Piscidin is Highly Active against Carbapenem-Resistant Acinetobacter baumannii and NDM-1-Producing Klebsiella pneumonia in a Systemic Septicaemia Infection Mouse Model
Abstract
:1. Introduction
2. Results
2.1. In Vitro Efficacy of TP3 and TP4
| (a) | |||||
|---|---|---|---|---|---|
| Bacterial Strain | TP3 | TP4 | Piscidin-1 | Ampicillin | Imipenem |
| (μg/mL) | (μg/mL) | (μg/mL) | (μg/mL) | (μg/mL) | |
| K. pneumoniae (YT32) | 3.125 | 3.125 | 50 | ND | <1.56 |
| E. coli (YT39) | <1.56 | <1.56 | 25 | ND | <1.56 |
| E. coli (YT154) | <1.56 | <1.56 | 3.125 | ND | <1.56 |
| A. baumannii (Icu53) | <1.56 | <1.56 | 6.25 | ND | <1.56 |
| A. baumannii (Sk44) | 12.5 | <1.56 | 3.125 | ND | 50 |
| K. pneumoniae (NDM-1) | 25 | 3.125 | 50 | ND | 3.125 |
| K. pneumoniae (blaNDM-1) | 3.125 | ND | 50 | ND | <1.56 |
| (b) | |||||
| Bacterial Strain | TP3 | TP4 | Piscidin-1 | Ampicillin | Imipenem |
| (μg/mL) | (μg/mL) | (μg/mL) | (μg/mL) | (μg/mL) | |
| K. pneumoniae (YT32) | 3.125 | 3.125 | 50 | ND | 1.56 |
| E. coli (YT39) | 1.56 | 1.56 | 25 | ND | 1.56 |
| E. coli (YT154) | 1.56 | 1.56 | 3.125 | ND | 1.56 |
| A. baumannii (Icu53) | 1.56 | 1.56 | 6.25 | ND | 12 |
| A. baumannii (Sk44) | 25 | 1.56 | 25 | ND | 50 |
| K. pneumoniae (NDM-1) | 25 | 3.125 | 50 | ND | 6 |
| K. pneumoniae (blaNDM-1) | 3.125 | ND | 50 | ND | 6 |
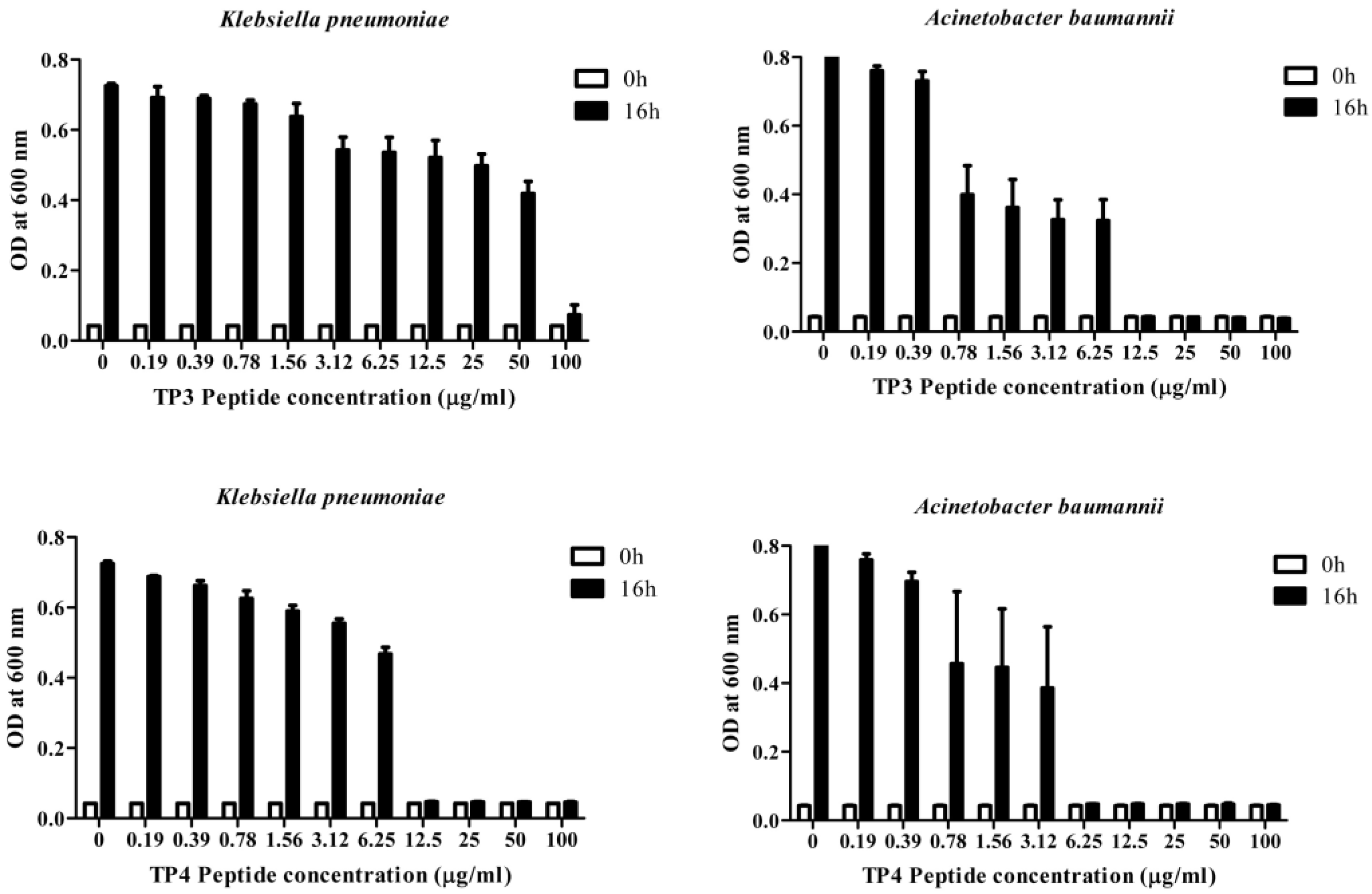
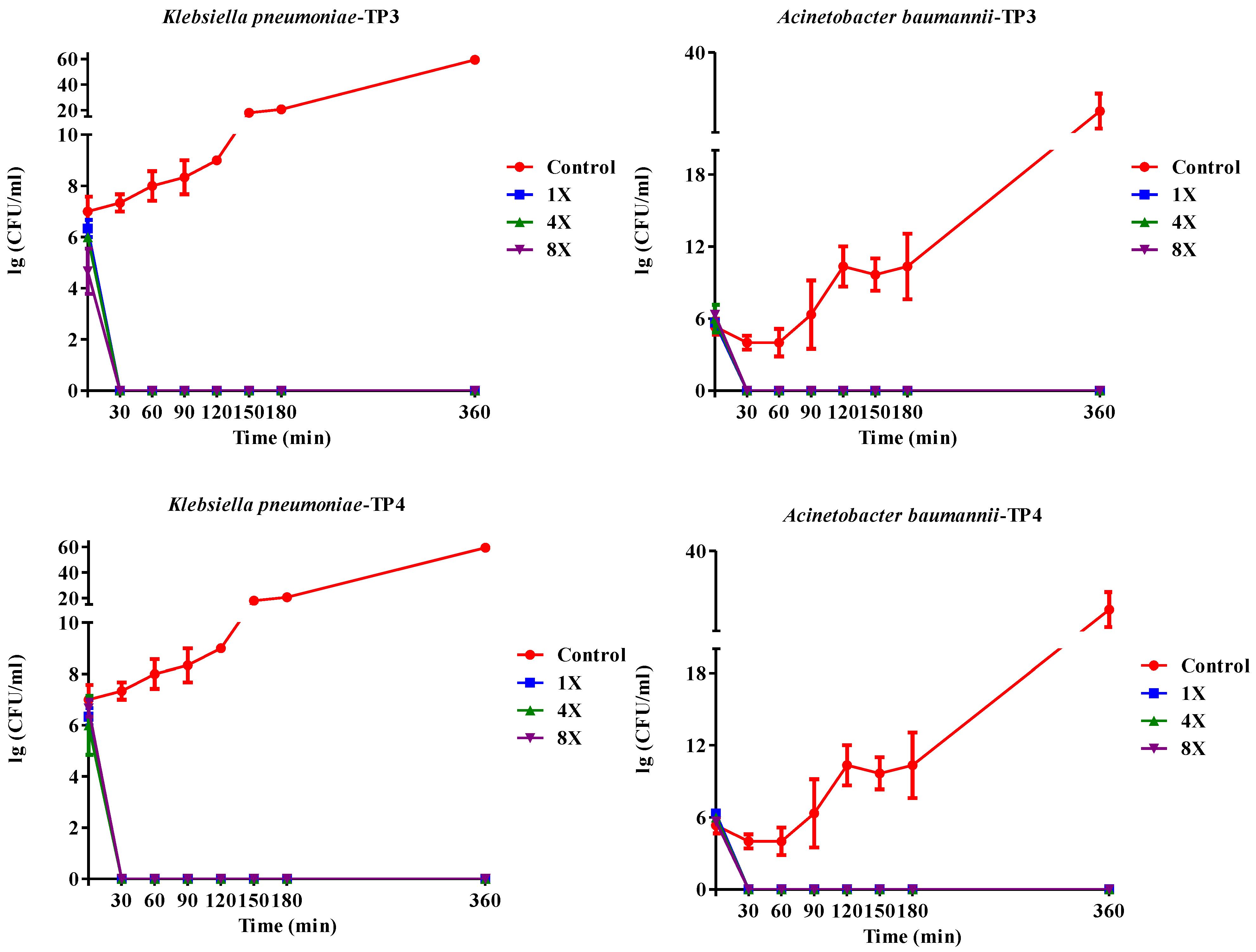
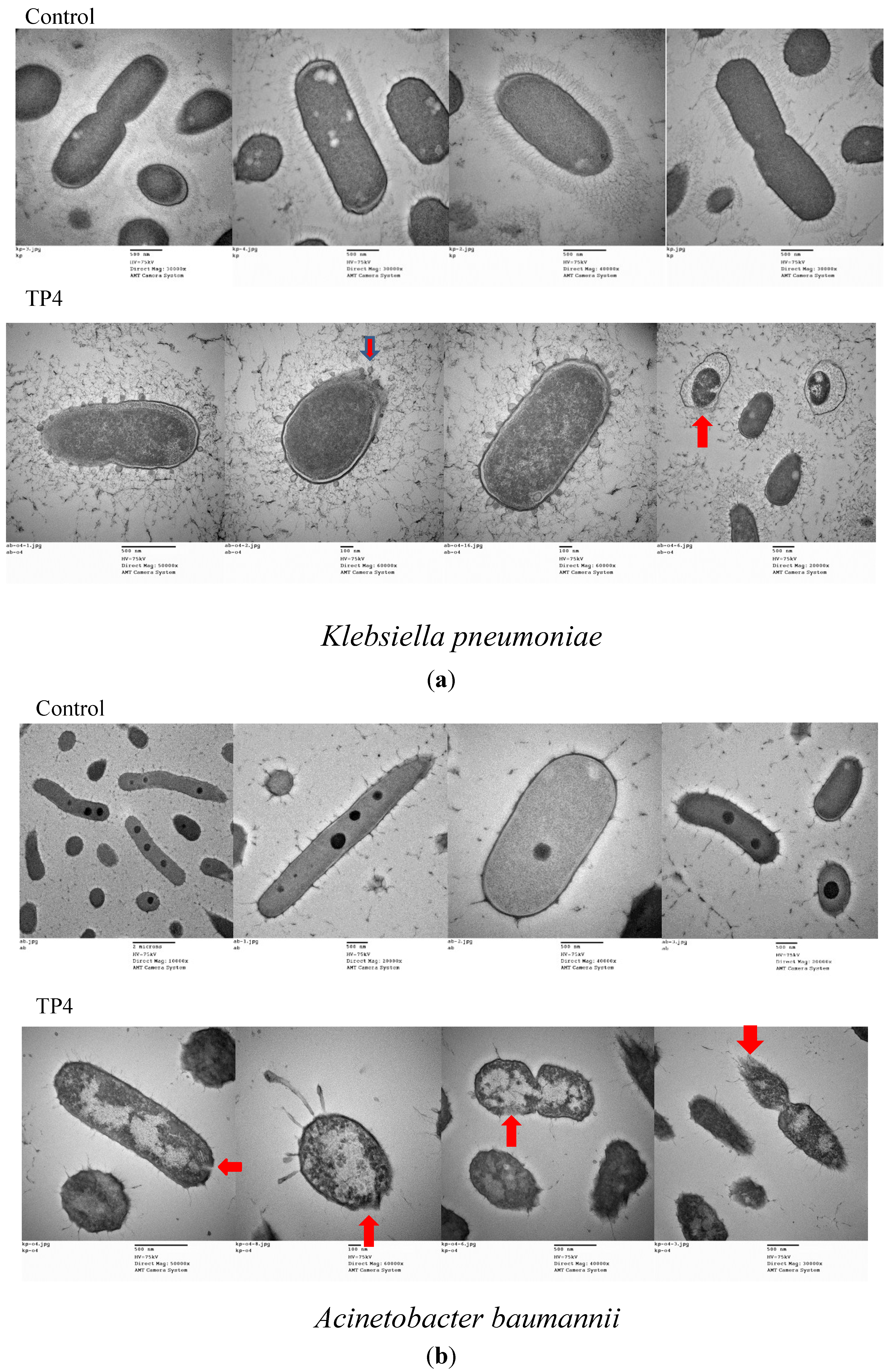
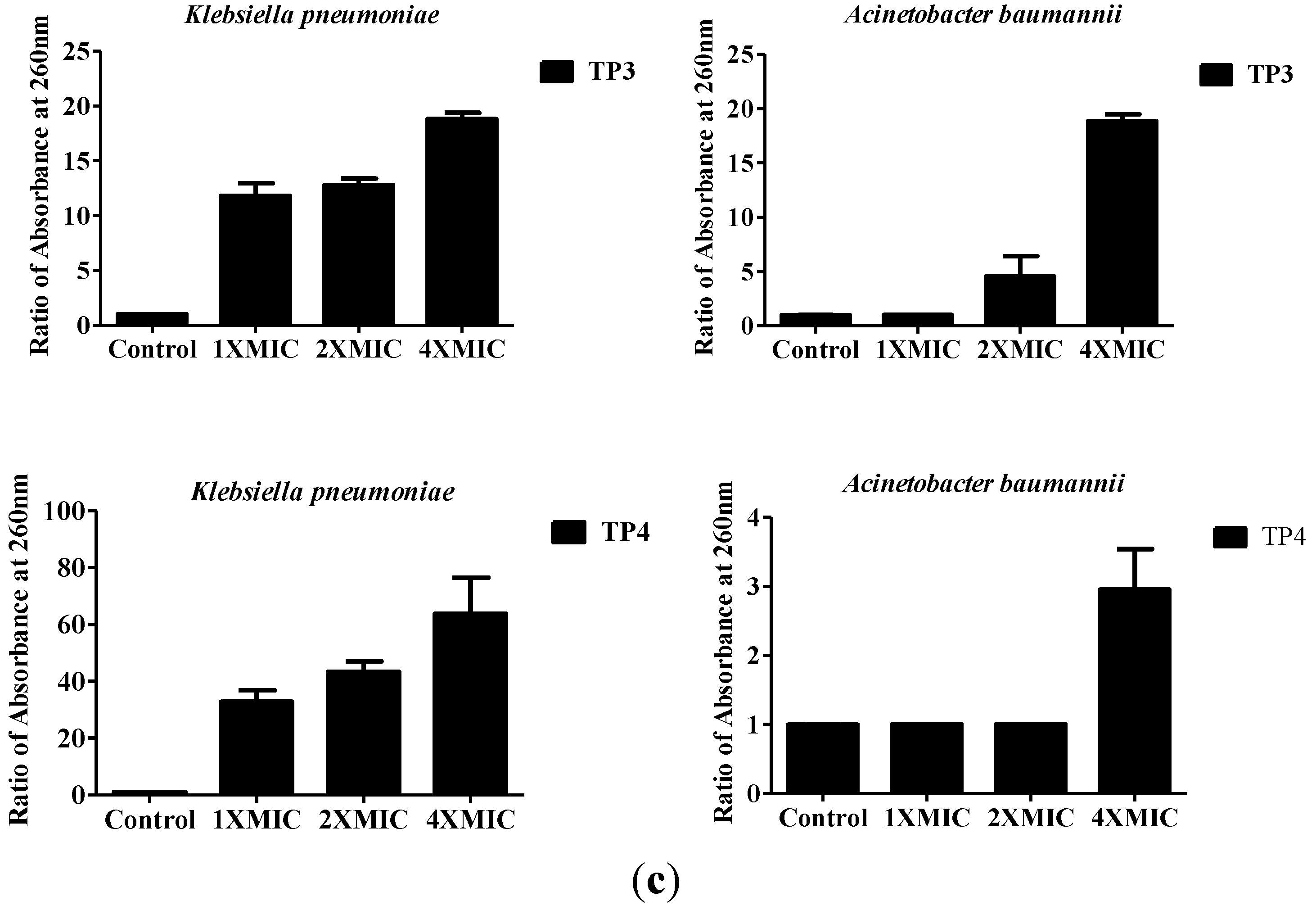
2.2. In Vivo Efficacy of TP3 and TP4 against Infections Caused by A. Baumannii (Sk44) and K. Pneumonia (NDM-1)
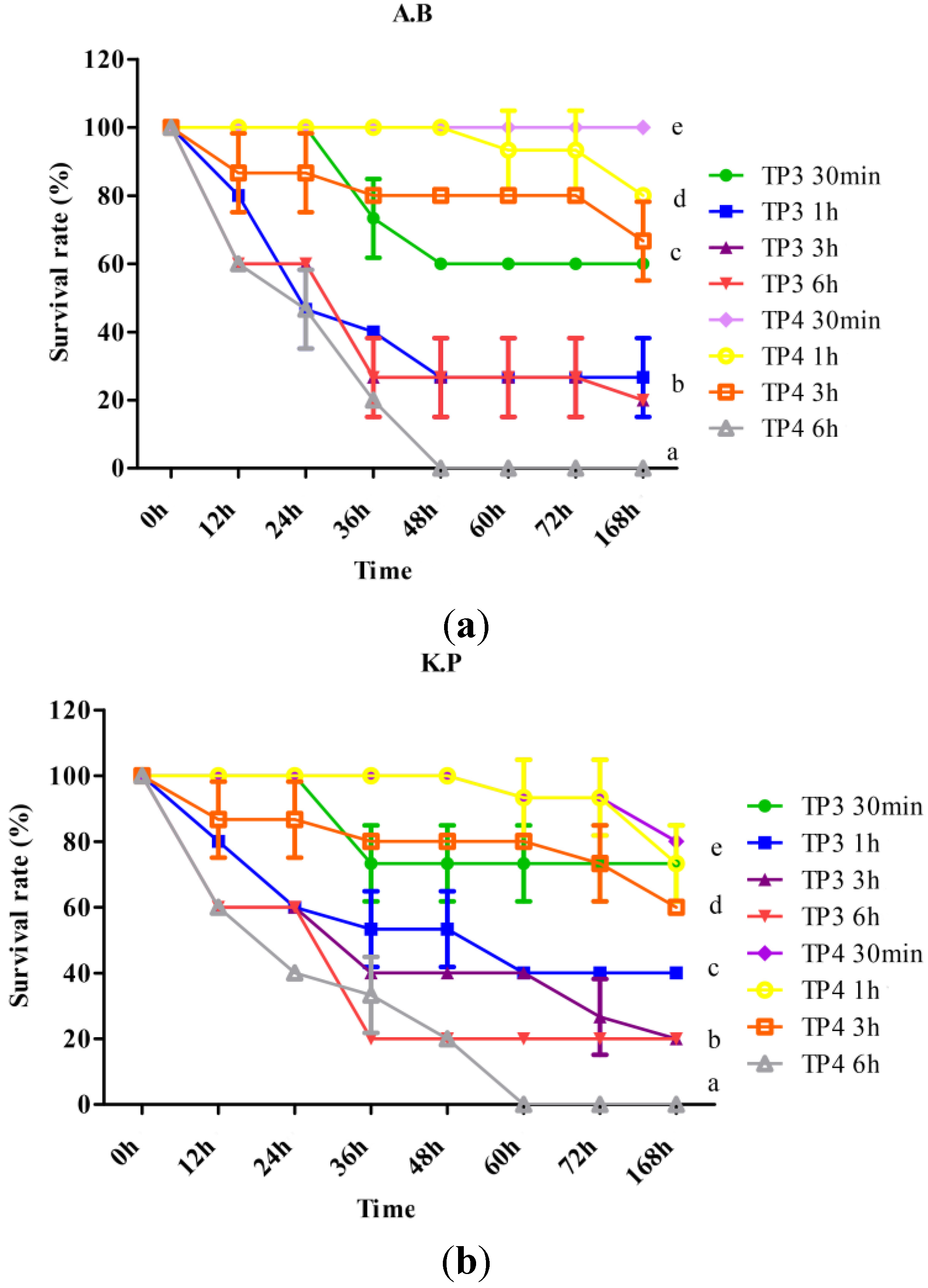
| Treatment | Survival (%) KP | Survival (%) AB |
|---|---|---|
| Control | 0 ± 0 | 0 ± 0 |
| TP3 | 71.1 ± 11.7 (P < 0.05) | 62.2 ± 3.8 (P < 0.05) |
| TP4 | 88.8 ± 19.24 (P < 0.05) | 93.3 ± 11.5 (P < 0.05) |
| AMP | 0 ± 0 | 8.8 ± 8.3 |
| IMP | 45.5 ± 32.03 (P < 0.05) | 46.6 ± 41.6 (P < 0.05) |
| TIG | 46.6 ± 41.63 (P < 0.05) | 65.5 ± 43.5 (P < 0.05) |
2.3. TP3 and TP4 Exhibit in Vivo Bacteriostatic Properties against A. Baumannii (Sk44) and K. Pneumonia (NDM-1)
| Treatment (KP) | Bacterial Count in Blood (CFU/mL) | Bacterial Count in Peritoneum (CFU/mL) | Bacterial Count in Spleen (CFU/mL) | Bacterial Count in Liver (CFU/mL) | Bacterial Count in mesenteric lymph nodes (CFU/mL) |
|---|---|---|---|---|---|
| No treatment | 4.2 × 104 ± 1.2 × 104 c | 3.8 × 107 ± 0.9 × 107 e | 6.6 × 107 ± 2.2 × 107 e | 6.9 × 107 ± 3.8 × 107 e | 6.5 × 107 ± 1.9 × 107 e |
| TP3 (150 μg/mouse) | 2.4 × 103 ± 0.2 × 103 b | 1.4 × 107 ± 0.2 × 107 e | 1.6 × 107 ± 1.3 × 107 e | 1.6 × 107 ± 0.2 × 107 e | 2.8 × 107 ± 1.5 × 107 e |
| TP4 (50 μg/mouse) | 0.2 × 102 ± 0.1 × 102 a | 8.6 × 106 ± 4.1 × 106 d | 2.3 × 107 ± 0.9 × 107 e | 1.2 × 107 ± 0.6 × 10 7 e | 1.4 × 107 ± 1.1 × 107 e |
| TIG (100 μg/mouse) | 0.2 × 102 ± 0.1 × 102 a | 7.0 × 106 ± 6.7 × 106 d | 2.3 × 107 ± 1.2 × 107 e | 6.2 × 106 ± 1.0 × 106 d | 1.5 × 107 ± 0.1 × 107 e |
| IMP (100 μg/mouse) | 2.3 × 102 ± 1.6 × 102 a | 9.4 × 106 ± 1.0 × 106 d | 1.2 × 107 ± 0.4 × 107 e | 4.2 × 106 ± 0.4 × 106 d | 1.3 × 107 ± 1.3 × 107 e |
| AMP (100 μg/mouse) | 3.2 × 104 ± 2.0 × 104 c | 3.2 × 107 ± 2.4 × 107 e | 3.1 × 107 ± 0.5 × 107 e | 1.7 × 106 ± 0.4 × 107 e | 2.3 × 107 ± 0.5 × 107 e |
| Treatment (AB) | Bacterial Count in Blood (CFU/mL) | Bacterial Count in Peritoneum (CFU/mL) | Bacterial Count in Spleen (CFU/mL) | Bacterial Count in Liver (CFU/mL) | Bacterial Count in mesenteric lymph nodes (CFU/mL) |
| No treatment | 3.6 × 106 ± 2.3 × 106 d | 7.3 × 108 ± 2.3 × 108 f | 7.8 × 108 ± 1.2 × 108 f | 3.3 × 109 ± 5.9 × 108 g | 9.2 × 108 ± 5.4 × 108 f |
| TP3 (150 μg/mouse) | 1.4 × 103 ± 0.1 × 103 b | 5.8 × 107 ± 2.9 × 107 e | 2.1 × 107 ± 1.0 × 107 e | 1.7 × 107 ± 0.8 × 107 e | 2.6 × 107 ± 1.0 × 107 e |
| TP4 (50 μg/mouse) | 0.2 × 102 ± 1.0 × 102 a | 7.8 × 107 ± 1.1 × 107 e | 9.2 × 107 ± 1.4 × 107 e | 2.4 × 106 ± 0.4 × 106 d | 1.1 × 107 ± 0.1 × 107 e |
| TIG (100 μg/mouse) | 0.4 × 102 ± 0.2 × 102 a | 1.4 × 107 ± 0.1 × 107 e | 2.3 × 107 ± 0.3 × 107 e | 2.8 × 107 ± 0.3 × 107 e | 1.7 × 106 ± 0.2 × 106 d |
| IMP (100 μg/mouse) | 3.2 × 104 ± 1.8 × 104 c | 3.8 × 107 ± 3.1 × 107 e | 2.4 × 108 ± 2.3 × 108 f | 2.1 × 108 ± 2.1 × 108 f | 4.7 × 107 ± 1.6 × 107 e |
| AMP (100 μg/mouse) | 2.4 × 106± 0.4 × 106 d | 5.9 × 107 ± 3.6 × 107 e | 2.3 × 108 ± 0.1 × 108 f | 3.0 × 109 ± 1.9 × 109 g | 4.0 × 108 ± 2.0 × 108 f |
2.4. TP3 and TP4 Do Not Exert Acute Toxic Effects in Mice
| Dose (mg/mouse) | Mice(n) Receiving TP3 | Mice(n) Receiving TP4 | Mice(n) Receiving IMP | Mice(n) Receiving TIG |
|---|---|---|---|---|
| 0.1 | 3, no effect | 3, no effect | 3, no effect | 3, no effect |
| 0.5 | 3, no effect | 3, no effect | 3, no effect | 3, no effect |
| 1 | 3, no effect | 3, no effect | 3, no effect | 3, no effect |
| 1.5 | 3, no effect | 2, no effect; 1, toxicity level 1 | 3, no effect | 2, no effect; 1, toxicity level 1 |
| 2 | 2, no effect; 1, toxicity level 1 | 2, no effect; 1, toxicity level 1 | 2, no effect; 1, toxicity level 1 | 1, no effect; 1, toxicity level 1; 1, toxicity level 2 |
3. Discussion
| (a) | ||||||
|---|---|---|---|---|---|---|
| Treatment | GOT (U/L) | GPT (U/L) | BUN (mg/dL) | CRE (mg/dL) | UA (mg/dL) | TBIL (mg/dL) |
| Control | 11.10 ± 4.60 | 6.10 ± 2.80 | 1.71 ± 0.09 | <0.10 | 0.47 ± 0.06 | 0.11 ± 0.05 |
| TP3 | 12.90 ± 5.90 (P > 0.05) | 7.80 ± 5.00 (P > 0.05) | 1.77 ± 0.10 (P > 0.05) | <0.10 | 0.55 ± 0.10 (P > 0.05) | 0.19 ± 0.05 (P > 0.05) |
| TP4 | 7.40 ± 2.20 (P > 0.05) | 5.80 ± 1.30 (P > 0.05) | 1.65 ± 0.08 (P > 0.05) | <0.10 | 0.52 ± 0.09 (P > 0.05) | 0.16 ± 0.05 (P > 0.05) |
| (b) | ||||||
| Treatment | GOT (U/L) | GPT (U/L) | BUN (mg/dL) | CRE (mg/dL) | UA (mg/dL) | TBIL (mg/dL) |
| Control | 8.67 ± 0.58 ab | 5.33 ± 0.58 ab | 1.67 ± 0.15 (P > 0.05) | <0.10 | 0.20 ± 0.1 a | 0.23 ± 0.06 c |
| TP3 | 10.60 ± 2.51 a | 6.60 ± 1.52 b | 1.73 ± 0.11 (P > 0.05) | <0.10 | 0.50 ± 0.10 c | 0.20 ± 0.00 bc |
| TP4 | 8.00 ± 2.00 ab | 6.30 ± 0.57 b | 1.90 ± 0.20 (P > 0.05) | <0.10 | 0.33 ± 0.05 abc | 0.13 ± 0.05 ab |
| TIG | 8.00 ± 1.73 b | 4.33 ± 0.57 a | 1.33 ± 1.09 (P > 0.05) | <0.10 | 0.43 ± 0.11 bc | 0.06 ± 0.00 a |
| IMP | 8.00 ± 1.00 ab | 5.30 ± 0.57 ab | 1.76 ± 0.15 (P > 0.05) | <0.10 | 0.26±0.11 ab | 0.10 ± 0.10 a |
| AMP | 8.00 ± 1.00 ab | 6.30 ± 0.57 b | 1.86 ± 0.05 (P > 0.05) | <0.10 | 0.20 ± 0.10 a | 0.10 ± 0.00 a |
4. Experimental Section
4.1. Materials and Microorganisms
4.2. Determination of Minimum Inhibitory Concentrations (MICs) and Killing Efficiency
4.3. Assessing Bacterial Membrane Integrity in the Presence of TP3 and TP4
4.4. Mouse Model of Sepsis
4.5. In Vivo Toxicity
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nordmann, P.; Poirel, L.; Toleman, M.A.; Walsh, T.R. Does broad-spectrum beta-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 2011, 66, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Shakil, S.; Azhar, E.I.; Tabrez, S.; Kamal, M.A.; Jabir, N.R.; Abuzenadah, A.M.; Damanhouri, G.A.; Alam, Q. New Delhi metallo-β-lactamase (NDM-1): An update. J. Chemother. 2011, 23, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Fiett, J.; Baraniak, A.; Izdebski, R.; Sitkiewicz, I.; Żabicka, D.; Meler, A.; Filczak, K.; Hryniewicz, W.; Gniadkowski, M. The first NDM metallo-β-lactamase-producing Enterobacteriaceae isolate in Poland: Evolution of IncFII-type plasmids carrying the blaNDM-1 gene. Antimicrob. Agents Chemother. 2014, 58, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Abbo, A.; Navon-Venezia, S.; Hammer-Muntz, O.; Krichali, T.; Siegman-Igra, Y.; Carmeli, Y. Multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 2005, 11, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Esposito, S.; Petrosillo, N.; Nicolini, L. Drug treatment for multidrug-resistant Acinetobacter baumannii infections. Future Microbiol. 2008, 3, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Abramson, M.A.; Beekmann, S.E.; Gallagher, G.; Riedel, S.; Diekema, D.J.; Quinn, J.P.; Doern, G.V. Antimicrobial resistance among Gram-negative Bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J. Clin. Microbiol. 2007, 45, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Huang, T.C.; Muthusamy, S.; Lee, J.F.; Duann, Y.F.; Lin, C.H. Piscidin-1, an antimicrobial peptide from fish (hybrid striped bass Morone saxatilis × M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zool. Sci. 2012, 29, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Pan, C.Y.; Rajanbabu, V.; Chan, Y.L.; Wu, C.J.; Chen, J.Y. Modulation of immune responses by the antimicrobial peptide, epinecidin (Epi)-1, and establishment of an Epi-1-based inactivated vaccine. Biomaterials 2011, 32, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, V.; Chen, J.Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Silphaduang, U.; Noga, E.J. Peptide antibiotics in mast cells of fish. Nature 2001, 414, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.F.; Jin, Y.; Xu, X.; Qiao, Y.; Wu, Y.; Mao, Y.; Su, Y.Q.; Wang, J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellf. Immunol. 2013, 35, 513–524. [Google Scholar] [CrossRef]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Chien, Y.L.; Pan, C.Y.; Lin, T.L.; Chen, J.Y.; Chiu, S.J.; Hui, C.F. Epinecidin-1, an antimicrobial peptide from fish (Epinephelus coioides) which has an antitumor effect like lytic peptides in human fibrosarcoma cells. Peptides 2009, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.C.; Lee, S.H.; Hour, A.L.; Pan, C.Y.; Lee, L.H.; Chen, J.Y. Five different piscidins from Nile Tilapia, Oreochromis niloticus: Analysis of their expressions and biological functions. PLoS ONE 2012, 7, e50263. [Google Scholar] [CrossRef] [PubMed]
- Mulero, I.; Noga, E.J.; Meseguer, J.; García-Ayala, A.; Mulero, V. The antimicrobial peptides piscidins are stored in the granules of professional phagocytic granulocytes of fish and are delivered to the bacteria-containing phagosome upon phagocytosis. Dev. Comp. Immunol. 2008, 32, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Silphaduang, U.; Colorni, A.; Noga, E.J. Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis. Aquat. Organ. 2006, 72, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Rastegar Lari, A.; Azimi, L.; Rahbar, M.; Fallah, F.; Alaghehbandan, R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: First report from Iran. Burns 2013, 39, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Dartois, N.; Gandjini, H.; Yan, J.L.; Korth-Bradley, J.; McGovern, P.C. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob. Agents Chemother. 2013, 57, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, R.; Borrás-Blasco, J.; Sirvent, A.E.; Padilla, S.; Navarro-Ruiz, A.; Solavera, J.; Amoros, F. Imipenem-induced Clostridium difficile diarrhea in a patient with chronic renal failure. Saudi. J. Kidney Dis. Transpl. 2011, 22, 541–543. [Google Scholar] [PubMed]
- Albur, M.; Noel, A.; Bowker, K.; MacGowan, A. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2012, 56, 3441–3443. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Pharmacokinetic considerations regarding tigecycline for multidrug-resistant (MDR) Klebsiella pneumoniae or MDR Acinetobacter baumannii urosepsis. J. Clin. Microbiol. 2009, 47, 1613. [Google Scholar] [CrossRef] [PubMed]
- Haeili, M.; Ghodousi, A.; Nomanpour, B.; Omrani, M.; Feizabadi, M.M. Drug resistance patterns of bacteria isolated from patients with nosocomial pneumonia at Tehran hospitals during 2009–2011. J. Infect. Dev. Ctries 2013, 7, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, K.; Macielag, M.; Weidner-Wells, M. Taking inventory: Antibacterial agents currently at or beyond phase 1. Curr. Opin. Microbiol. 2004, 7, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr.; Wade, J.D.; Lin, F.; Condie, B.A.; Hanrieder, J.; Hoffmann, R. Designer antibacterial peptides kill fluoroquinolone-resistant clinical isolates. J. Med. Chem. 2005, 48, 5349–5359. [Google Scholar] [CrossRef] [PubMed]
- Ostorhazi, E.; Rozgonyi, F.; Sztodola, A.; Harmos, F.; Kovalszky, I.; Szabo, D.; Knappe, D.; Hoffmann, R.; Cassone, M.; Wade, J.D.; et al. Preclinical advantages of intramuscularly administered peptide A3-APO over existing therapies in Acinetobacter baumannii wound infections. J. Antimicrob. Chemother. 2010, 65, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Khara, J.S.; Wang, Y.; Ke, X.Y.; Liu, S.; Newton, S.M.; Langford, P.R.; Yang, Y.Y.; Ee, P.L. Anti-mycobacterial activities of synthetic cationic α-helical peptides and their synergism with rifampicin. Biomaterials 2014, 35, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Batoni, G.; Esin, S.; Luperini, F.; Pardini, M.; Bottai, D.; Florio, W.; Giuca, M.R.; Gabriele, M.; Campa, M. Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob. Agents Chemother. 2003, 47, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Batoni, G.; Esin, S.; Florio, W.; Bottai, D.; Favilli, F.; Campa, M. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 2006, 50, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, P.; Rasmussen, M.; Björck, L. alpha2-Macroglobulin-proteinase complexes protect Streptococcus pyogenes from killing by the antimicrobial peptide LL-37. J. Biol. Chem. 2004, 279, 52820–52823. [Google Scholar] [CrossRef] [PubMed]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: Natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef]
- Lebel, G.; Piché, F.; Frenette, M.; Gottschalk, M.; Grenier, D. Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 2013, 50, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Byl, B.; Clevenbergh, P.; Kentos, A.; Jacobs, F.; Marchant, A.; Vincent, J.L.; Thys, J.P. Ceftazidime- and imipenem-induced endotoxin release during treatment of gram-negative infections. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, V.; Pan, C.Y.; Lee, S.C.; Lin, W.J.; Lin, C.C.; Li, C.L.; Chen, J.Y. Tilapia hepcidin 2–3 peptide modulates lipopolysaccharide-induced cytokines and inhibits tumor necrosis factor-alpha through cyclooxygenase-2 and phosphodiesterase 4D. J. Biol. Chem. 2010, 285, 30577–30586. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Falciani, C.; Mantengoli, E.; Bindi, S.; Brunetti, J.; Iozzi, S.; Rossolini, G.M.; Bracci, L. A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. FASEB J. 2010, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Mocchegiani, F.; D’Amato, G.; Del Prete, M.S.; Orlando, F.; Kamysz, W.; Lukasiak, J.; Saba, V.; et al. Administration of protegrin peptide IB-367 to prevent endotoxin induced mortality in bile duct ligated rats. Gut 2003, 52, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, A.; Papo, N.; Shai, Y. In vitro activity and potency of an intravenously injected antimicrobial peptide and its dl amino acid analog in mice infected with bacteria. Antimicrob. Agents Chemother. 2004, 48, 3127–3129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Lee, W.H.; Zhang, Y. Efficacy of OH-CATH30 and its analogs against drug-resistant bacteria in vitro and in mouse models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Nicasio, A.M.; Crandon, J.L.; Nicolau, D.P. In vivo pharmacodynamic profile of tigecycline against phenotypically diverse Escherichia coli and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2009, 53, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Garrison, M.W.; Neumiller, J.J.; Setter, S.M. Tigecycline: An investigational glycylcycline antimicrobial with activity against resistant gram-positive organisms. Clin. Ther. 2005, 27, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Sader, H.S.; Fritsche, T.R. Tigecycline activity tested against 11808 bacterial pathogens recently collected from US medical centers. Diagn. Microbiol. Infect. Dis. 2008, 60, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Chen, T.L.; Chen, I.C.; Huang, M.S.; Wang, F.D.; Fung, C.P.; Lee, S.D. First identification of a patient colonized with Klebsiella pneumoniae carrying blaNDM-1 in Taiwan. J. Chin. Med. Assoc. 2010, 73, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chen, T.L.; Lee, Y.T.; Huang, L.; Kuo, S.C.; Yu, K.W.; Hsueh, P.R.; Dou, H.Y.; Su, I.J.; Fung, C.P. Dissemination of multidrug-resistant Acinetobacter baumannii carrying BlaOxA-23 from hospitals in central Taiwan. J. Microbiol. Immunol. Infect. 2013, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chen, J.Y.; Lin, T.L.; Lin, C.H. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides 2009, 30, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Rajanbabu, V.; Chen, J.Y.; Her, G.M.; Nan, F.H. Evaluation of the epinecidin-1 peptide as an active ingredient in cleaning solutions against pathogens. Peptides 2010, 31, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. M7-A6, 6th ed.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003. [Google Scholar]
- Huang, Y.; Wiradharma, N.; Xu, K.; Ji, Z.; Bi, S.; Li, L.; Yang, Y.Y.; Fan, W. Cationic amphiphilic alpha-helical peptides for the treatment of carbapenem-resistant Acinetobacter baumannii infection. Biomaterials 2012, 33, 8841–8847. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.-Y.; Chen, J.-C.; Chen, T.-L.; Wu, J.-L.; Hui, C.-F.; Chen, J.-Y. Piscidin is Highly Active against Carbapenem-Resistant Acinetobacter baumannii and NDM-1-Producing Klebsiella pneumonia in a Systemic Septicaemia Infection Mouse Model. Mar. Drugs 2015, 13, 2287-2305. https://doi.org/10.3390/md13042287
Pan C-Y, Chen J-C, Chen T-L, Wu J-L, Hui C-F, Chen J-Y. Piscidin is Highly Active against Carbapenem-Resistant Acinetobacter baumannii and NDM-1-Producing Klebsiella pneumonia in a Systemic Septicaemia Infection Mouse Model. Marine Drugs. 2015; 13(4):2287-2305. https://doi.org/10.3390/md13042287
Chicago/Turabian StylePan, Chieh-Yu, Jian-Chyi Chen, Te-Li Chen, Jen-Leih Wu, Cho-Fat Hui, and Jyh-Yih Chen. 2015. "Piscidin is Highly Active against Carbapenem-Resistant Acinetobacter baumannii and NDM-1-Producing Klebsiella pneumonia in a Systemic Septicaemia Infection Mouse Model" Marine Drugs 13, no. 4: 2287-2305. https://doi.org/10.3390/md13042287





