, 30, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, H. Targeted metagenomics: A high-resolution metagenomics approach for specific gene clusters in complex microbial communities. Environ. Microbiol. 2012, 14, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Mori, T.; Ruckert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.; Heycke, N.; Schmitt, S.; et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.; Taylor, T.D. Functional assignment of metagenomic data: Challenges and applications. Brief. Bioinform. 2012, 13, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.B.; Lo, C.C.; Chain, P.S. Next generation sequencing and bioinformatic bottlenecks: The current state of metagenomic data analysis. Curr. Opin. Biotechnol. 2012, 23, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Teeling, H.; Glockner, F.O. Current opportunities and challenges in microbial metagenome analysis—A bioinformatic perspective. Brief. Bioinf. 2012, 13, 728–742. [Google Scholar] [CrossRef]
- Wooley, J.C.; Godzik, A.; Friedberg, I. A primer on metagenomics. PLoS Comput. Biol. 2010, 6, e1000667. [Google Scholar] [CrossRef] [PubMed]
- Wooley, J.C.; Ye, Y. Metagenomics: Facts and artifacts, and computational challenges*. J. Comput. Sci. Technol. 2009, 25, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Biggins, J.B.; Ternei, M.A.; Brady, S.F. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J. Am. Chem. Soc. 2012, 134, 13192–13195. [Google Scholar] [CrossRef] [PubMed]
- Gatte-Picchi, D.; Weiz, A.; Ishida, K.; Hertweck, C.; Dittmann, E. Functional analysis of environmental DNA-derived microviridins provides new insights into the diversity of the tricyclic peptide family. Appl. Environ. Microbiol. 2014, 80, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.Y.; Sanchez, J.F.; Wang, C.C.; Keller, N.P. Toward awakening cryptic secondary metabolite gene clusters in filamentous fungi. Methods Enzymol. 2012, 517, 303–324. [Google Scholar] [PubMed]
- Luo, Y.; Huang, H.; Liang, J.; Wang, M.; Lu, L.; Shao, Z.; Cobb, R.E.; Zhao, H. Activation and characterization of a cryptic polycyclic tetramate macrolactam biosynthetic gene cluster. Nat. Commun. 2013, 4, 2894. [Google Scholar] [PubMed]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Medema, M.H.; Kazempour, D.; Fischbach, M.A.; Breitling, R.; Takano, E.; Weber, T. AntiSMASH 2.0—A versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013, 41, W204–W212. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; van Raaphorst, R.; Takano, E.; Breitling, R. Computational tools for the synthetic design of biochemical pathways. Nat. Rev. Microbiol. 2012, 10, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Egan, F.; Reen, F.J.; O’Gara, F. Tle distribution and diversity in metagenomic datasets reveal niche specialization. Environ. Microbiol. Rep. 2014, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, I.C.; Voet, T. Single cell genomics: Advances and future perspectives. PLoS Genet. 2014, 10, e1004126. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fujita, D.; Hanagata, N. Perspectives and challenges of emerging single-molecule DNA sequencing technologies. Small 2009, 5, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Non-genetic heterogeneity of cells in development: More than just noise. Development 2009, 136, 3853–3862. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tolar, B.B.; Swan, B.K.; Zhang, C.L.; Stepanauskas, R.; Ann Moran, M.; Hollibaugh, J.T. Single-cell genomics shedding light on marine Thaumarchaeota diversification. ISME J. 2014, 8, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Price, D.C.; Stepanauskas, R.; Rajah, V.D.; Sieracki, M.E.; Wilson, W.H.; Yang, E.C.; Duffy, S.; Bhattacharya, D. Single-cell genomics reveals organismal interactions in uncultivated marine protists. Science 2011, 332, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.S.; Price, D.C.; Schliep, A.; Cai, G.; Korobeynikov, A.; Yoon, H.S.; Yang, E.C.; Bhattacharya, D. Single cell genome analysis of an uncultured heterotrophic Stramenopile. Sci. Rep. 2014, 4, 4780. [Google Scholar] [PubMed]
- Barone, R.; de Santi, C.; Palma Esposito, F.; Tedesco, P.; Galati, F.; Visone, M.; DiScala, A.; de Pascale, D. Marine metagenomics, a valuable tool for enzymes and bioactive compounds discovery. Front. Mar. Sci. 2014, 1, 1–6. [Google Scholar] [CrossRef]
- Grindberg, R.V.; Ishoey, T.; Brinza, D.; Esquenazi, E.; Coates, R.C.; Liu, W.T.; Gerwick, L.; Dorrestein, P.C.; Pevzner, P.; Lasken, R.; et al. Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS ONE 2011, 6, e18565. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Jenssen, H.; Hilpert, K.; Cheung, W.A.; Pante, N.; Hancock, R.E.W.; Cherkasov, A. Identification of novel antibacterial peptides by chemoinformatics and machine learning. J. Med. Chem. 2009, 52, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B. Machine learning methods in chemoinformatics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 468–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frecer, V. QSAR analysis of antimicrobial and haemolytic effects of cyclic cationic antimicrobial peptides derived from protegrin-1. Bioorgan. Med. Chem. 2006, 14, 6065–6074. [Google Scholar] [CrossRef]
- Frecer, V.; Ho, B.; Ding, J.L. De novo design of potent antimicrobial peptides. Antimicrob. Agents Chemother. 2004, 48, 3349–3357. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, N.; Kaznessis, Y. Protegrin structure-activity relationships: Using homology models of synthetic sequences to determine structural characteristics important for activity. Peptides 2005, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sushko, Y.; Novotarskyi, S.; Korner, R.; Vogt, J.; Abdelaziz, A.; Tetko, I.V. Prediction-driven matched molecular pairs to interpret QSARs and aid the molecular optimization process. J. Cheminf. 2014, 6, 48. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef] [PubMed]
- Kersten, R.D.; Yang, Y.L.; Xu, Y.; Cimermancic, P.; Nam, S.J.; Fenical, W.; Fischbach, M.A.; Moore, B.S.; Dorrestein, P.C. A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nat. Chem. Biol. 2011, 7, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Paalvast, Y.; Nguyen, D.D.; Melnik, A.; Dorrestein, P.C.; Takano, E.; Breitling, R. Pep2path: Automated mass spectrometry-guided genome mining of peptidic natural products. PLoS Comput. Biol. 2014, 10, e1003822. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Collins, J.J. Synthetic biology: Applications come of age. Nat. Rev. Genet. 2010, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Neumann-Staubitz, P. Synthetic biology approaches in drug discovery and pharmaceutical biotechnology. Appl. Microbiol. Biotechnol. 2010, 87, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Bloch, J.F.; Tardieu-Guigues, E. Marine biotechnologies and synthetic biology, new issues for a fair and equitable profit-sharing commercial use. Mar. Genomics 2014, 17, 79–83. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reen, F.J.; Gutiérrez-Barranquero, J.A.; Dobson, A.D.W.; Adams, C.; O'Gara, F. Emerging Concepts Promising New Horizons for Marine Biodiscovery and Synthetic Biology. Mar. Drugs 2015, 13, 2924-2954. https://doi.org/10.3390/md13052924
Reen FJ, Gutiérrez-Barranquero JA, Dobson ADW, Adams C, O'Gara F. Emerging Concepts Promising New Horizons for Marine Biodiscovery and Synthetic Biology. Marine Drugs. 2015; 13(5):2924-2954. https://doi.org/10.3390/md13052924
Chicago/Turabian StyleReen, F. Jerry, José A. Gutiérrez-Barranquero, Alan D. W. Dobson, Claire Adams, and Fergal O'Gara. 2015. "Emerging Concepts Promising New Horizons for Marine Biodiscovery and Synthetic Biology" Marine Drugs 13, no. 5: 2924-2954. https://doi.org/10.3390/md13052924